-
PDF
- Split View
-
Views
-
Cite
Cite
Jennilee B. Robinson, Marina E. Eremeeva, Patrick E. Olson, Scott A. Thornton, Michael J. Medina, John W. Sumner, Gregory A. Dasch, New Approaches to Detection and Identification of Rickettsia africae and Ehrlichia ruminantium in Amblyomma variegatum (Acari: Ixodidae) Ticks From the Caribbean , Journal of Medical Entomology, Volume 46, Issue 4, 1 July 2009, Pages 942–951, https://doi.org/10.1603/033.046.0429
Close - Share Icon Share
Abstract
Imported from Africa in the 1700s and despite frequent modern eradication efforts, Amblyomma variegatum (F.) spread through the Caribbean by cattle transport, small ruminants, and migrating birds. A. variegatum is a vector for Rickettsia africae, the causative agent of African tick bite fever, and Ehrlichia ruminantium, the causative agent of heartwater. We examined 95 A. variegatum and six Rhipicephalus (Boophilus) microplus (Canestrini) collected from cattle at an abattoir in Antigua. Engorged tick extracts adsorbed on Nobotu filter paper strips and new nested polymerase chain reaction (PCR) assays for E. ruminantium and Dermatophilus congolensis were used to evaluate these ticks for the presence of these pathogenic bacteria. Amblyomma ticks (62.4%) contained R. africae DNA by PCR/restriction fragment length polymorphism analysis and DNA sequencing of the OmpA and 17-kDa antigen genes. Twenty Amblyomma and two Rh. microplus contained E. ruminantium DNA. No E. chaffeensis, Anaplasma phagocytophilum, Coxiella burnetii, or D. congolensis DNA was detected in these ticks. The continued presence of Am. variegatum in the Caribbean poses a significant risk of infection in cattle with E. ruminantium and in humans by R. africae. Eradication efforts are essential to prevent the further spread of Am. variegatum.
Amblyomma variegatum (F.), the tropical bont tick, is currently present on many of the Leeward and Windward Islands of the Caribbean, and it is widespread in sub-Saharan Africa. Originally introduced to the New World with cattle imported from Africa to Guadeloupe and Antigua in the 18th century, it has spread to the other Caribbean islands because of inter-island transport of cattle or other small ruminants and attachment to migratory birds, particularly the cattle egret, Bubulcus ibis (Corn et al. 1996, Maillard and Maillard 1998, Bram et al. 2002). The tropical bont tick is associated with two major diseases in the Caribbean: heartwater, caused by Ehrlichia (Cowdria) ruminantium, and acute dermatophilosis, caused by the Actinomycete Dermatophilus congolensis (Camus and Barre 1995). Heartwater is a devastating and fatal disease of wild and domestic ruminants and was probably introduced into the Caribbean along with Am. variegatum from Africa. This disease does not occur on all the islands where Am. variegatum is present but has been a continuing agricultural problem on the islands of Antigua, Guadeloupe, and Marie-Galante. Heartwater is responsible for substantial losses throughout its distribution in Africa and the West Indies because of high livestock mortality and expensive vector control programs (Camus and Barre 1995). D. congolensis is present in the environment, and severe disease in livestock occurs virtually everywhere Am. variegatum is present (Camus and Barre 1995). Currently North, Central, and South America are free of heartwater. The importation of E. ruminantium into these countries, where other Amblyomma spec but not Am. americanum or Am. cajennense (Mahan et al. 2000) may become efficient indigenous vectors, and competent reservoirs such as white-tailed deer or other ruminants are widespread, could be catastrophic to agriculture (Bram et al. 2002).
