-
PDF
- Split View
-
Views
-
Cite
Cite
X. Porcasi, S. S. Catalá, H. Hrellac, M. C. Scavuzzo, D. E. Gorla, Infestation of Rural Houses by Triatoma Infestans (Hemiptera: Reduviidae) in Southern Area of Gran Chaco in Argentina , Journal of Medical Entomology, Volume 43, Issue 5, 1 September 2006, Pages 1060–1067, https://doi.org/10.1093/jmedent/43.5.1060
Close - Share Icon Share
Abstract
The impact of control activities against Triatoma infestans (Klug) (Hemiptera: Reduviidae) in South America has a marked contrast within and outside the Gran Chaco region. Development of a geographic information system, as part of an improvement in control program activities, allowed analysis of the spatial pattern of house infestations by T. infestans before and after house spraying with deltamethrin in the San Martín Department (an arid Chaco region of central Argentina). The overall peridomestic infestation index decreased from 48.2 to 28.2% after insecticide application. House infestation was spatially clustered in regions with low or high infestation levels that were located east and southwest of the department, respectively. This pattern was detected both before and after the insecticide application. Three environmental variables calculated from a temporal series of MODIS imagery (average of night temperature, maximum of day temperature, and temporal variation of vegetation index) were capable of correctly discriminating 96% of the places belonging to either high or low house infestation observed after the insecticide application.
Control of Chagas disease in Latin America has been highly successful through the multinational initiatives in the Southern Cone, Andean Pact, and Central America, under the coordination of World Health Organization (WHO)/Pan American Health Organization (Dias et al. 2002). The initiatives worked with a common technical agenda, by vector control with pyrethroid insecticides, control of blood banks to reduce transfusional transmission, and parasitological treatment of infected people <15 yr of age, for which available drugs can be effective. This strategy has led to interruption of the vectorial transmission of the disease in Chile and Uruguay, most of Brazil, and several provinces of Argentina outside the Gran Chaco region (WHO 1995, 1997, 1998; Dias and Schofield 1999; Incosur 2004).
Success of the initiative for the elimination of domestic infestation by Triatoma infestans (Hemiptera: Reduviidae), the main vector of Chagas disease in the Southern Cone of South America, contrasts with the recent increase of Chagas disease case numbers in the Gran Chaco region of Argentina (Gürtler et al. 2005). In this country, the organizational changes of the control programs, initiated during the early 1980s, from vertical to decentralized structures where responsibility is dispersed over many provincial and municipal authorities, have led to a progressive reduction of vector control activities. At the end of 2001, Argentina entered an economic crisis that has led to a marked reduction of the health budget, which has limited the vector control activities. In addition, problems have occurred in the elimination of peridomestic populations of T. infestans within the Gran Chaco region with the existing methods. These problems had long been identified by the studies of Gürtler et al. (2004), and there is now a marked contrast between the epidemiological situation in the Gran Chaco and elsewhere in Argentina.
The Gran Chaco is a natural subarid region of ≈1 million km2 (representing the second largest biome in America after the Amazon region) that extends over parts of Bolivia, Paraguay, Brazil, and Argentina. Human population density is low, usually averaging fewer than five people per square kilometer. The typical vegetation is an open mosaic of woodland and grasslands, although overgrazing and overexploitation for timber has left many areas with impoverished scrubland. In such areas, the local communities tend to rely on goat production, which is associated with use of brushwood corrals that often harbor high densities of T. infestans (Bucher and Schofield 1981).
The region of Los Llanos in the province of La Rioja is located to the south of the Gran Chaco and constitutes one of the most arid and poorest regions of Argentina. As part of the Chagas disease vector control program, rural houses in this region were first sprayed with organochlorine insecticides in the late 1950s to early 1960s and extensively sprayed with pyrethroid insecticides during the 1980s (Segura 2002). By the early 1990s, the national program for the control of Chagas disease decentralized the activities of vector control, transferring the responsibility to the provinces. For La Rioja, this decentralization resulted in a virtual cessation of vector control activities during the next 15 yr, except for individual houses irregularly sprayed by nonprofessional personnel.
