-
PDF
- Split View
-
Views
-
Cite
Cite
Bradley A. Mullens, Alec C. Gerry, Life History and Seasonal Abundance of Fannia benjamini Complex (Diptera: Muscidae) in Southern California , Journal of Medical Entomology, Volume 43, Issue 2, 1 March 2006, Pages 192–199, https://doi.org/10.1093/jmedent/43.2.192
Close - Share Icon Share
Abstract
Seasonal abundance and life history of Fannia benjamini complex (Diptera: Muscidae) was studied in the coastal mountain community of La Habra Heights in Los Angeles County, California, with additional observations in drier, inland mountains in the Woodcrest area, Riverside County. The dominant species (>95% of fly collections) was Fannia conspicua Malloch, whereas Fannia benjamini Malloch also was present. Both species could be collected nearly year-round by netting adults (almost exclusively females) attracted to people, although F. benjamini was relatively more common in cooler weather (November-April). In La Habra Heights, adult activity peaked in June and July (both species), whereas peak activity in Woodcrest was late April through June for F. conspicua and February through April for F. benjamini. Field-collected adult females provided only water died within 2–4 d, whereas females provided with water plus a dry milk and sugar mixture survived up to 128–130 d in the laboratory. Males of F. conspicua (1–40 individuals) swarmed at heights of 0.5–4.0 m near Aptenia cordifolia (L.f.) Schwantes, an exotic, succulent, low-growing ground cover plant. Eggs of F. conspicua were deposited singly on older, decaying leaves of this plant, and adult F. conspicua emerged from Aptenia-covered areas in the field. In the laboratory, larvae of F. conspicua grazed on microbial surface films associated with Aptenia. Emergence of F. conspicua from field-collected soil-Aptenia mixtures (held in the laboratory) occurred from 1.5 to 5 mo after collection. Widespread Aptenia planting since the 1980s has probably resulted in F. conspicua becoming a severe human nuisance in some areas.
The Fannia benjamini Malloch complex (Diptera: Muscidae) is found primarily in upland coastal mountain and Sierra foothill habitats of California, with some distributed in Baja, Mexico, and Arizona (Chillcott 1960). Commonly called "canyon flies," they range from 3.5 to 4.5 mm in length, and the complex consists of seven species (Turner 1976). One member of the complex, F. thelaziae Turner, supports development of nematodes (eyeworms) in the genus Thelazia, which infect a variety of mammals, including people (Weinmann et al. 1974, Turner 1976). Where they are abundant, these flies also are very significant human and animal nuisance pests, flying around and sometimes landing on the face and other parts of the body.
Despite their abundance and pest status, very little is known about "canyon fly" biology. Winkler and Wagner (1961) reared F. benjamini complex from eggs derived from wild-caught females, and superficially described larval development in the laboratory. Poorbaugh (1969) reared F. benjamini through two laboratory generations, describing some aspects of its immature development, laboratory mating behavior, and larval feeding behavior. Garcia and Rodovsky (1962) observed adult feeding behavior of wild-caught adult female F. benjamini and made preliminary observations of their orientation to feeding tabanids, two-dimensional visual targets, and survival and fecundity.
However, there have been no systematic studies of "canyon fly" activity. The immature development sites in the field have remained unknown, with the exception of a single pupa of F. benjamini taken from a wood rat nest (Chillcott 1960). The current study was designed to determine species present in selected locations in southern California, to document their seasonal adult activity, and to investigate other life history aspects, particularly immature developmental sites.
Materials and Methods
Study Sites.