Rickettsia africae is another pathogenic bacterium that is transmitted by Am. variegatum. This spotted fever group rickettsia causes African tickbite fever (ATBF) in humans (Kelly et al. 1996). Several studies have shown that R. africae is very prevalent in Am. hebraeum and Am. variegatum ticks in sub-Saharan Africa, where it is a major threat to the health of tourists traveling in the region (Raoult et al. 2001). In 1992, >30% of U.S. Army personnel participating in a field exercise in Botswana became ill with a nonrash tick typhus-like illness caused by R. africae (Smoak et al. 1996; G.A.D., unpublished observations). In 1998, a case of ATBF was diagnosed in a French woman after visiting Guadeloupe, French West Indies (Parola et al. 1998). A survey of Am. variegatum ticks on that island yielded 27% positive for R. africae either by culture or polymerase chain reaction (PCR) (Parola et al. 1999). Kelly et al. (2003) tested Am. variegatum collected from St. Kitts and Nevis in 1999 and 2002 and found that 41% of ticks were infected with R. africae. Similarly, 56% of Am. variegatum collected from Martinique in 2002 were positive for R. africae by PCR (Parola et al. 2003).
A field deployment to the island nation of Antigua in July 2000 was conducted by a team from the U.S. Navy Environmental and Preventive Medicine Unit No. 5 (NEPMU-5) as a part of a large annual U.S. military exercise called New Horizons. This training includes sending construction personnel to several locations in the Caribbean and Central and South America for local building projects. Besides providing needed support for one of these units, the NEPMU-5 team conducted directed analyses for specified infectious disease threats on the island of Antigua (Thornton et al. 2001). In this paper, we report the analysis of ticks collected by NEPMU-5 personnel for the presence of infectious agents that may be threatening both animals and humans on Antigua using tick extracts collected on Nobotu filter paper strips and describe the development of new molecular assays for the detection of E. ruminantium and D. congolensis.
Materials and Methods
Tick Collection.
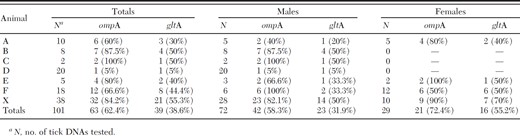
Ticks were removed from cattle at an abattoir in Antigua’s capitol city of St. Johns in July 2000. Seven different infested host animals (designated A-F) were examined over a 6-d period. Approximately 80% of the cattle processed at the facility had no ticks. Ninety-five adult Am. variegatum were collected including 23 females and 72 males. One animal, F, had 6 engorged females of Rhipicephalus (Boophilus) microplus (Canestrini) and 12 A. variegatum ticks. The Amblyomma ticks were identified using a standard taxonomic key in the field. The Rhipicephalus (Boophilus) microplus were identified by Dr. J. Keirans of the National Tick Collection (Georgia Southern University, Statesboro, GA) based on three voucher specimens preserved in 70% ethanol.
Tick Blots.
Tick blots were prepared by NEPMU-5 in Antigua. Partially engorged ticks were individually placed in sterile 12 by 75-mm tubes containing 1 ml sterile saline and homogenized by crushing repeatedly with wooden applicator sticks. Engorged Amblyomma females were homogenized in sterile 50-ml conical tubes with no saline addition. A Nobotu paper filter strip, shaped like a key with a hole in the wide top portion for stringing, was used to absorb the resulting liquid up to the beginning of the wide part. The strips were suspended, six to eight at a time, on a new applicator stick to dry. Methanol was dripped onto the strips, and the strips were allowed to air dry and placed in individual 4-ml cryovials. Vials were stored and shipped to the Centers for Disease Control and Prevention at ambient temperature. No live ticks or fresh tick material were brought back for isolation attempts to avoid any risk of introducing ticks infected with E. ruminantium or R. africae into the continental United States.
DNA Extraction.
Tick DNA was extracted from the filter paper strips in 3-ml tubes using the QIAamp DNeasy kit (Qiagen, Valencia, CA). To remove the DNA from the paper, 0.4 ml of 0.01 M phosphate-buffered saline (PBS; pH 7.2), 0.4 ml of Buffer AL (Qiagen), and 40 μl of Proteinase K (Qiagen) were added to the screw cap vial containing the filter strip and digested at 70°C for 10 min. Then the fluid was aspirated and placed in a clean tube. Next, 0.5 ml of PBS was added to the filter paper and boiled for 5 min. The two eluates were combined, mixed with 0.5 ml of absolute ethanol, and transferred onto a Qiagen column in two consecutive aliquots separated by centrifugation. DNA was eluted with two volumes of 100 μl of Tris-EDTA following the manufacturer’s protocol. The DNA was stored at 4°C until PCR was performed.