By 2002, the government of La Rioja reactivated the Chagas disease vector control activities, identifying as the main target for vector control the eight provincial departments within the region of Los Llanos. By the beginning of 2004, professional teams from the provincial Ministry of Health had sprayed all houses (intradomicile and peridomestic structures) of the San Martín Department. In parallel, blood samples were taken from all children <15 yr of age (estimated coverage >95%) and also from pregnant and fertile women, to carry out serological tests according to the standard national protocol for Chagas disease. Serological tests included indirect hemagglutination and enzyme-linked immunosorbent assay as primary tests, and indirect immunofluorescent antibody test in case of discordance between the former two assays. The serological results showed that 8.65% (n = 104) of children between 6 mo and 4 yr of age, 7.92% (n = 164) of children 5–9 yr of age, and 16.7% (n = 162) of children between 10 and 14 yr of age were infected with T. cruzi (H.H., unpublished data). The serological results (especially in the age class 6 mo-4 yr) indicate that the vectorial transmission was very active in the region. All cases were serologically reconfirmed and are currently under parasitological treatment with benznidazole.
The procedure for data collection in the Chagas program of La Rioja showed problems in the recent past, including loss of vector control data. As part of the improvement of the program activities, a new protocol for data collection was designed, including the development of a spatial database where relevant data of the control program could be stored and analyzed.
The objective of the current study was to analyze the spatial pattern of house infestation by T. infestans before and after house spraying with pyrethroid insecticides at the scale of the San Martín Department (La Rioja, Argentina).
Materials and Methods
The study was carried out in the Department of San Martín, an area 5,034 km2, south of La Rioja Province in central Argentina (Fig. 1). According to the last national census of 2001, there were a total of 4,921 people living in 1,120 houses in the department. Most of the population lives in dispersed communities of <25 houses. Only one community (Ulapes) has >40 houses, where 2,674 people lived in 488 houses.
Location of the San Martin Department within the La Rioja Province (Argentina). The inset shows the Gran Chaco region (hatched pattern), where active vectorial transmission occurs heterogeneously around the region in Argentina.
All houses within this department were inspected by a professional team of two technical staff members of the provincial vector control program of La Rioja. Infestation by T. infestans in the domestic and peridomestic structures was recorded by active search through manual collection and use of a dislodgant spray (0.2% aqueous tetramethrin). A house was considered infested when at least one live specimen of T. infestans (nymph or adult) was found either in an intra or peridomestic structure. No timed collection of triatomines was carried out, so no estimate of triatomine population abundance is available for the baseline data. All houses within the department were treated with deltamethrin (25 mg [AI]/m2) by the technical staff of the provincial vector control program of La Rioja (spray coverage 94.8% of the houses) using 6-liter manual compression sprayers with Schmitt 8002 fan nozzles. Insecticide was applied to indoor surfaces (walls and roof), including room furniture, external walls, and peridomestic structures (goat and pig corrals, chicken coops, and grain and tool deposits used by dogs and chickens to sleep). House spraying was carried out between November 2003 and June 2004.
Latitude and longitude coordinates of 151 localities (defined as a site with a house or group of houses separated from another house or group by at least 500 m) were recorded with a Garmin 38 global positioning system and used to build a geographic database. These localities were the units of analysis and were considered infested if at least one house of the group was infested by T. infestans either in the intradomicile or a peridomestic structure.
One year after spraying the houses with deltamethrin (between November 2004 and February 2005), 69% of the localities recorded before the insecticide application were visited by a research team to carry out an active search for T. infestans in peridomestic structures (especially goat corrals and chicken coops). Because of the large dispersion of the rural houses in the area, 100% coverage was not possible for the research team. Localities were selected to obtain the maximum spatial coverage of the department, leaving aside the most distant localities. The search was confined to peridomestic structures, because these structures have shown the highest likelihood of harboring residual populations (Cecere et al. 1997). For each house, the search involved two investigators and was aided by the use of a dislodgant spray (0.2% aqueous tetramethrin) up to 15 min. When a live T. infestans (nymph or adult) was found in a peridomestic structure, the search was interrupted, and the house was recorded as infested. A locality was considered infested if at least one peridomestic structure was positive for T. infestans.
Because localities were not uniformly distributed around the department and the house infestation rate was not necessarily randomly distributed among localities, spatial analysis of the house infestation rate was carried out with the Bernoulli model of the SaTScan, version 4.0 statistics (Information Management Services 2003). This point-pattern analysis seeks the existence of groups of localities with significantly higher or lower infestation rates than the departmental average. To carry out the analysis, the scan statistics calculate the number of infested houses in circles of increasing radii and compares this number with the expected number of houses predicted by the binomial probability distribution (Kulldorff and Nagarwalla 1995, Kulldorff 1997).
