-
PDF
- Split View
-
Views
-
Cite
Cite
Haribalan Perumalsamy, Nam-Jin Kim, Young-Joon Ahn, Larvicidal Activity of Compounds Isolated From Asarum heterotropoides Against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (Diptera: Culicidae) , Journal of Medical Entomology, Volume 46, Issue 6, 1 November 2009, Pages 1420–1423, https://doi.org/10.1603/033.046.0624
Close - Share Icon Share
Abstract
The toxicity of several compounds isolated from Asarum heterotropoides root steam distillate to third-instar larvae of Culex pipiens pallens Coquillett, Aedes aegypti (L.), and Ochlerotatus togoi Theobald was examined using a direct contact mortality bioassay. Safrole was the most toxic constituent to Cx. p. pallens and Ae. aegypti larvae, whereas terpinolene was most toxic to Oc. togoi. However, LC50 values of these three mosquito larvae to both essential oils as well as the remainder of the 26 compounds identified in A. heterotropoides were considerably greater than for fenthion or temephos. However, we suggest that constituents of A. heterotropoides root steam distillate merit further study as potential mosquito larvicides due to global efforts to reduce the level of highly toxic synthetic pesticides in the aquatic environment.
Plant essential oils and their constituents have been suggested as alternative sources for larval mosquito control products, in part, because they constitute a potential source of bioactive chemicals that often produce only minor adverse effects on nontarget organisms and the environment. Essential oils often act at multiple and novel target sites, thereby reducing the potential for resistance (Sukumar et al. 1991, Wink 1993, Kostyukovsky et al. 2002, Priestley et al. 2003, Isman 2006). These oils are widely available with some being relatively inexpensive compared with plant extracts such as pyrethrum and neem (Isman 2001). Much effort has been focused on essential oils and their constituents as potential sources of commercial larvicides because they meet the criteria of minimum risk pesticides (USEPA 1996, 2004). In a preliminary study by us, steam distillate from the root of Asarum heterotropoides was shown to elicit larvicidal activity against third-instar larvae of Culex pipiens pallens Coquillett (unpublished data).
We report here on the toxicity of 17 essential oil constituents isolated from A. heterotropoides root steam distillate as well as additional 11 previously known compounds isolated from this plant species against early third-instar larvae of Aedes aegypti (L.), Ochlerotatus togoi Theobald, and Cx. p. pallens. All constituents from A. heterotropoides were compared with two of widely used larvicides: fenthion and temephos.
Materials and Methods
Steam Distillation.
Air-dried root (600 g) of A. heterotropoides was pulverized and subjected to steam distillation at 100°C for 2 h by using a Clevenger-type apparatus. The volatile oil was dried over anhydrous sodium sulfate and stored in a sealed vial at 4°C until use. The yield of the distillate was 2.98% based on dried weight of the plant.
Gas Chromatography (GC).
A GC 2010 gas chromatograph (Shimadzu, Kyoto, Japan), equipped with splitless injector, was used to separate and detect the constituents of the steam distillate. Isolates were separated with a 30 m by 0.25 mm i.d. (df = 0.25 μm) DB-5 MS bonded phase, fused-silica capillary column (J&W Scientific, Ringoes, NJ). Flow rate of the helium carrier gas was 1.1 ml/min. Oven temperature was held at 50°C for 5 min, ramped to 280°C at a rate of 5°C/min, and then maintained at 280°C for 10 min. The injector temperature was 280°C. Steam distillate constituents were identified by coelution of authenticated samples after coinjection.
CG-Mass Spectroscopy (MS).
GC-MS analysis was performed using a GC 2010 gas chromatograph-QP 2010 mass spectrometer (Shimadzu). The capillary column and temperature conditions for the GC-MS analysis were the same as described above for GC analysis. Helium carrier gas column head pressure was 8.89 psi (61.3 kPa). The ion source temperature was 200°C. Interface temperature was maintained at 290°C and mass spectra obtained at 70 eV. The sector mass analyzer was set to scan from 50 to 650 amu every 0.50 s. Chemical constituents were identified by comparison of mass spectra of each peak with those of authentic samples from a mass spectra library (Anonymous 2000).