Dermatophilus congolensis (van Saceghem) Gordon (ATCC #14637) was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and grown on agar plates supplemented with 5% sheep blood at 37°C. DNA was extracted from cultured D. congolensis cells using the QIAamp DNeasy kit (Qiagen). Cells were scraped from the sheep blood agar plates and suspended in 100 μl of 0.01 M PBS. The Qiagen protocol was followed, and the DNA was eluted in two volumes of 200 μl Buffer AE (Qiagen), diluted with an additional 100 μl of Tris-EDTA, resulting in a final volume of 0.5 ml, and was stored at 4°C.
PCR.
Amplification mixtures of 25 μl consisting of GeneAmp DNA amplification reagents (N801–0055; Perkin-Elmer Cetus, Norwalk, CT) were used for all PCR with the following reagents (final concentrations): 9.25 μl water, 2.5 μl of 10× buffer (1.5 mM MgCl2, 50 mM KCl, 10 mM Tris, pH 8.3, 0.001% gelatin), 4 μl of dNTP mixture (0.2 mM of each dNTP), 3.0 μl (1.0 μM) of each forward and reverse primer, 0.25 μl of AmpliTaq DNA Polymerase (1.25 U), and 3 μl of template DNA. Reactions were performed on the model PTC-200 Programmable Thermal Controller (MJ Research, Watertown, MA).
For detection of rickettsial DNA, fragments of the OmpA and GltA genes were amplified from the tick DNA extracts as described in Table 1. Additionally, the 17-kDa protein gene was amplified for DNA sequencing from four tick DNA extracts that were strongly positive in both ompA and gltA assays.
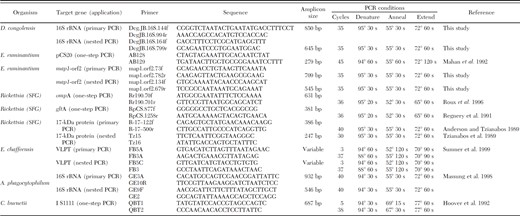

PCR of the E. ruminantium pCS20 gene was performed as previously described (Mahan et al. 1992). For nested PCR amplification of the E. ruminantium map1-like gene (orf-2), consensus primers were designed by locating conserved regions within a ClustalW 1.74 Multiple Sequence Alignment of the map1 and map1- like genes of E. ruminantium isolates including Welgevonden isolate (AF 125274), Crystal Springs isolate (AF125275), Highway isolate (AF125276), LemcoT3 isolate (AF125277), Um Banein isolate (AF125278), and an Antigua isolate obtained in 1985 (AF125279).
For amplification of D. congolensis 16S rDNA, primers were designed by locating conserved regions within a ClustalW 1.74 Multiple Sequence Alignment of D. congolensis (L40615), Dermatophilus crocodyli (AF226615), Dermatophilus chelonae (AJ243919), and the closely related Streptomyces ambifaciens (M27245).
DNA from tick blot extracts was also tested for the presence of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and Coxiella burnetii as described in Table 1.
All of the PCR amplicons were resolved at 80 V for 30 min in a 1% UltraPure agarose gel (Invitrogen, Carlsbad, CA) in 1× Tris-Borate-EDTA and visualized with 1 μM ethidium bromide.
Preparation of Reference Reagents.
DNA from E. ruminantium Crystal Springs isolate was kindly provided by Dr. Robert Massung (Centers for Disease Control and Prevention, Atlanta, GA), and map1-like and pCS20 gene fragments were PCR amplified and ligated into PCR 2.1 vector plasmids according to the manufacturer’s instructions (Invitrogen). Transformation of INVαF’ E. coli cells was performed, and recombinant colonies were selected on 150-mm LB agar plates supplemented with 20 μl of 100 mg/ml amplicillin and 40 μl of 40 mg/ml X-gal (Novagen, San Diego, CA). Plasmids were isolated from the recombinant cells with the QIAprep Spin Miniprep Kit (Qiagen) and stored in 25 μl of Tris-EDTA at 4°C for use as control DNA in PCR screening reactions.