Considering the potential association of the persistent reinfestation of houses within the Gran Chaco region with particular environmental conditions, and findings of previous studies showing that remotely sensed variables are associated with the geographic distribution of T. infestans (Gorla 2002), an environmental characterization was performed over the whole department to study possible associations between infestation rates and climatic and ground coverage properties. The environmental characterization was obtained using satellite estimates of land surface temperature (LST) (day and night) and normalized difference vegetation index (NDVI) for each locality. LST is a measure of energy emissivity of the land surface expressed as brightness temperature, and the NDVI is a measure of green vegetation coverage (Hay 2000). LST and NDVI values were extracted from a time series between 1 January and 31 December 2003 of MODIS imagery downloaded from the Earth Observing System Data Gateway (http://edcimswww.cr.usgs.gov/pub/imswelcome). NDVI images are 16-d composites with a spatial resolution of 250 by 250 m, whereas LST are 8-d composites with a spatial resolution of 1 by 1 km. Values of the original LST image set were transformed to degrees centigrade, and NDVI imagery was transformed to values in the range 0 to 1. All image processing used the ENVI, version 3.5 (Research Systems Inc. 2002) and Idrisi32 (Clark Labs 2002) software packages.
The mean, maximum, minimum, and standard deviation of day and night LST as well as LST range (difference between day and night LST) were extracted from the pixel image (1 by 1 km) that each locality occupied. The same statistics were used for the NDVI but extracting each pixel value from a 5 by 5 pixel grid (1.56 km2) to characterize each locality. The environmental properties of individual localities and locality groups with high and low infestation by T. infestans identified with the spatial cluster analysis were used to perform a forward stepwise discriminant analysis using Statistica for Windows, version 5.5 (Stafsoft 2000).
Results
Of the 716 rural and periurban houses of the department, 259 (36.2%) were positive for T. infestans at the moment of the insecticide application. The house infestation pattern was highly heterogeneous. Among the 183 urban houses inspected and treated in the city of Ulapes, only two (located at the periphery of the city) were found infested. The rest of the infested houses (257) were located in the rural area. For the rest of the analysis, the data set on the houses of Ulapes is excluded.
Houses in the rural area are highly dispersed and organized in small groups; 82% of them are arranged in groups of 1–15 houses. Approximately 70% of the houses occur as localities with one or two houses, with a mean distance to the nearest neighbor locality of 1,831 m. Of the overall total of 533 rural houses, 48.2% (257) of them were infested by T. infestans before the insecticide application (Fig. 2).

Localities infested by T. infestans before the insecticide application that remained positive (closed circles), or were recorded as negative after the insecticide application (open circles). Localities noninfested before the insecticide application that were recorded as positive (closed squares) or remained negative after the insecticide application (open squares).
The pattern of house infestation before the insecticide application showed two clusters of high house infestation rate. A primary cluster was identified to the southwest of the department, where 15 localities showed a house infestation rate of 68.4% (n = 52 houses). The cluster was centered at 31° 48′48″ S, 66° 32′23″ W and had a radius size of 14.6 km. The statistical test showed the cluster in the border of significance (P = 0.06). A secondary cluster of high house infestation rate, composed by four localities with 100% of infested houses (n = 12) (P = 0.017) was located at the northwest of the department, near Ulapes (31° 35′48″ S, 66° 9′44″ W, with 4.56-km radius). A highly significant (P < 0.001) cluster of low house infestation rate (11.4%) was located at the northeast of the department, including four localities (with 35 houses); it was centered at 31° 30′42″S, 65° 54′31″ W and had a 5.9-km radius (Fig. 3A).
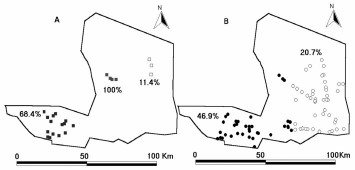
(A) Cluster of high infestation (primary and secondary clusters: 100 and 68.4% infestation, respectively), as closed squares and cluster of low infestation (11.4%) as open squares before the insecticide application. (B) Cluster of high infestation (46.9%), as closed circles and cluster of low infestation (20.7%) as open circles after the insecticide application.