Primary study sites were two residences and a horse riding ring area in coastal mountain habitat in the city of La Habra Heights, Los Angeles County (33° 57′ N, 117° 57′ W, 280-m elevation). This area has numerous hills and canyons with both endemic and exotic trees, including coast live oak, Quercus agrifolia Nee; sycamore, Platanus racemosa Nutt.; Brazilian pepper, Schinus terebinthifolius Raddi; and Eucalyptus spp., with arroyo willow, Salix lasiolepis Benth, and black willow, Salix nigra Marsh, in the drainages. The natural habitat in treeless areas is coastal sage scrub, where common plants include California buckwheat, Eriogonum fasciculatum Benth, and California sagebrush, Artemisia californica Less. The area receives ≈41 cm of rainfall per year, and >80% of this rainfall occurs between November and April. Average high and low air temperatures in January are 20.8 and 7.6°C and in July 28.0 and 17.3°C, respectively.
Other observations were made in the Woodcrest area of western Riverside County (33° 53′ N, 117° 21′ W, 430-m elevation). This area is topographically somewhat similar to La Habra Heights, but is drier, hotter in summer, and cooler in winter. Natural areas (without irrigation) have essentially no trees except in the drainages, where sycamore, Eucalyptus spp., and black willow are common. The sage scrub habitat is more sparse but with many of the same plants. The area receives 27 cm of rain per year, also almost all between fall and spring. Average high and low temperatures in January are 19.6 and 5.7°C and in July 30.7 and 17.3°C, respectively.
Seasonal Adult Activity.
Because adult "canyon flies" are attracted to the vicinity of people or animals, their activity was documented by collecting flies attracted to the researchers using a short-handled, 45-cm diameter aerial sweep net. Collections initially were made from both residences and the horse riding area in La Habra Heights. Weekly collections were made beginning 13 May 2003 and continued through 14 August 2003. Collections continued at the horse riding area and were made every 2 wk between 26 August and 29 January 2004. Weekly collections at the horse riding area resumed on 4 March 2004 and continued through 19 August 2004. Collections generally were made in the morning between 0900 and 1030 hours.
On each sample date, a researcher stopped for 5 min at each of four consecutive, regular locations situated 30–75 m apart. Flies attracted to the person were collected by net, killed in a cooler with dry ice, and placed in a labeled vial for transport back to the laboratory for counting and identification. Flies were identified using keys in Turner (1976) and through comparison with identified material in the California Academy of Sciences, San Francisco, CA.
The two researchers were compared in La Habra Heights for attraction and netting efficiency on three dates in July and August 2003. On those dates, the two researchers stood ≈4 m apart and netted flies for matched 5-min periods (n = 10). Numbers of "canyon flies" collected were compared using a paired t-test.
Net collections also were made in Woodcrest, where periodic observations were made on relative adult activity between April 2003 and July 2004. These collections, although not done regularly as in La Habra heights, allowed determination of species present and some assessment of seasonal activity in a drier, inland southern California location.
Adult Longevity.
On the morning of 24 July 2003, a large number of F. conspicua adults were collected from a researcher at the horse riding area in La Habra Heights. These were returned to the laboratory and set up in 237-ml cardboard containers (20 females per container and five containers per treatment) with different food and water sources (treatments) as follows: 1) a deionized water vial with wick, 2) a water vial plus a small dish of dry milk and sugar, 3) 10% sugar water in a vial, 4) liquid whole milk on tissues plus 10% sugar water in a vial, and 5) fresh beef liver plus 10% sugar water in a vial. The liver and whole milk were replaced every 2 d. Flies were maintained at 23 ± 1°C and natural photoperiod and monitored every 1 to 2 d for mortality (dead flies were removed) until all had died. The numbers of adult females dying in the different food treatments were subjected to survival analysis.
Observations on Mating Behavior and Immature Development Sites.
Observations of male swarming behavior (location, time of day, and approximate number of individuals) were made in the field in May and June 2003 and 2004 in Woodcrest, and in July 2004 at the horse riding area in La Habra Heights.