Restriction Digestion of PCR-Amplified DNA.
Restriction fragment length polymorphism (RFLP) analysis of the rickettsial ompA amplicon was performed to further characterize the species origin of the fragments (Roux et al. 1996). Restriction digests were performed in 20-μl reactions, which included 5 μl of amplified DNA and 15 μl of reaction mixture containing 2 μl of 10× buffer, 0.2 μl BSA if required, and 5–10 U of restriction endonucleases RsaI, PstI, or AluI (New England Biolabs, Ipswich, MA). The amplified DNA was added to the reaction mixture last, and the tubes were incubated at 37°C for 2 h and at ambient temperature overnight. The restriction fragments were resolved by gel electrophoresis in 3% Nusieve 3:1 GTG Agarose (Cambrex, Charles City, IA) in 1× Tris- Borate-EDTA at 70 V for 90 min and visualized with ethidium bromide staining.
Sequencing of PCR-Amplified DNA.
Amplified DNA was purified from excess primers and PCR reagents using the Wizard SV Gel and PCR Clean Up System (Promega, Madison, WI). The sequence reaction was set up using reagents from 2.0 or 3.0 Big Dye Terminator Sequencing Kits according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). The products were purified using the DyeEx Spin Kit (Qiagen). Purified sequencing products were resolved using an ABI Prism 3100 Sequencer. Sequences were submitted to NCBI GenBank with the following accession numbers: R. africae 17-kDa protein genes from four separate ticks—tick 128 (EU072682), tick 129 (EU072683), tick 130 (EU072684), and tick 131 (EU072685), R. africae OmpA gene sequence (EU622980), and E. ruminantium map1-like (orf2) tick 127 (EU072681).
Results
Detection and Identification of Spotted Fever Group Rickettsiae.
Spotted fever group rickettsia DNA was amplified from 62.4% (63 of 101) of extracted DNA samples using OmpA gene primers Rr190.70p and Rr190.701n, whereas amplicons of the GltA gene were obtained for 38.6% (39 of 101) of the samples (Table 2). ompA amplicons were obtained from all samples that gave a positive gltA amplicon. Spotted fever group rickettsiae were detected only in DNA from Am. variegatum ticks and not in any DNA from Rh. microplus.

Restriction endonucleases RsaI, PstI, and AluI were used to characterize the identity of the rickettsial ompA amplicons based on in silico prediction of RFLP patterns (Fig. 1). Predicted endonuclease RsaI digestion patterns of the 632-bp OmpA gene amplicon of R. africae consists of two fragments of 308 and 324 bp. This pattern, which on the agarose gel appears as a single band, is characteristic for a cluster of rickettsiae including R. africae, R. parkeri, unnamed spotted fever group isolate S, and R. sibirica subsp. mongolotimonae. RFLP patterns predicted for endonuclease PstI, fragments of 78, 81, 83, 123, and 267 bp, are characteristic for rickettsiae including R. africae, R. parkeri, R. sibirica subsp. mongolotimonae, R. sibirica, and unnamed spotted fever group isolate S. On the agarose gel, the PstI disgest fragments of 78, 81, and 83 bp appear as a single band. Digestion with restriction endonuclease AluI yielded a pattern of 86, 124, 208, and 214 bp, which was characteristic for R. africae R. sibirica, R. sibirica subsp. mongolotimonae, unnamed spotted fever group isolate S, and R. conorii Malish 7. The 208- and 214-bp fragments appear as a single band on the agarose gel. The AluI assay allows differentiation of R. africae from R. parkeri (RFLP pattern is five bands of 56, 86, 124, 153, and 214 bp). Figure 1 shows representative results of restriction digest of ompA amplicons, as well as the predicted RFLP patterns for other rickettsiae.