Evaluation of the peridomestic structures of the treated houses (excluding Ulapes) 1 yr after the spraying showed an overall infestation rate of 28.2% (108 of 383 houses). The house infestation pattern showed one significant cluster (P = 0.02) of high infestation to the southwest of the department where 130 houses in 36 localities showed 46.9% infestation by T. infestans, centered at (31° 42′ S, 66° 31′28″ W) and a 41.0-km radius. Another highly significant cluster (P = 0.001) of low house infestation (20.7%) was located east of the department, including 150 houses in 48 localities (Fig. 3B); it was centered at 31° 42′57″ S, 65° 55′59″ W and had a 27.9-km radius.
A comparison of house infestation before and after the spraying intervention of 89 localities showed that 47 localities that were infested before the spraying, 46.8% (n = 22) remained infested 1 yr later. Of the 42 localities that were not infested before the spraying, 69% (n = 29) remained uninfested 1 yr later (Fig. 2).
All localities included in the cluster of low infestation recorded before spraying were included in the low infestation cluster after spraying to the east of the San Martín Department. All localities of the primary and secondary clusters of high infestation were included in a single high infestation cluster after spraying to the southwest of the San Martín Department.
A similar spatial analysis carried out on the serological data showed no significant clustering. Of the 49 seropositive children <15 yr of age, 26 (of 125 tested) lived within the area of the low house infestation and 16 (of 62 tested) lived in the area of the high house infestation (Programa Chagas La Rioja, unpublished data). The difference between the proportion of seropositives (0.21 for low and 0.26 for high house infestation) was not significant.
An environmental characterization of the areas showing low and high house infestation rates was carried out, by using the values of LST and vegetation index (NDVI) for the pixels belonging to the localities of each cluster identified. The cluster of high infestation included 33 1-km pixels and 900 250-m pixels, whereas the cluster of low infestation included 25 1-km pixels and 1,125 250-m pixels. Because the prespraying clusters were included within the postspraying clusters, no analysis was carried out on the prespraying pattern of house infestation.
Figure 4 shows the strong seasonal variation of the LST (day and night). Maximum day LST reached >40°C during the summer (December–March) and minimum night LST dropped below zero during the winter (July–September). Average day LST was similar in clusters of high and low house infestation, but night LST was an average 2°C lower in the low infestation cluster than in the high infestation cluster. Daily range of LST (LST day – LST night) was on average 2.4°C wider in the area of high house infestation than in the low infestation.
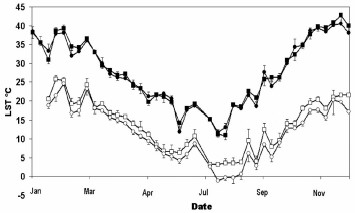
Annual variation (2003) of diurnal land surface temperature (LST day) produced by the MODIS sensor (closed symbols) and LST night (open symbols) in locality groups of high (squares) and low (circles) house infestation rate. LST values are 8-d composites.
NDVI also showed strong seasonality and very low values (between 0.25 and 0.5). Figures were similar in the eastern and western areas from midsummer to end of spring. NDVI values diverged from the beginning of the spring (September), when the rainy period began. Vegetation response to rainfall was more rapid in the east than in the west, and average values by the beginning of summer were higher in the east than in the west (Fig. 5).
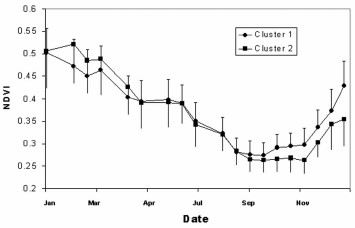
Annual variation (2003) of 16-d composites of the vegetation index (NDVI) in locality groups of high (squares) and low (circles) house infestation rate.
An analysis of variance (ANOVA) of the LST (day and night) and the NDVI, after discounting the temporal variability, showed that variability among localities in the southwestern area (higher house infestation) is 1.13 times higher for the LST day, 1.41 times higher for the LST night, and 3.93 times higher for the NDVI (all values P < 0.01).
