-
PDF
- Split View
-
Views
-
Cite
Cite
Alexandra Chaskopoulou, Roberto M. Pereira, Michael E. Scharf, Philip G. Koehler, Vapor Toxicity of Three Prototype Volatile Insecticidal Compounds to House Fly (Diptera: Muscidae), Journal of Medical Entomology, Volume 46, Issue 6, 1 November 2009, Pages 1400–1406, https://doi.org/10.1603/033.046.0621
Close - Share Icon Share
Abstract
Vapor toxicities of two prototype formate esters, ethylene glycol di-formate (EGDF) and heptyl formate, and the prototype heterobicyclic menthofuran were determined against laboratory-reared adult house flies, Musca domestica L. (Diptera: Muscidae). Toxicities were compared between house flies and other dipteran species tested previously. The organophosphate fumigant insecticide dichlorvos (DDVP) was used as a positive control. Although less toxic than DDVP, all three prototype compounds were toxic to house flies. Most toxic was menthofuran (LC50 = 3.70 mg/liter air), followed by EGDF (LC50 = 9.27 mg/liter air) and heptyl formate (LC50 = 32.62 mg/liter air). The toxicity of menthofuran when applied after piperonyl butoxide increased 1.5-fold. The toxicities of heptyl formate and EGDF when applied after S,S,S-tributyl phosphorotrithioate decreased by 1.5- and 2-folds, respectively. These results are slightly different from results obtained for other dipterans, further supporting previous hypotheses that toxicity may have some species specificity. Ceramic porous rods impregnated with heptyl formate were used to evaluate the effectiveness of controlled vapor release for a single dose of heptyl formate against house flies. Controlled vapor release of heptyl formate can be used successfully to provide prolonged house fly mortality. Even though the prototype compounds did not exhibit toxicity as high as that of DDVP, their toxicities to dipterans, along with their reduced mammalian toxicities relative to DDVP, make them potential DDVP replacement candidates.
Volatile insecticides have been commonly used as fumigants for the control of structural pests and the protection of stored agricultural commodities. However, fumigants and other volatile insecticidal materials have been mostly ignored for the control of medically important pests such as mosquitoes and flies. Dichlorvos (DDVP) is a volatile insecticide mostly studied on mosquitoes and flies (Miles et al. 1962, Maddock et al. 1963, Brooks and Schoof 1964, Matthysse and McClain 1973). Dichlorvos is an organophosphate insecticide registered in 1948 (USEPA 2006) and commonly formulated in resin strips. This active ingredient has been classified by USEPA as a probable human carcinogen; and in 2006, its use in homes was restricted to confined spaces such as wardrobes, cupboards, and closets (USEPA 2006). Thus, there is a need for a less hazardous replacement for dichlorvos. Currently, low-molecular-weight, highly volatile formates, acetates, and heterobicyclics are under examination as replacements for dichlorvos.
Thirty prototype low-molecular-weight compounds with insecticidal activity were tested previously against Drosophila melanogaster (Meigen) (Scharf et al. 2006) and two mosquitoes species, Culex quinquefasciatus (Say) and Aedes aegypti (L.) (Chaskopoulou et al. 2009). The compounds belonged to six different classes: heterobicyclics, formates, acetates, propionates, butyrates, and valerates. Although there was some variation observed among species with respect to the most toxic materials, formate esters and heterobicyclics were the most toxic groups to both Drosophila and mosquitoes.
Formate esters and heterobicyclics can be naturally occurring compounds. Esters are found in fruits some with a floral, apple scent (Ashurst and Taylor 1999) and are used commercially as flavoring agents in food products. Menthofuran is found in peppermint oil (Wright 1999); has a musty, nutty odor; and is used as a flavoring/odor agent in coffee and chocolate products. At low levels, they are currently considered safe for human consumption; therefore, provided that they are sufficiently toxic, these compounds are ideal candidates for development as DDVP substitutes. One objective in this research was to determine vapor toxicities of three active compounds, two formate esters (ethylene glycol diformate [EGDF] and heptyl formate) and one heterobicyclic (menthofuran), against adult house flies. The second objective was to determine the effectiveness of controlled vapor release of heptyl formate impregnated in porous ceramic rods against house flies.