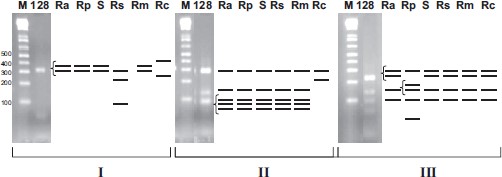
RFLP characterization of omp APCR amplicons (632 bp). Gel electrophoresis of restriction endonuclease digested DNA from tick 128 with the predicted patterns for spotted fever group rickettsiae R. africae (Ra), R. parkeri (Rp), R. sibirica (Rs), R. sibirica subsp. mongolotimonae (Rm), unnamed spotted fever group isolate S (S), and R. conorii Malish 7 (Rc), M, DNA size marker. (I) Restriction endonuclease RsaI digest. (II) Restriction endonuclease PstI digest. (III) Restriction endonuclease AluI digest. Bracketed lines resolve as single bands on the gel.
Nucleotide sequences for amplicons of the 17-kDa protein gene from four different Antiguan Am. variegatum ticks, tick 128, tick 129, tick 130, and tick 131, as well as the amplicon from control R. africae DNA from the Ethiopian isolate strain ESF 2500 were compared with GenBank sequences for R. conorii Malish 7 (M28480), R. rickettsii (M28479), R. amblyommii (U11013), and R. parkeri (U17008). The sequences of the 17-kDa protein gene DNA were identical for the four Antiguan ticks and the R. africae control DNA. These differed from the R. amblyommii sequence at six nucleotide positions and from the R. rickettsii, R. parkeri, and R. conorii Malish 7 sequences at one identical nucleotide position. Second, we also sequenced the 632-bp OmpA gene amplicon from six PCR-positive Am. variegatum. Nucleotide sequences, in addition to the RFLP analysis with AluI, confirmed that the spotted fever agent in infected Am. variegatum ticks was R. africae.
Detection and Identification of E. ruminantium.
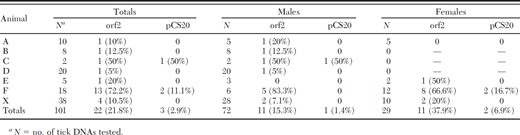
A nested PCR assay was designed to amplify the map1-like (orf-2) gene of E. ruminantium (Fig. 2) because we only obtained 2.9% (3 of 101) positive results using the direct pCS20 gene PCR assay (Table 3). With the nested assay, we were able to detect E. ruminantium DNA in a total of 21.8% (22 of 101) of ticks and in ticks from all seven infested animals. Two of the ticks from animal F containing E. ruminantium DNA were fully engorged Rh. microplus females and 11 of 12 Am. variegatum from this animal were also positive by the E. ruminantium PCR assay.
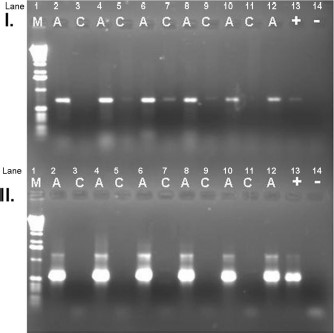
Gel electrophoresis of map1-like (orf2) amplicons. M, DNA marker; A, DNA amplified from Antigua tick DNA extracts; C, DNA amplified from individual Ix. scapularis tick extracts (expected to contain no E. ruminantium DNA); (+), amplified positive control from plasmids containing orf2 amplicon from control DNA (Crystal Springs isolate); (−), negative control (water was substituted for DNA in PCR mixture). (I) Primary round of amplification. (II) Secondary (nested) round of amplification.

Sequence analysis was done on DNA amplified with the map1-like (orf-2) PCR assay. The orf-2 sequence amplified from tick 127 was compared with GenBank sequences of the E. ruminantium Welgevonden isolate (AF125274), Crystal Springs isolate (AF125275), Highway isolate (AF125276), LemcoT3 isolate (AF125277), Um Banein isolate (AF125278), and an Antigua isolate from 1985 (AF125279) (Fig. 3). The sequences of this small conserved DNA region were identical for the DNA from tick 127, the LemcoT3 isolate, and the Welgevonden isolate. The 1985 Antigua isolate differed at a single nucleotide position. The Um Banein isolate differed at three nucleotide positions, and the Highway and Crystal Springs isolates differed at four identical nucleotide positions.
