-
PDF
- Split View
-
Views
-
Cite
Cite
Jefferson A. Vaughan, Jeffrey A. Bell, Michael J. Turell, Dave D. Chadee, Passage of Ingested Mansonella ozzardi (Spirurida: Onchocercidae) Microfilariae Through the Midgut of Aedes aegypti (Diptera: Culicidae) , Journal of Medical Entomology, Volume 44, Issue 1, 1 January 2007, Pages 111–116, https://doi.org/10.1093/jmedent/41.5.111
Close - Share Icon Share
Abstract
When virus and microfilariae are ingested concurrently by a mosquito, microfilariae (mf) may penetrate the mosquito midgut and introduce virus directly into the mosquito hemocoel, allowing mosquitoes to become infectious much sooner than normal and enhancing transmission of viruses by mosquitoes. Mansonella ozzardi (Manson) is a benign filarial nematode parasite of humans in Latin America and is transmitted by black flies (Diptera: Simuliidae) and biting midges (Diptera: Ceratopogonidae). Because M. ozzardi and dengue are sympatric, we wanted to know whether M. ozzardi mf had the ability to penetrate the midgut of Aedes aegypti (L.) (Diptera: Culicidae) and thus play a potential role in the enhancement of dengue transmission. To test this, the F1 progeny from locally collected Ae. aegypti were fed on M. ozzardi-infected human males in an endemic village in northern Trinidad. Mosquitoes were dissected at various times after feeding and examined for mf in the midguts and thoraces. Microfilariae penetrated the midguts of 43% of 63 mosquitoes that ingested mf. Overall, 11% of mf penetrated the midgut by 17 h after being ingested. The intensity of midgut penetration was positively correlated to the numbers of mf ingested. Because midgut penetration is a key requirement for mf enhancement to occur, the potential exists that M. ozzardi could be involved in the enhancement of dengue virus transmission.
Concurrent ingestion of microfilariae (mf) and arboviruses by vector arthropods has been shown to result in significantly more efficient transmission of the virus than when the same dose of virus was ingested alone (Mellor and Boorman 1980; Turell et al. 1984, 1987; Zytoon et al. 1993; Vaughan and Turell 1996; Vaughan et al. 1999). This has been referred to as microfilarial enhancement of arboviral transmission. As part of their normal developmental cycle in a vector, most mf species penetrate the vector midgut after being ingested and migrate to a preferred site of development (e.g., flight muscle and fat body). If a microfilaremic host is also viremic when fed upon by a vector, then there is the possibility that penetration of the midgut by mf will allow some of the ingested virus to enter directly into the vector hemocoel. The facilitated movement of virus directly into the hemocoel can have two important epidemiological consequences. First, it can increase vector competence by allowing virus to bypass both midgut infection and midgut escape barriers (Chamberlain and Sudia 1961, Kramer et al. 1981), transforming otherwise incompetent vector species into competent vector species and thus increasing the number of vector species involved in an arbovirus transmission cycle. Second, mf enhancement can accelerate arboviral development within a vector and shorten the time required for a virus-exposed mosquito to become infectious (i.e., shorten the extrinsic incubation period [EIP]). Because the EIP affects transmission exponentially (MacDonald 1952), small reductions in EIP can lead to large increases in vectorial capacity, even within natural arbovirus–vector systems.