Little is known of the mode of action of these esters. However, previous research provided evidence of both P450-based activation and deactivation of menthofuran in susceptible and resistant strains of D. melanogaster (Nguyen et al. 2007). Evidence of esterase-based activation of formate esters also was shown on adult rice weevil, Sitophilus oryzae (L.); adult lesser grain borer, Rhyzopertha dominica (F.) (Haritos and Dojchinov 2003); and D. melanogaster (Nguyen et al. 2007; Song and Scharf 2008a). Although the molecular modes of action remain unknown, both formate esters and heterobicyclics cause toxicity via neurological disruption. In addition, the formate esters disrupt mitochondrial function (Song and Scharf 2008a,b, 2009). Elucidation of the effects of piperonyl butoxide (PBO) on menthofuran and S,S,S-tributyl-phosphorotrithioate (DEF) on EGDF and heptyl formate may provide evidence of the activation and/or detoxification of these volatile compounds. Therefore, the third objective was to investigate the effects of PBO, a P450 inhibitor, and DEF, an esterase inhibitor, on the toxicities of the three insecticides.
Materials and Methods
Chemicals.
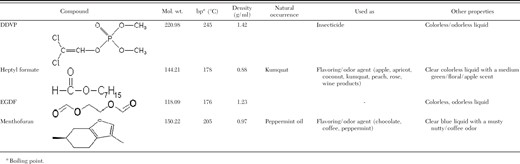
Three compounds: two formate esters (EGDF and heptyl formate) and one heterobicyclic (menthofuran) were obtained from Aldrich Chemical (Milwaukee, WI). DDVP (Chem Service, West Chester, PA) was used as a positive control. All compounds were >99% purity and in a liquid form (Table 1). Compounds were used undiluted or stock solutions were prepared in acetone at concentrations of 250 or 10 μg/μl when necessary. All compounds and stock solutions were held at –20°C in glass vials with rubber lined caps to prevent volatilization until used in experiments.

The insecticide synergists DEF (Mobay Chemical Co., Kansas City, MO) and PBO (MGK Inc., Minneapolis, MN) were >95% pure. DEF and PBO stock solutions were prepared at 100 μg/ml in acetone.
Ceramic Rods.
Hydrophilic, ceramic, porous rods (7.5 × 1.3 cm in diameter; Small Parts, Inc., Miami, FL) were used to provide controlled vapor release of heptyl formate. The ceramic rod pores were 2.5 μm in diameter and represented 38% of the rod volume. Before treatment with insecticides, all sides except the top end of the rods were wrapped tightly with aluminum foil to decrease the insecticide release rate.
Insects.
Flies (horse-teaching-unit [HTU] strain of M. domestica) reared at the University of Florida in Gainesville were used in all experiments. Flies were reared on a photoperiod of 12:12 (L:D) h at 26°C and ≈55% RH. Fly larvae were fed on a medium containing 3 liters of wheat bran, 1.5 liters of water, and 250 ml of dairy calf feed (Calf Manna, Manna Pro. Corp., St. Louis, MO) pellets. Fly pupae were separated from the medium and placed into screened rearing cages (40.6 by 26.7 by 26.7 cm) for emergence. Fly adults were maintained on a 2:1 granulated sugar:powdered milk diet, with water provided ad libitum. Before each treatment 3- to 5-d-old adult house flies were aspirated from their cages and placed into plastic deli cups on ice until their activity was reduced. Chilling on ice is a preferred method to immobilize flies for more prolonged bioassay handling because, unlike other methods, chilling does not stress the flies significantly (Harris et al. 1965, Mount et al. 1976, Smith and Olson 1982). Female flies were removed from the deli cups by using a feather tip forceps. At minimum, 300 female flies were tested in each bioassay with each insecticidal compound.