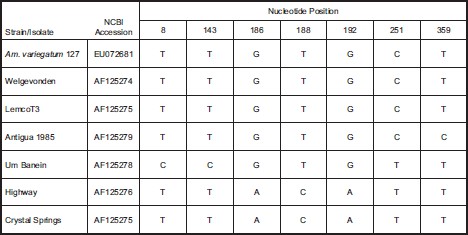
Differences found from a clustal nucleotide sequence alignment comparing the map1-like (orf2) gene fragment from Antigua tick 127 to other E. ruminantium strains: Welgevonden isolate (AF125274), Crystal Springs isolate (AF125275), Highway isolate (AF125276), LemcoT3 isolate (AF125277),UmBanein isolate (AF125278), and the Antigua isolate from 1985 (AF125279). Nucleotide position numbering is given based on nucleotide order within the PCR amplified gene fragment.
Some Am. variegatum ticks from this study were coinfected with both R. africae and E. ruminantium. Eighteen of 22 (81.8%) ticks that were positive by PCR for the presence of E. ruminantium DNA also had detectable levels of rickettsial DNA. Overall, 18 of 95 (19%) of the Am. variegatum in this study were coinfected. Coinfected ticks were removed from five of the seven bovines in this study. No D. congolensis, E. chaffeensis, A. phagocytophilum, or C. burnetii DNA was detected in any of the tick blot extracts.
Discussion
Amblyomma ticks have aggressive host seeking behaviors, in which ticks actively converge on hosts instead of using the more passive strategy of ambush from vegetation (Jensenius et al. 2003). Humans are accidental hosts for these ticks but are often bitten. Humans are at risk from the bites of various species of Amblyomma ticks because of the threat of infection with various species of Rickettsia and Ehrlichia. Am. variegatum feeds primarily on hosts other than humans including cattle, as well as many other wild and domestic animals such as horses, sheep, donkeys, pigs, lizards, and birds (Jensenius et al. 2003). Am. variegatum ticks transmit R. africae to humans in Africa and the Caribbean. R. africae is often a relatively mild rickettsial infection presenting with multiple eschars, fever, and rash (Kelly et al. 1996). However, recent reports of ATBF-related myocarditits and subacute neuropathy suggest that this disease could be a more serious threat to public health than originally considered (Bellini et al. 2005, Jensenius et al. 2006).
In this study, we collected blood-engorged tick samples on filter paper for detection of infectious agents. Previously, this technique has been used to preserve blood samples for serology for multiple organisms, including rickettsiae (Fenollar and Raoult 1999). This technique was important for this study, as well as similar field studies, because it was an efficient way to preserve tick specimens while in the field where refrigeration was not readily available. Second, because the filter paper blots were treated with methanol, it allowed us to aseptically transport DNA from exotic ticks into the continental United States for analysis without having to import actual ticks or potentially infectious blood samples. Engorged ticks can be problematic for molecular analysis because residual blood components in DNA extracts can inhibit PCR reactions. The high level of sensitivity achieved in direct ompA assays, even in the case of heavily engorged females, suggested inhibitors are largely removed with our procedure without the significant loss of pathogen DNA.
Tick blots were screened by PCR for the presence of SFG rickettsiae DNA. We showed that, for detection of SFG DNA from tick blot extracts, the ompA PCR assay is more sensitive than the gltA PCR. By ompA PCR, we found 63 of 101 (62.4%) ticks collected in Antigua were positive for SFG rickettsiae. To identify the SFG rickettsiae that we detected in the tick blots by PCR, samples with strong PCR amplicons of the OmpA protein gene were digested with three restriction endonucleases that allowed species-specific identification based on RFLP patterns. Compared with nucleotide sequencing of each PCR-positive isolate, RFLP provides a quick, less expensive, and technically less demanding alternative, an important consideration in the developing world for field studies. To further confirm the RFLP results and verify the specific identity of the SFG rickettsiae in the Am. variegatum ticks as R. africae, nucleotide sequencing of the OmpA protein and 17-kDa protein genes was also done on select PCR amplicons.