A stepwise discriminant analysis to identify high and low infestation localities (as estimated by the SaTScan statistic) by using environmental variables as predictors produced a significant model (F3,74 = 97.53; P < 0.0001) that correctly classified 96.1% of the localities belonging to a high or low infestation cluster (Table 1). The environmental variables with most weight in the discrimination of the two clusters of high and low infestation were the LST night average, LST day maximum, and the temporal variance of the NDVI. The model showed a better performance in the classification of groups of low (100% correct) than for high (90.91% correct) peridomestic infestation (Table 2).
Environmental variables selected by the forward stepwise discriminate analysis of high and low house infestation by T. infestans in the San Martín Department (La Rioja)
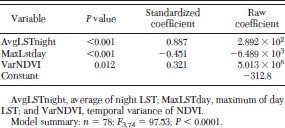
Environmental variables selected by the forward stepwise discriminate analysis of high and low house infestation by T. infestans in the San Martín Department (La Rioja)

Classification matrix of localities, assigned to low or high house infestation areas by a linear discriminant model based on the three variables selected (see Table 1)
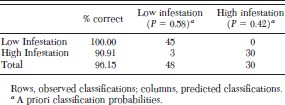
Classification matrix of localities, assigned to low or high house infestation areas by a linear discriminant model based on the three variables selected (see Table 1)

Discussion
The success of the Southern Cone Initiative in the control of T. infestans in areas outside the Gran Chaco region contrasts with the failure of complete control of this species within the region. Since the launch of the initiative in 1991, Uruguay and Chile have been certified free of vectorial transmission of Chagas disease, as have 10 states of Brazil, and the Argentinean provinces of Jujuy, La Pampa, Neuquén, Rio Negro, and Entre Ríos (Dias and Schofield 1999, Incosur 2004). The progressive interruption of the vectorial transmission shows a clear spatial pattern, leaving a cleared territory outside the Gran Chaco and a core area where the disease is still actively transmitted by T. infestans within the region, with regular (and in some places, increasing) occurrence of acute cases (Gürtler et al. 2005). This scenario should receive urgent attention by health authorities, especially oriented toward the epidemiological situation of Chagas disease in the Gran Chaco, either for vector control and vigilance, detection of human infections, or parasitological treatment where necessary.
The persistence of infestation by T. infestans in the Gran Chaco is probably associated with interruptions of the local control programs, but it also suggests that this species is particularly well adapted to the habitats offered by this region. In a sense, peridomestic populations of T. infestans in the Gran Chaco may represent population refuges where the current control activities have low efficiency (Gürtler et al. 2004). This may reflect the colonization route used by T. infestans to disperse around the southern cone of South America. Schofield (1988) suggested that the dispersion center of the species was located in the Cochabamba valleys of Bolivia, from where the species dispersed following the trade routes during pre- and postcolonial periods. Recent findings indicate that there could be two main dispersal routes, associated with different cytotypes of T. infestans (Panzera et al. 2004, Bargues et al. 2006): a northern route derived from the selvatic foci of the Cochabamba valleys, invading southern Peru and the northern regions of Chile, and a southern route originating from the Bolivian–Paraguayan–Argentinian Chaco, that dispersed over the rest of the southern cone. The vector populations derived from the Chaco dispersal center seem to represent a particularly successful T. infestans type that colonized large areas of the southern cone, but the success of control programs outside the Gran Chaco implies that these vector populations may be better adapted to Chaco habitats than elsewhere.
After 15 yr of virtual interruption in the vector control activities in the San Martín Department (La Rioja), house infestation rate by T. infestans in the rural area rose to almost 50% and the vectorial transmission of Chagas disease remained active and high. Recent data on other provincial departments within the region show a similar situation (H.H. et al., unpublished data).
The pattern of house infestation within the rural area of the San Martín Department before the insecticide application showed a heterogeneous distribution, with areas of high and low infestation rates. This pattern could be a consequence of a combination of local living conditions, awareness of the presence of vectors by the house owners, differential efficiency of local (nonprofessional) control interventions, and environmental factors. At the departmental scale, two significant clusters were present: one cluster with high infestation rate at the west and the other cluster with low infestation rate at the east.