Chemicals.
Seventeen compounds identified in the A. heterotropoides root steam distillate were used in this study: borneol, camphene, 3,5-dimethoxy toluene, eucarvone, (+)-limonene, pentadecane, α-phellandrene, (–)-β-pinene, methyleugenol, 3,4,5-trimethoxy toluene, and safrole were purchased from Sigma-Aldrich (St. Louis, MO); estragole, myristicin, (+)-α-pinene, (+)-β-pinene, and γ-terpinene were purchased from Fluka (Buchs, Switzerland); and (–)-α-pinene was purchased from Wako Pure Chemicals (Osaka, Japan). In addition another 11 previously known essential oil compounds from A. heterotropoides (Tang and Eisenbrand 1992, Gong et al. 2006) also were used in this study: β-asarone, Δ3-carene, fenchene, and linalool were purchased from Sigma-Aldrich; terpinen-4-ol and verbenone were purchased from Fluka; 1,8-cineole and α-terpineol were purchased from Wako Pure Chemicals; and β-caryophyllene, myrcene, and terpinolene were purchased from Tokyo Chemical Industry (Tokyo, Japan). Fenthion (98.4% [AI]) and temephos (97.3% [AI]) were supplied by Supelco (Bellefonte, PA) and Riedel (Seelze, Germany), respectively. Triton X-100 was obtained from Shinyo Pure Chemicals (Osaka, Japan). All other chemicals were reagent grade and available commercially.
Mosquitoes.
Stock cultures of Ae. aegypti, Cx. p. pallens, and Oc. togoi (originally obtained from the National Institute of Health, Korea Centers for Disease Control and Prevention (Seoul, Korea) in 1999 and 2006, respectively) were maintained in the laboratory without exposure to insecticides (Yang et al. 2004). Adult mosquitoes were maintained on a 10% sugar solution and blood fed on live mice. Larvae were reared in plastic trays (24 by 35 by 5 cm) containing 0.5 g of sterilized diet (40-mesh chick chow powder/yeast, 4:1 by weight). All species were reared at 27 ± 1°C, 65–75% RH, and a photoperiod of 16:8 (L:D) h.
Bioassay.
A direct contact mortality bioassay (Kim et al. 2008) was used to evaluate the toxicity of all compounds using early third instars of each mosquito species. In brief, each compound was dissolved in methanol then further diluted in distilled water containing Triton X-100 (20 μl/liter). Groups of 20 mosquito larvae of each species were separately put into paper cups (270 ml) containing each test compound solution (250 ml). The toxicity of each test compound was determined with six concentrations ranging from 1 to 200 ppm. Fenthion and temephos served as standard references and were similarly formulated as the essential oils. Controls (i.e., no essential oil or insecticide) consisted of the methanol-Triton X-100 carrier solution in distilled water.
Treated and control larvae were held at the same conditions used for colony maintenance. Larvae were considered to be dead if they did not move when prodded with fine wooden dowel at 24 h posttreatment. Because all bioassays could not be conducted at the same time, treatments were blocked over time with a separate control treatment included in each block. Freshly prepared compound solutions were used for each block of bioassays (Robertson and Preisler 1992).
Data Analysis.
Concentration mortality data were subjected to probit analysis (SAS Institute 2004). The LC50 values of each species, and their treatments, were considered to be significantly different from one another when their 95% confidence limits (CL) failed to overlap.
Results and Discussion
Chemical Constituents of A. heterotropoides Root Steam Distillate.
Asarom root steam distillate was composed of three major and 14 minor constituents of which methyleugenol, safrole, and 3,5-dimethoxytoluene, made up ≈51% (Table 1).