There are several possibilities for how the ticks acquired the rickettsial DNA that we detected in this study. Efficient transovarial transmission does occur in Amblyomma species for SFG rickettsiae as shown for R. parkeri and R. amblyommii in Am. americanum and R. africae in Am. hebraeum (Kelly and Mason 1991, Goddard 2003; G.A.D., unpublished data). In transovarial and transtadial transmission, the tick itself is the main reservoir host of rickettsial species in endemic foci. Although transovarial transmission does occur with rickettsiae, Rickettsia may not always be detectable in unengorged ticks collected in the field because the bacterial load may be too low for detection by PCR assays. However, during blood feeding, "reactivated" Rickettsia may become more easily detected as they multiply in response to bloodmeal acquisition by their tick host (Hayes and Burgdorfer 1982). Alternatively, the ticks were infected by feeding on a rickettsemic animal, which could have been either the animal they were collected from or a host animal from a previous life stage. Kelly et al. (1991) reported that cattle can be asymptomatically infected with rickettsiae isolated from Am. hebraeum and can maintain a transient dose-dependent rickettsemia for up to 32 d. A third possibility is that the rickettsiae were acquired by the Am. variegatum ticks through cofeeding. Cofeeding might cause infection of uninfected ticks feeding at the same time with infected ticks on the same nonbacteremic or nonviremic host, as has been observed in Ixodid ticks with multiple viruses and bacteria (Philip 1959, Labuda et al. 1997, Patrican 1997, Niebylski et al. 1999).
Tick blots were also screened by PCR for the presence of E. ruminantium DNA. We found that, although pCS20 DNA may be an effective probe for testing blood samples for E. ruminantium infection, this assay was inefficient in detecting the DNA present in an extract from a tick blot. Therefore, a nested PCR assay for the map1-like (orf2) gene was designed for this study. With this assay, we were able to detect E. ruminantium DNA in 22 of 101(21.8%) tick blots. Two of the positive ticks were Rh. microplus. In this study, we examined a total of six female Rh. microplus ticks. Rh. microplus is not considered to be a vector for E. ruminantium. The Rh. microplus ticks were collected only from animal F, and 100% (6 of 6) of the female and 83.3% (5 of 6) of the male Am. variegatum ticks collected from this animal also tested positive for E. ruminantium, indicating that animal F was possibly infected with E. ruminantium, and this bacteria was being acquired by the ticks as they took their bloodmeal. Am. variegatum ticks can only be infected with E. ruminantium through cofeeding with infected ticks or by acquiring the bacteria from feeding on an infected animal because the heartwater bacterium is not transovarially transmitted (Bezuidenhout 1987). The sequencing of the map1-like (orf2) gene amplified from Antigua tick DNA extracts showed the sequence obtained in this study is identical except at one nucleotide position to the 1985 E. ruminantium isolate from Antigua, suggesting that the same strain of E. ruminantium may have persisted on Antigua despite the intensive tick eradication efforts (Sulsona et al. 1999).
No other bacterial DNAs were detected in tick blot DNA extracts, although the samples were screened for the presence of D. congolensis, A. phagocytophilum, E. chaffeensis, and C. burnetii. Of these bacteria, D. congolensis has been specifically cited as a veterinary health threat in the Caribbean that is specifically associated with the presence of Am. variegatum ticks (Camus and Barre 1995). Several studies have suggested that Am. variegatum can exacerbate the condition of an infected animal by activating inflammatory immune responses in the skin that increase the severity of D. congolensis lesions (Walker and Lloyd 1993, Koney et al. 1994). In this case the tick is not actually a host for the bacteria and therefore its DNA might not be present in the tick blot extracts.