Most laboratory studies of mf enhancement have used various arboviruses with Brugia spp. mf and Aedes/Ochlerotatus spp. mosquitoes as experimental model systems (Turell et al. 1984, 1987; Vaughan and Turell 1996; Vaughan et al. 1999). But the concept of mf enhancement could apply, in theory, to almost any filarial–vector system (Mellor and Boorman 1980) where filarial and arboviral infections are sympatric. An important consideration is whether the mf can penetrate the midgut and enter the hemocoel and thus allow the virus to bypass the midgut. Further development of the parasite is irrelevant, i.e., a mosquito species does not have to be a competent vector of filariasis for mf enhancement to occur. This was illustrated in a study examining the ability of co-ingested mf of Brugia malayi to affect the ability of Ochlerotatus taeniorhynchus (Wiedemann) (Diptera: Culicidae) transmit Venezuelan equine encephalomyelitis virus (family Togaviridae, genus Alphavirus, VEEV) (Vaughan et al. 1999). A 30-fold increase in VEEV transmission rate was observed in the mosquitoes ingesting both agents compared with mosquitoes ingesting VEEV alone, yet ingested B. malayi mf never developed to larval (L3) stage within Oc. taeniorhynchus and were essentially all dead by 48 h after ingestion (J.A.V., unpublished data). Potentially, there could be innumerable combinations of parasite–vector-virus in nature where mf enhancement could apply, including combinations with mf not normally associated with mosquitoes.
Dengue is the most important mosquito-borne viral disease of humans and involves cyclical transmission of the virus between humans and mosquito vectors [primarily Aedes aegypti (L.)]. There are also several human filarial diseases that are sympatric with dengue, including lymphatic filariasis (Wuchereria bancrofti, Brugia malayi, and Brugia timori), loiasis (Loa loa), onchocerciasis (Onchocerca volvulus), and mansonelliasis (Mansonella ozzardi, Mansonella perstans, and Mansonella streptocerca). To understand the potential that these human filarial infections may have on the mf enhancement of dengue transmission, it is important to understand the ability of these mf species to penetrate the midgut of Ae. aegypti. It is well established that Wuchereria and Brugia mf can readily penetrate the midgut of Ae. aegypti mosquitoes (Ramachandran and Zaini 1967, McGreevy et al. 1982, Lowichik and Lowrie 1988, Zielke 1992, Calheiros et al. 1998). Indeed, laboratory studies have shown that co-ingestion of B. malayi mf and dengue 2 virus can enhance virus transmission by Ae. aegypti (Turell et al. 1987). Less is known about the ability of the other, nonmosquito-borne human filarial species to penetrate the midgut of Ae. aegypti mosquitoes. Mansonella ozzardi is a benign filarial infection of humans throughout tropical Latin America that is transmitted by black flies (Diptera: Simuliidae: Simulium spp.) and/or biting midges (Diptera: Ceratopogonidae: Culicoides spp.) (Nelson and Davies 1976, Nathan 1978, Shelley et al. 1980, Shelley and Coscaron 2001). Although not generally associated with mosquitoes, M. ozzardi occurs in areas where several mosquito-borne arboviruses are transmitted, including dengue virus. In this report, we describe the ability of M. ozzardi mf to penetrate the midgut of a co-indigenous strain of Ae. aegypti mosquitoes.
Materials and Methods
Study Site.
The research was conducted on-site within the village of Blanchisseuse (10° 78′ N, 61° 31′ W), a discrete rural community on the coastal foothills of Trinidad's forested Northern Range Mountains. The village is endemic for M. ozzardi and has been a study site for Mansonella research for many years (Nathan et al. 1982, Chadee et al. 1995, Gonzalez et al. 1999).
Mosquitoes.
Engorged Ae. aegypti females were collected on-site in Blanchisseuse and returned to the Insect Control Division, Ministry of Public Health, in Saint Joseph, Trinidad and Tobago, where the females were allowed to lay eggs and larvae were reared to adults. To safeguard against the potential of using mosquitoes that may have been vertically infected with dengue or other flavivirus, the parents of the F1 mosquitoes (n = 115) were frozen and shipped overnight to the University of North Dakota where they were tested by polymerase chain reaction (PCR) against flavivirus RNA by using the primer sets and methodology described by Scaramozzino et al. (2001). The RNA extracted from locally caught Culex tarsalis (Coquillett) (Diptera: Culicidae) infected with West Nile virus (Bell et al. 2005) served as positive controls. All Ae. aegypti parent mosquitoes tested negative, ensuring that all F1 mosquitoes used in this trial were derived from flavivirus-free stock.
Mosquito Blood Feeding.