Chemical constituents of A. heterotropoides root steam distillate identified by GC and GC-MS
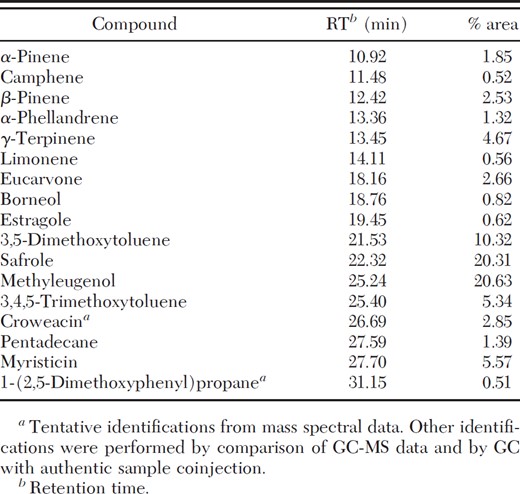
Chemical constituents of A. heterotropoides root steam distillate identified by GC and GC-MS

Larvicidal Activity of Test Compounds.
Safrole was the most toxic compound against larval Cx. p. pallens with similar toxicity produced by terpinolene, γ-terpinene, (–)-β-pinene, (+)-limonene, Δ3-carene, and α-phellandrene (Table 2).
Toxicity of A. heterotropoides root steam distillate, its constituents, and two commercially available larvicides to early third-instar larvae of Cx. p. fallens at 24-h exposure
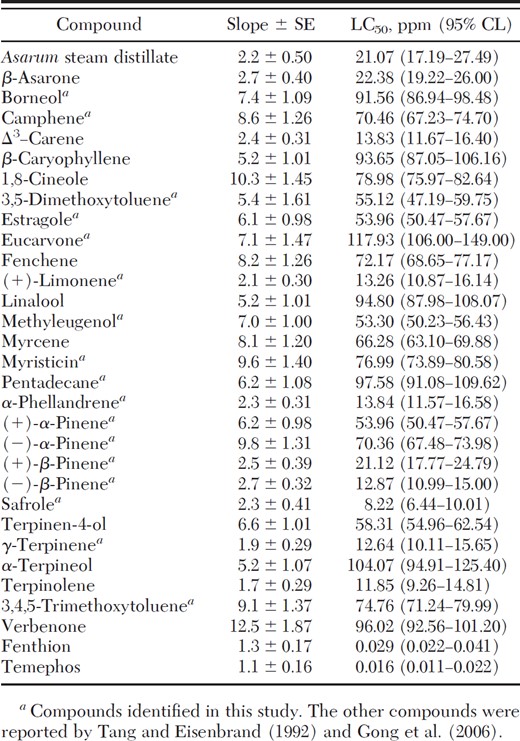
Toxicity of A. heterotropoides root steam distillate, its constituents, and two commercially available larvicides to early third-instar larvae of Cx. p. fallens at 24-h exposure

Against early third-instar larvae of Ae. aegypti, safrole was the most toxic compound, followed by terpinolene, (–)-β-pinene, γ-terpinene, and Δ3-carene (Table 3).