Coinfection with spotted fever group rickettsiae and E. ruminantium was detected in 18 of 95 (19%) of the Am. variegatum ticks in this study. Coinfection in ticks has been documented previously with multiple pathogens such as Borrelia, Anaplasma, and Ehrlichia in both Ixodes and Amblyomma species ticks (Schulze et al. 2005). To the best of our knowledge, this is the first report of coinfection with R. africae and E. ruminantium in Amblyomma ticks.
Although the occurrence of R. africae and E. ruminantium infection in these samples was high, 80% of the animals in the sampling period at the St. Johns abattoir had no ticks. Visits to the local hospitals and to the Antiguan Ministry of Health yielded no patients or knowledge of ATBF-like cases. However, dengue and leptospirosis do occur and may be easily confused clinically with ATBF. Samples of one Antiguan and nine U.S. military personnel who had been in the country 5 mo, with at least some symptoms of illness, were tested for possible exposure to spotted fever group rickettsia. None of the patients had any recollection of tick bite, and a dipstick assay for serological response to SFG rickettsiae gave negative results for each subject (Thornton et al. 2001).
The Caribbean Amblyomma Programme (CAP) is an international multiagency effort to eliminate threats of Amblyomma-transmitted diseases through tick eradication (Pegram et al. 2004). CAP was started in 1994 and has successfully eradicated Am. variegatum from a majority of the Caribbean islands enrolled in the program. CAP certified the islands of St. Kitts, St. Lucia, Anguilla, Montserrat, Barbados, and Dominica officially free of the tropical bont tick by 2003, whereas three islands, Antigua, St. Martin, and Nevis, are still infested (Pegram et al. 2004). The island of Antigua has had significant trouble maintaining an active eradication campaign. Eradication efforts began in Antigua in 1997, but because of poor compliance and management, all funding for eradication efforts was suspended by December 2001. In 2002, a new initiative focused only on a single parish, St. Phillip, was begun. However, significant amounts of new funding were never secured, and this project came to an end in December 2005 (R. Pegram, personal communication). Since then, limited funding has been used to generate an island-wide registry of farmers. Most recently, the Antiguan government and Ministry of Agriculture have implemented several new initiatives including a cost recovery program where farmers buy already-stocked Bayticol acaricide at cost to make funds to replenish the purchased stock, and a seasonal tick control program where island-wide treatment only takes place during the July to September rainy season when tick population is the highest. In 2007, CAP issued Bayticol to all enrolled islands, and Antigua has chosen to distribute 12-mo supplies of Bayticol free of charge to all registered farmers (R. Lloyd, personal communication). The most recent statement issued from the 13th meeting of the Amblyomma Programme Council (APC) emphasizes that the tropical bont tick still constitutes a threat locally in the Caribbean, as well as by spread to nearby continents, and avows the APC’s continued commitment to surveillance and control.
Despite the lack of known human cases of ATBF in Antigua, our data suggest that the likelihood of both R. africae and E. ruminantium infections in Antigua is great enough to demand concern from those interested in preventing disease threats in the Caribbean and the spread of these diseases to North and South America. This is especially important when noting that Bubulcus ibis, the cattle egret, can be found throughout the continental United States, especially in the south and California (National Geographic Society 2000). However, usually larvae and nymphs ticks feed on these birds and thus are unlikely to be infected with E. ruminantium because it must be acquired horizontally from a host animal. However, the threat for the importation of R. africae to the mainland continents by bird migration is a continued public health concern because the organism is maintained in the tick population at a high rate through transovarial transmission. Animals are not the only hosts that can import exotic ticks. Recently, American tourists have reported returning to America with exotic tick species such as Am. hebraeum attached to them (Burridge et al. 2002). In summary, the threat of the tropical bont tick is real, both on Antigua and to the surrounding tick-free countries, and continuing eradication efforts should be sustained.
Acknowledgements
The authors thank J. Keirans for identification of the Rh. (Boophilus) microplus ticks, T. Parakh for laboratory assistance, and R. Massung for E. ruminantium DNA. J.B.R. thanks the Oak Ridge Associated Universities, Oakridge, TN, for administering her undergraduate internship.
The findings and conclusions presented in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the Department of the Defense, or the Department of the Navy.
References Cited