Before this research was conducted, the study protocol received approval from the Institutional Ethics Committee of the Ministry of Health, Trinidad and Tobago and the Human Subjects Research Review Board of the University of North Dakota. Potential subjects (males older than 19-yr old) were recruited from villagers known from previous surveys to harbor M. ozzardi infections. Two days before mosquito blood feeding, 200 μl of blood was obtained from each of six subjects via fingerprick, spread on a microscope slide, air-dried overnight, and stained with Giemsa stain, and the total number of mf on each slide was counted to determine absolute density of mf in the peripheral blood for each person. Mosquitoes were allowed 15–20 min to feed from a screened cage, based on procedures approved by the two Institutional Review Boards. Most of the mosquito feedings were conducted in the evening (2030–2230 hours). Two of volunteers with the highest microfilaremias allowed mosquitoes to feed on their arms the following afternoon (1500–1700 hours) to test for possible mf periodicity that might affect the level of mf uptake by a diurnal feeding mosquito such as Ae. aegypti.
Assessment of Microfilarial Ingestion and Penetration.
Engorged mosquitoes were held at ambient temperature. Beginning 1 h after the feeding, three people (two dissecting mosquitoes; one counting mf) collected paired data for each mosquito (i.e., mf counts for bloodmeal and carcass). Mosquitoes were aspirated into ethanol, transferred to saline, and individual midguts were carefully excised with fine-tipped jeweler's forceps, taking care not to rupture the midgut during the process. Only "clean" dissections where midguts were excised intact were included in the data set. Individual midguts were transferred to a glass slide, compressed with a coverslip, and examined for mf at 100–400× magnification. The carcasses were thoroughly teased apart with needles and similarly examined for mf. Microfilariae were relatively easy to find when they were alive and wriggling, but dead or moribund mf often required careful inspection. For each mosquito, mf were counted as either being contained within the bloodmeal or in the hemocoel. The sum of the two counts represented the total number of mf ingested by an individual mosquito. To ensure accuracy, counts were rechecked by more than one observer.
Statistical Analyses.
Microfilarial counts for both numbers ingested and numbers penetrating the midgut were not normally distributed (Shapiro–Wilk normality tests; W statistic ≥ 0.63; P < 0.0001) nor did transformation of data by logarithms or square roots normalize the data. Kruskal–Wallis tests were used to compare medians. Infection prevalences were compared using chi-square or Fisher exact tests. Spearman rank correlation (rs) was used to test for density relationships. A 0.05 level of significance was used for all statistical tests (Statistix version 8, Analytical Software, Tallahassee, FL).
Results
Ingestion of Microfilariae.
Based on the results from two volunteers that were fed upon twice, there were no differences among mosquitoes between nighttime and daytime feedings with respect to either quantities of mf ingested (Wilcoxon rank sum tests, P > 0.27) or prevalences of mf ingested (Fisher exact tests, P > 0.63) (data not shown). Because there was no evidence to suggest that M. ozzardi in Trinidad were nocturnally periodic, data for the different times of day were pooled for each of these volunteers in subsequent analyses. Microfilaremias in the six volunteers ranged from 3 to 394 mf per 200 μl of blood (Table 1). Only three of a total of 87 mosquitoes dissected were lost to "dirty" dissections. Overall, mf were detected in 63 of the 84 engorged mosquitoes successfully dissected and examined. The percentages of engorged mosquitoes that ingested mf ranged from 0 (volunteer 5) to 94% (volunteer 2) and were positively correlated with microfilaremia of the volunteers (rs = 0.83, n = 6, P = 0.03). The mean numbers ingested were mostly within the range expected, based on host microfilaremia and bloodmeal volumes, and there was no indication that Ae. aegypti concentrated M. ozzardi mf during feeding. There was a significant difference among the six volunteers in the quantity of mf ingested by the mosquitoes that fed on them (range, 0–8.6 mf per mosquito, Kruskal–Wallis, H = 27.1, P < 0.0001) and in general, the quantity ingested increased with increasing host microfilaremia, although the correlation was not statistically significant (rs = 0.81, n = 6, P = 0.06).