Toxicity of A. heterotropoides root steam distillate, its constituents, and two commercially available larvicides to early third-instar larvae of Ae. aegyptii at 24-h exposure
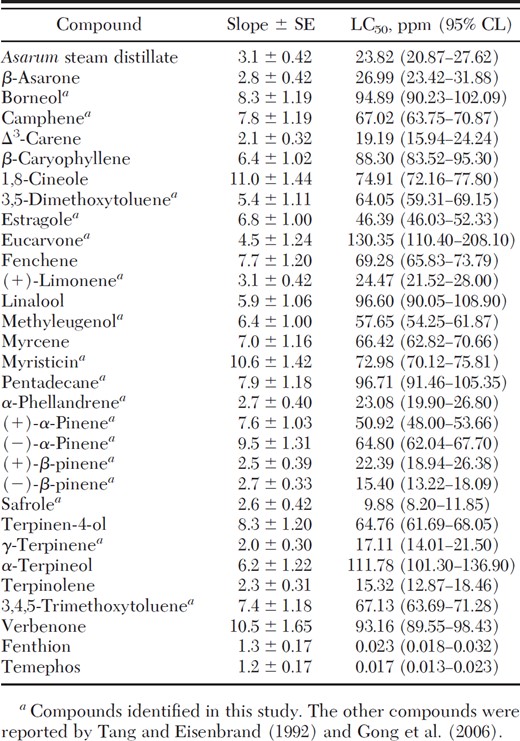
Toxicity of A. heterotropoides root steam distillate, its constituents, and two commercially available larvicides to early third-instar larvae of Ae. aegyptii at 24-h exposure

Terpinolene was the most toxic compound against larval Oc. togoi, followed by γ-terpinene, safrole, α-phellandrene, Δ3-carene, (–)-β-pinene, and (+)-limonene (Table 4). The toxicity of all distillate constituents to all three mosquito species was less than fenthion or temephos. There was no mortality in controls for any the species in this study.
Toxicity of A. heterotropoides root steam distillate, its constituents, and two commercially available larvicides to early third-instar larvae of O. togoi at 24-h exposure
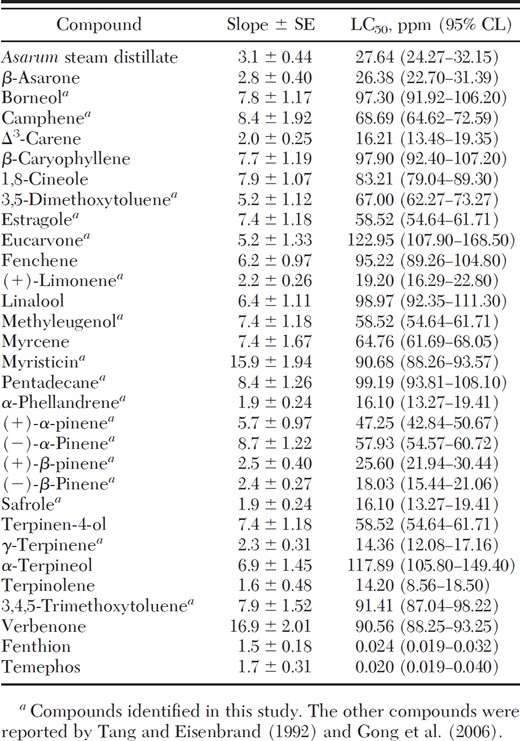
Toxicity of A. heterotropoides root steam distillate, its constituents, and two commercially available larvicides to early third-instar larvae of O. togoi at 24-h exposure

This is the first report of larvicidal activity of A. heterotropoides steam distillate and its active constituents against Cx. p. pallens, Ae. aegypti, and Oc. togoi.
Differential susceptibility to the these plant constituents was observed. However, for the practical use of these essential oils, as larvicides, to proceed, further research is needed to establish their safety in relation to human health, nontarget aquatic organisms, and the environment. Historically, A. heterotropoides has been used as an analgesic and antitussive agent for the treatment of influenza, headache, rheumatic pain, and asthma (Tang and Eisenbrand 1992). However, one of the constituents of this plant species, β-asarone is a human carcinogen (Anonymous 1968). The modes of action need to be established for the more active steam distillates described here as well as the development of formulations that may improve larvicidal potency especially compared with products currently in the marketplace for this purpose.
Acknowledgements
This work was supported by the Ministry of Education, Science and Technology of Korean Government (Plant Diversity Research Center of 21st Century Frontier Research Program, Brain Korea 21 Project and World Class University Program) (to Y.-J.A.).
References Cited