Bioassays.
Main Bioassay Setup. Bioassay methods were adapted from Scharf et al. (2006) and Nguyen et al. (2007). For the house fly bioassay (Fig. 1), 10 females were transferred from the deli cups into 125-ml plastic vials. A cotton wick (≈1.5 cm in length) dipped in 10% (wt:vol) solution of sugar water and supported by a toothpick (6.3 cm in length) was placed in the vials to provide nutrition and moisture to flies. Vials with an opening of ≈2.6 cm in diameter were closed with caps covered with common fiberglass window screening (≈1.6-mm mesh). The window screening prevented insect escape while allowing for gas exchange. The flies were allowed 1 h to recover from the effects of the ice before the treatment. Then, each fly vial was placed into 1-liter Mason glass jar along with a piece of untreated filter paper (55 mm in diameter).
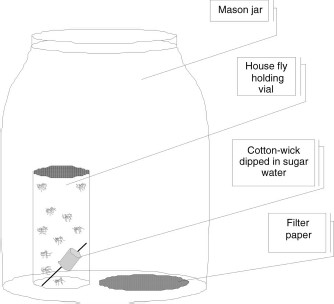
Mason glass jar setup for determination of vapor toxicity against house flies. The Mason glass jar after treatment was closed tightly with the lid inverted (the rubber gasket side up).
Before closing the glass jar, the filter paper was treated with insecticidal solution by using 100-μl micropipettes (Eppendorf, Westbury, NY). The concentration of the insecticidal solution and the volume of the active ingredient applied varied depending on the material being tested. Preliminary evaluation was conducted to determine the best dose range for each compound that provided 20-80% mortality. Undiluted heptyl formate and EGDF were applied at doses of 20-50 and 2–12 μl, respectively, corresponding to a vapor phase concentration of 18-44 and 2.5–15 mg/liter air in the jars, respectively, assuming 100% volatization. Menthofuran was applied at 12–20 μl of a 250 μg/μl acetone solution, corresponding to 3-5 mg/liter air in the jars. DDVP was applied at 10–20 μl of a 10 μg/μl acetone solution, corresponding to 0.1–0.2 mg/liter air in the jars. There were also blank controls in which filter papers received no chemical at all, and solvent controls that received a volume of acetone identical to the highest insecticide solution volume (up to 20 μl). After the filter papers were treated, the jars were closed tightly. Previous studies indicated high control mortality when jar lids were placed with the rubber gasket side down, presumably due to CO2 buildup (Chaskopoulou 2007); thus, lids were inverted with the gasket side up. Mortality was recorded after 24 h. To determine mortality, the jars were shaken for a minimum of 15 s before fly movement was observed. The criterion for a fly death was the cessation of movement, and the number of dead flies for each trial was recorded.
DEF and PBO Bioassay Setup. The DEF and PBO bioassay was conducted similarly to the above-mentioned protocol except that the house flies were exposed to DEF or PBO just before their exposure to the insecticides. Also, 125-ml glass vials were used to prevent absorption of DEF or PBO into the plastic. An aliquot of 100 μl of 10% DEF or PBO stock solution was pipetted into vials using a 100-μl micropipette (Eppendorf) before the introduction of flies, so that each vial contained 10 μg of either DEF or PBO. Previous studies have shown that these quantities of DEF or PBO cause no mortality in Drosophila after 24 h of exposure (Nguyen et al. 2007). After addition of DEF or PBO, the vials were rolled on their sides within a fume hood to ensure equal distribution of the products on the inner surfaces until the acetone evaporated. Once the acetone evaporated, 10 house flies and a cotton wick were added to the vials as described above and vials were set aside for an hour to allow for DEF or PBO to inhibit house fly detoxification enzymes. Along with the blank and the solvent control, DEF and PBO controls also were obtained where the flies were only exposed to DEF or PBO. After the DEF or PBO exposure, insecticide bioassays proceeded as described above for exposures to menthofuran, EGDF, and heptyl formate.