Ingestion of M. ozzardi mf by endemic F1 Ae. aegypti mosquitoes fed on the forearms of six different microfilaremic adult male human volunteers, Blanchisseuse Trinidad, 2004
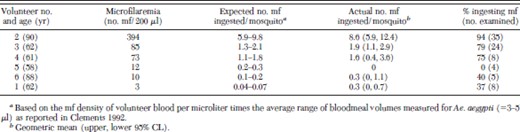
Ingestion of M. ozzardi mf by endemic F1 Ae. aegypti mosquitoes fed on the forearms of six different microfilaremic adult male human volunteers, Blanchisseuse Trinidad, 2004

Mf Penetration of the Mosquito Midgut.
Only mosquitoes that had ingested mf were included in the analyses of mf penetration of the mosquito midgut. Overall, M. ozzardi mf penetrated the midguts in 43% of 63 mosquitoes that ingested mf (Table 2). Of the 539 total mf ingested by the 63 mosquitoes, 11% penetrated the midgut and moved into the hemocoel. There was a significant positive correlation between the numbers of mf ingested and the numbers of mf that penetrated the midgut (rs = 0.45, n = 63, P < 0.001) (Fig. 1). Most mf found within mosquito hemocoels were located in the vicinity of the thoracic flight muscles. Examination of mf penetration at selected intervals indicated that mf penetration of the mosquito midgut increased over the first 2 h, remained relatively constant up to 6 h, and then decreased at 14–17 h (Table 3). Unlike mf observed wriggling in the hemocoel during earlier time points, most mf observed in the hemocoel after 14 h were immobile and assumed a rigidly straight body position. Melanized mf were never observed in either the midguts or the hemocoel.
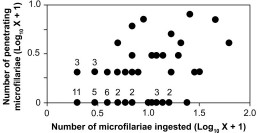
Density relationship between the number of M. ozzardi mf ingested by Ae. aegypti mosquitoes and the number of mf that penetrated the mosquito midgut and move into the hemocoel (n = 63). Some mosquitoes yielded the same values for mf ingested and mf escaped. Thus, some data points have associated superscripts that indicate the number of mosquitoes having those values in common.
Penetration of ingested M. ozzardi mf through the midgut of resident Ae. aegypti mosquitoes fed on microfilaremic human volunteers, Blanchisseuse Trinidad, 2004
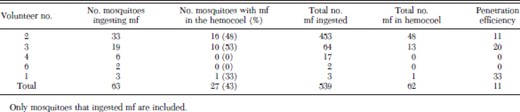
Penetration of ingested M. ozzardi mf through the midgut of resident Ae. aegypti mosquitoes fed on microfilaremic human volunteers, Blanchisseuse Trinidad, 2004

Penetration of ingested M. ozzardi mf through the midgut of resident Ae. aegypti mosquitoes at selected intervals after feeding on microfilaremic human volunteers, Blanchisseuse Trinidad, 2004.

Penetration of ingested M. ozzardi mf through the midgut of resident Ae. aegypti mosquitoes at selected intervals after feeding on microfilaremic human volunteers, Blanchisseuse Trinidad, 2004.

Discussion
Mosquitoes are not considered to be involved in the transmission of M. ozzardi. Nevertheless, we demonstrated that ingested M. ozzardi mf readily penetrated the midguts of native Ae. aegypti mosquitoes. Virtually all mf that had penetrated the midguts were either moribund or dead by 14 h after the bloodmeal, confirming that Ae. aegypti are not likely to be involved in the natural transmission of this filarial worm. Thus, the importance of this study has nothing to do with transmission of M. ozzardi, but rather it addresses the broader question of whether a nonmosquito borne filarial species, like M. ozzardi, could enhance the transmission of arboviruses from a vertebrate host (e.g., human) to mosquitoes (e.g., Ae. aegypti).