One year after the control intervention, the outcome of the insecticide application was not homogeneous around the San Martín Department, with clusters of high and low infestation rate, similar to the clusters occurring before the intervention. The main difference was that the cluster sizes were larger after the intervention. The persistence of this clustering pattern after the control intervention suggest that the efficiency of the insecticide application (higher where infestation rate was lower, lower where the infestation rate was higher) is not homogeneously distributed around the San Martín Department. Under these circumstances, the distribution of the infestation rate seems to be associated with structural causes, e.g., complexity of the peridomestic structures and/or environmental features. The clustered distribution of the infestation also could be responding to the abundance of T. infestans populations. Cecere et al. (2002) showed that the abundance of domestic populations before insecticide treatment was associated with the percentage of infested houses after spraying.
A high proportion of localities (47%) remained infested 1 yr after the insecticide application. A similar situation was reported for T. dimidiata populations in Guatemala (Nakagawa et al. 2003, 2005), where clusters of highly infested villages were found continually infested after three insecticide applications. Within these localities, the control efficiency after the first spraying was ≈58%.
Descriptive statistics for the annual series of day and night LST (LST day and LST night, in °C) and vegetation index (NDVI) for the group of localities within high and low house infestation rates
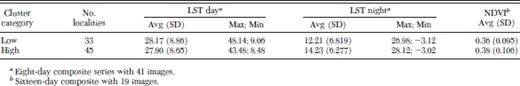
Descriptive statistics for the annual series of day and night LST (LST day and LST night, in °C) and vegetation index (NDVI) for the group of localities within high and low house infestation rates

The results observed in this study confirm the low spraying efficacy in peridomestic structures at the Chaco region, as was shown by Gürtler et al. (2004). They suggested a decreased effectiveness because of the dust covering the treated surface, combined with degradation of the active ingredient when directly exposed to the sun's radiation.
In the current study, 14% localities in total recorded as not infested before the insecticide application were found infested after the spraying, which probably represents failures in the detection of T. infestans in the complex peridomestic ecotopes before the insecticide application. If these houses were not infested before the insecticide application, reinfestation from untreated sites or from residual populations from an external source could be suspected. However, the mean distance to the nearest infested localities is ≈4 km, much larger than the expected range for active dispersal by T. infestans (Schofield et al. 1992, Cecere et al. 2004). In addition, fourth and fifth instars were found in most of these localities (data not shown), which seems to suggest survivorship after insecticide application. The observed pattern could have been the outcome of passive dispersal from a distant source, followed by a rapid vector population growth, although this seems less likely because of the short time elapsed between the two entomological surveys (including a cold winter season in between). Failure of infestation detection seems more plausible, probably as a consequence of low population abundance, known to produce false negative results because of the low sensibility of sampling methods based on active searches (Gürtler et al. 1995).
The identification of the infestation rate clustering at the departmental level by using a geographic information system offers several advantages over the traditional reporting system currently in use by the vector control programs in Argentina. It allows for a clear identification of heterogeneity in the infestation rate distribution that could be used as a basis for risk stratification and differential allocation of resources. The GIS database also allows a more efficient mechanism for monitoring vector control activities, compared with procedures based on hard-copy forms. Other activities carried out by the Chagas disease control program (e.g., parasitological treatments) or not connected with the program (e.g., vaccination programs) could use the same information base.
This study showed that the area with higher infestation presented a wider range of temperature, higher night temperatures, and lower and temporally more variable vegetation index than localities included in the cluster with lower infestation. The occurrence of a lower population abundance where night temperature is lower reflects the results reported by Gorla (1992) that showed a significant density-independent effect of monthly minimum temperature over the mortality of T. infestans populations developed under natural climatic conditions of the Gran Chaco region. Two variables related with LST night average and LST day maximum were able to describe the postspraying pattern of house infestation.
The model produced by the discriminate analysis suggests that at the landscape scale, climatic and ground cover variables could give indications about the frequency of house infestation by T. infestans. Further studies to clarify the relationship of these landscape indicators and the disease epidemiology would give a good base to produce a risk map of the disease in the dispersed rural areas of the Gran Chaco.
Acknowledgements
This study was carried out with partial support of the ECLAT network and the Consejo Nacional de Ciencia y Técnica (CONICET) of Argentina and the Government of La Rioja. X.P. holds a fellowship from CONICET and CONAE. D.E.G. is a researcher of CONICET. We thank M. Lamfri (Instituto Gulich) for the technical support with MODIS image processing and V. Rodriguez (CRILAR) for the field support. We acknowledge the support of David J. Rogers and group at the Tala Research Group, Oxford University (Oxford, United Kingdom).
References Cited