Controlled Vapor Release of Heptyl Formate.
Before bioassays, the release rate of heptyl formate from ceramic rods was estimated. Within a fume hood, rods were weighed, impregnated with heptyl formate and then reweighed. The decrease in the rod weight was recorded every 10 min for 3 h, and insecticidal release rate was calculated. Regression analysis (SAS Institute 2003) via y = 0.6x - 0.3 and R = 0.9994, where y represents heptyl formate weight in milligrams and x represents time of release in minutes, determined that at least 4.5 d would be required for 4 g of heptyl formate to be released from rods.
For the rod bioassay, the setup was as described before, but flies were exposed to insecticide from a single rod impregnated with 3.8 g of heptyl formate. A blank control (rods with no heptyl formate) also was prepared. Additional flies in separate jars were exposed to a filter paper treated with 0.95 g of heptyl formate, which is the amount of heptyl formate estimated to be released from a single rod within 24 h, or 3.8 g of heptyl formate, the same amount used in the rods. All insecticide-treated materials were used immediately after being treated (day 0) and then transferred daily to glass vials with new flies for eight additional days. Fly mortality was determined after 24-h exposure, after which the treated materials were transferred to new jars with new insects.
Data Analysis.
In those cases where control mortality was observed, data were adjusted using Abbott's formula (Abbott 1925). When control mortality exceeded 10%, the replicates corresponding to that control were discarded. Based on the concentration of the insecticide per volume of air in the bioassay, the LC50 and LC90 values, with 95% confidence intervals (CI) of each insecticide, with and without DEF or PBO, were estimated with Probit analysis (SAS Institute 2003). PoloPlus 2.0 (LeOra Software 2005) was used to calculate the potency ratios of the LC50 with and without DEF or PBO. Nonoverlapping 95% confidence limits (CL) were used to determine significant differences in the LCs for all experiments. For the controlled release bioassay, Student—Newman–Keuls (SNK) tests were performed to determine the day when significant decrease in house fly mortality occurred (SAS Institute 2003).
Results and Discussion
Vapor Toxicity.
All three prototype compounds were toxic to female house flies (Table 2). The heterobicyclic menthofuran was the most toxic compound followed by the formate esters EGDF and heptyl formate. The positive control, the organophosphate DDVP, demonstrated considerably higher toxicity than any of the prototype compounds tested. Specifically, DDVP was 25 times more toxic than menthofuran. Even though DDVP performed better than the prototype compounds, its high mammalian toxicity, with an oral LD50 for rat of 17 mg/kg (Material Safety Data Sheet, Chem Service, 2003), has resulted in its application being restricted to confined spaces where little human activity takes place (USEPA 2006). However, the prototype compounds tested here are characterized by a balanced combination of good insect toxicity and low mammalian toxicity, because they have been primarily used as food additives. For example, heptyl formate has an oral LD50 for rat equal of 1510 mg/kg (Material Safety Data Sheet, Sigma-Aldrich, 2005), and the other volatile compounds have no reported mammalian LD50 but have been used as food additives. Therefore, these prototype compounds are not as hazardous to mammals as DDVP and may be viable alternatives as vapor toxicants for use in houses and other public areas.