It has been shown in laboratory studies that mosquitoes that feed on vertebrates co-infected with mf and an arbovirus are significantly more efficient vectors of the virus than mosquitoes that ingest virus alone (Turell et al. 1984, 1987; Zytoon et al. 1993; Vaughan and Turell 1996, Vaughan et al. 1999). For this enhancing effect to happen, the mf must be able to penetrate the mosquito's midgut. To our knowledge, the only study examining M. ozzardi in mosquitoes was by Davis (1928) who dissected triatomine bugs and mosquitoes at various times after feeding them on microfilaremic people and examined the insects for developing filariae in the hemocoel. A small percentage of some mosquito species (including Ae. aegypti), contained stunted, developmentally arrested, or dead worms in the thorax. Once it was discovered that Culicoides were the vectors (Buckley 1934), no further studies were published on possible relationships between M. ozzardi and mosquitoes. Our study corroborates and expands that of Davis (1928) and shows that M. ozzardi mf are ingested during the course of blood feeding by Ae. aegypti mosquitoes and are capable of penetrating the midgut and moving into the hemocoel. Thus, the potential exists for M. ozzardi to be involved in the mf enhancement of anthroponotic viruses such as the dengue viruses.
There are other reasons to suspect that M. ozzardi infections could potentially interact with dengue transmission. Throughout most of its distribution in the Western Hemisphere, dengue is sympatric with M. ozzardi. Therefore, it is not unreasonable to assume that dually infected humans may exist wherever dengue and mansonelliasis overlap, particularly if the prevalence of M. ozzardi infection is high. In Trinidad, prevalence of mansonelliasis has been recorded as high as 11–31% (Nathan et al. 1979, Gonzalez et al. 1999). Mansonelliasis is typically a rural disease due to the distribution of its vector (i.e., biting midges), whereas dengue tends to be a more urban disease because its principal vector, Ae. aegypti, is synanthropic. However, in Trinidad, Ae. aegypti abundance (as measured by Breteau index) and dengue incidence (i.e., per capita number of new cases) have been observed to be distributed equally between rural and urban areas during recent dengue outbreaks (Brown et al. 2004, Chadee et al. 2005). In addition, the mf of M. ozzardi are aperiodic (Nathan et al. 1978). Thus, M. ozzardi mf are present in human peripheral circulation during the time when Ae. aegypti mosquitoes are most likely to feed (i.e., during daytime), unlike the mf of most strains of Wuchereria bancrofti and Brugia spp. that display nocturnal periodicity.
It is still unknown whether M. ozzardi infections can actually enhance dengue transmission. Proof will require vector competence studies by using M. ozzardi mf, similar to studies described by Turell et al. 1984, who demonstrated enhancement of dengue virus infectivity to Ae. aegypti by B. malayi mf. We hypothesize that the primary effect of mf enhancement on dengue transmission will be a reduction in the extrinsic incubation period of dengue viruses within mosquito species that are already vectors of dengue (e.g., Ae. aegypti and Aedes albopictus Skuse). All evidence to date indicates that intrathoracically inoculated dengue viruses only replicate efficiently in Aedes spp. mosquitoes (Rosen et al. 1985, Huang et al. 1992). Therefore, it seems unlikely that in the case of dengue, mf enhancement can alter the vector competence of otherwise refractory mosquito species, i.e., mf enhancement probably will not create new dengue vectors. Even so, shortening the extrinsic incubation period of dengue within the vector may still produce a significant impact on dengue epidemiology.
Acknowledgements
We thank Robin Persad and Lester James, Ministry of Health, Saint Joseph, Trinidad and Tobago, for collecting and rearing of mosquitoes and invaluable help with the logistics of this study. Nathan Mickelson, University of North Dakota, assayed mosquitoes for flaviviruses. This project was funded in part by a University of North Dakota Faculty Research Seed Money Award (to J.A.V.) and North Dakota EPSCoR (to J.A.B.).
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
References Cited