Vapor toxicity of EGDF, heptyl formate, and menthofuran with and without the synergistic effect of DEF or PBO to house flies
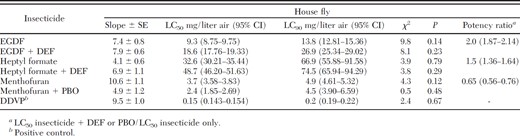
Vapor toxicity of EGDF, heptyl formate, and menthofuran with and without the synergistic effect of DEF or PBO to house flies

This study is the first report of the toxic effects of heptyl formate, EGDF, and menthofuran vapors on adult house flies. Scharf at al. (2006) and Chaskopoulou et al. (2009) reported the toxicities of these compounds and 27 others to D. melanogaster and adult mosquitoes of two species (Cx. quinquefasciatus and Ae. aegypti), respectively. Results presented here and previously reveal that the volatile compounds exhibit different toxicological profiles against adult house flies, Drosophila and mosquitoes. Overall, house flies were least susceptible to the prototype insecticides compared with the other three dipteran species (Fig. 2). The heterobicyclic menthofuran was the most toxic compound tested on house flies, D. melanogaster, and Cx. quinquefasciatus; however, this was not the case with Ae. aegypti which was more susceptible to EGDF. Also, the positive control, DDVP, consistently demonstrated the highest toxicity to all insect species. The performance of EGDF and heptyl formate varied depending on the insect tested and did not show any specific trend; in Ae. aegypti and house flies, EGDF was more toxic than heptyl formate, where as in Cx. quinquefasciatus and D. melanogaster, heptyl formate was more toxic than EGDF.

Comparisons of four different vapor toxicants against four dipteran species. Drosophila and mosquito data retrieved from Scharf et al. (2006) and Chaskopoulou et al. (2009), respectively.
Several explanations are possible for the differences in toxicological profiles among house flies, D. melanogaster, and mosquitoes, such as differences in insect handling techniques, and species differences in acquiring, metabolizing, and detoxifying insecticides (Chaskopoulou et al. 2009). The mosquitoes and house flies were anesthetized with ice, whereas D. melanogaster were anesthetized with CO2. Mosquitoes and house flies had to remain immobilized to be sexed. The preferred method for more prolonged handling is chilling because it does not cause significant stress (Harris et al. 1965, Mount et al. 1976, Smith and Olson 1982).
Volatile insecticides such as those described here normally enter the insect body through the spiracles (Matsumura 1980). Therefore, the susceptibility of an insect to a vapor toxicant may be correlated with its rate of respiration (Vincent and Lindgren 1965), in addition to potential correlation with body weight. Little information is available on the respiratory patterns of insects with body weight of ≈1 mg, such as D. melanogaster (Williams et al. 1997, Williams and Bradley 1998, Lehmann et al. 2000, Fielden et al. 2001) or on house flies. Perhaps house flies, D. melanogaster, and mosquitoes have different respiration rates, possibly allowing different amounts of insecticide to enter their body, and resulting in the toxicity differences among these insects as we observed.
Differences in the detoxification systems of house flies, D. melanogaster, and mosquitoes also may contribute to differences in toxicity we have observed among species. The two most significant reactions involving the detoxification and metabolism of insecticides are NADPH-requiring cytochrome P450 oxidation and esterase-based hydrolysis (Feyereisen 2005, Oakeshott et al. 2005). The activities of these two enzymatic systems vary among different insect species, resulting in species differences in susceptibility to various insecticidal compounds (Casida et al. 1976, Brooks 1986, Valles et al. 1997, Scharf et al. 2000). Our findings suggest that, because of apparent species differences in acquisition and metabolism, prototype insecticides must be tested directly on the targeted species and field populations to obtain a realistic assessment of insecticidal performance.
DEF and PBO Effects on Toxicity.
When applied after DEF, heptyl formate and EGDF toxicities decreased (LC50 increased) significantly (Table 2). The toxicity of heptyl formate decreased by 1.5-fold, whereas the toxicity of EGDF decreased by 2-fold. This provides supporting evidence of esterase-based activation of both heptyl formate and EGDF. These findings are in agreement with Haritos and Dojchinov (2003), Nguyen et al. (2007), and Song and Scharf (2008) who demonstrated esterase-based activation of some formate esters. Haritos and Dojchinov (2003) also demonstrated that formate esters are more toxic than other alkyl esters, in part due to hydrolysis to formic acid. This may explain why EGDF was significantly more toxic than heptyl formate to house flies. EGDF can liberate two molecules of formic acid (a highly toxic compound that disrupts mitochondrial function; Nicholls, 1975), whereas heptyl formate contains only one formic acid. Therefore, hydrolysis of EGDF by esterase action theoretically releases twice as much formic acid as heptyl formate. Also, Song and Scharf (2008, 2009) showed in vivo and in vitro liberation of formic acid from a broad range of formate esters and found that formic acid caused significant neuroexcitation on the larval house fly nervous system, as well as mitochondrial disruption.
Also, our findings show that PBO, which inhibits cytochrome P450 enzymes, significantly increased the toxicity of the heterobicyclic menthofuran. Specifically, the toxicity of menthofuran increased 1.5-fold after preexposing flies to PBO. Currently, although it is known to cause neurological disruption in larvae (Song and Scharf 2008b), the molecular mode of action of menthofuran in adult house flies is not known. A role for cytochrome P450 in both in activation and deactivation of menthofuran has been identified on D. melanogaster (Nguyen et al. 2007). However, our results suggest that menthofuran is only deactivated by the P450 enzymatic system in female house flies.
Controlled Vapor Release of Heptyl Formate.
Untreated controls caused no mortality throughout the duration of the controlled vapor release experiment (Table 3). The filter paper treated with 0.95 g of heptyl formate produced 100% mortality initially, but no mortality after aging. The filter paper treated with 3.8 g of heptyl formate produced 100% mortality through the first 2 d but no mortality afterward. The ceramic rod impregnated with 3.8 g of heptyl formate caused mortality throughout the duration of the experiment. On the eighth day, a significant decrease in house fly mortality (64%) was seen in relation to mortality on day 0 (100%).
Percentage of mortality caused by controlled vapor release of heptyl formate to house flies over 9 d
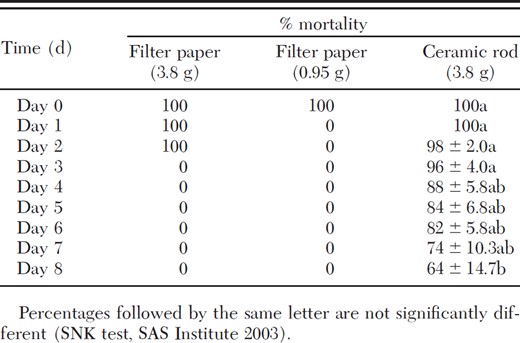
Percentage of mortality caused by controlled vapor release of heptyl formate to house flies over 9 d

Although we estimated that controlled release of heptyl formate from the ceramic rods would last only 4 d, it lasted at least 8 d in our experiments. The actual release rate of heptyl formate probably followed a logarithmic curve, and not the linear release rate we estimated, which only represented the initial 3 h. As time passed, it is likely that the release rate gradually decreased and therefore resulted in prolonged fly mortality. In the future, these compounds may need to be embedded in specialized plastic polymers, similar to resin strips used with DDVP, so prolonged release of vapors can be achieved, resulting in increased insecticide release time and exposure.
Even though the prototype compounds tested here did not exhibit vapor toxicity as high as DDVP, they showed good potential as alternative vapor toxicants against house flies. Because they are food additives, considered safe for human consumption in small doses, and have pleasant, fruity odors, these compounds may be good candidates to replace DDVP, especially in places where DDVP use has been restricted. The potential of these prototype compounds as contact toxicants also should be investigated to further define their potential as tools for the control of public health pests such as house flies and mosquitoes.
Acknowledgements
We thank Jeff Hertz, Mark Mitola, and Ryan Welch for house fly rearing support. This work was funded by grant W81XWH-04-1-0868 from the Deployed War-Fighter Protection program of the U.S. Armed Forces Pest Management Board.
References Cited