Large, tent-type emergence traps (Fig. 1) were designed and deployed in spring and summer 2004. Fiber-reinforced, brown plastic tarps (either 1.5 by 2.2 m or 2.5 by 3.1 m) formed the "skirt" of the trap and had a 12-cm hole cut in the center. Marine plywood (two layers each 1.9 cm in thickness) was used as a top. The rectangular plywood pieces (25.4 by 15.2 cm) had holes cut and offset toward one end- the top hole was 10.9 cm in diameter and the bottom hole was 11.7 cm in diameter. A fitted 2-liter clear polystyrene jar lid (screw top) nested tightly in the opening, and a conical piece of clear plastic was formed into a cone (glued to the lid) to impede exit of flies that made their way into the trap container. One side of the jar itself had a hole 9 cm in diameter and covered by 16 mesh window screen to provide ventilation. The jars thus could be easily removed and capped with a solid lid to obtain collected insects.

Emergence trap used to survey for Fannia spp. immature developmental sites.
The tarp was held securely between the two pieces of plywood, which were fastened together using bolts. The underside of the plywood end opposite the hole had a metal screw base affixed, and a 1.3-cm-diameter, sturdy plastic pipe was screwed into it. The pipe was 61 cm in length for the larger tarp and 46 cm in length for the smaller tarp.
To deploy the trap, a 1-m-long piece of metal reinforcing bar (1 cm in diameter) could be hammered into the soil, and the pipe slid down onto it. This formed a tentlike center pole. Tent stakes were pounded through the grommet holes at the edges of the tarp, securely holding the trap in position. The edges of the tarp were buried using soil. The 10 traps were deployed in a variety of locations thought to be possible developmental sites for canyon flies. These included organic debris accumulations beneath trees and shrubs, moist areas near the bottom of drainages, loose soil into which seasonal grass growth had been plowed for fire control, hillside areas with gopher burrowing activity, and hillside stands of Aptenia cordifolia (L.f.) Schwantes, an exotic, succulent ground cover planted extensively in the area since the 1980s for esthetics, erosion, and fire control. Traps were left in place for 2–6 wk and then moved to new locations.
Emergence from Field Soil or Vegetation.
Samples were taken from 0.25-m2 Aptenia-covered hillside areas on 30 July and 6 August 2004. Twelve small soil samples (80–150 g) from the top 6 cm under Aptenia were removed on 30 July, returned to the laboratory, weighed, oven-dried (50°C) for 3 d, and reweighed to calculate percentage of moisture. Separate samples of soil and vegetation (4 liters each) from the same general areas were removed on 6 August. These were held in the laboratory for fly emergence in plastic dishpans with emergence tops (Mullens et al. 1996) and checked every 1–3 d until 2 January 2005 (149 d after initial collection). Sample 1 contained mostly vegetation (Aptenia and pine needles) and was misted with deionized water periodically (usually every 1 to 2 d) beginning on 6 August. This sample was allowed to dry out from 24 October (80 d after initial collection) through 19 November (106 d after collection) and then rewet and misted again through 2 January. Sample 2 contained Aptenia and soil (top 6 cm of soil). It also was misted every 1 to 2 d beginning on 6 August. Three samples consisting primarily of the soil collected beneath Aptenia (top 6 cm of soil with some Aptenia leaves and roots) also were taken. One of these samples (sample 3) was misted beginning on 6 August, the second (sample 4) was misted beginning on 7 September (31 d after collection), and the third (sample 5) was misted beginning on 10 October (64 d after collection). Canyon flies were collected every 1–3 d to determine numbers and sex of emerging flies and how long emergence from a given sample might continue.
Results
Species and Seasonal Abundance.
Two species in the complex, F. benjamini s.s. and F. conspicua, were collected in southern California. F. benjamini could be separated morphologically from F. conspicua because of the orange base of the arista in conspicua (black in benjamini). Specimens of F. conspicua also tended to be slightly smaller and usually had pale, yellowish coloring laterally on the dorsal, anterior abdomen (gray in benjamini). Voucher specimens have been deposited in the Department of Entomology Research Museum, University of California, Riverside.
Overall, F. conspicua was far more common. It comprised between 94 and 98% of the total Fannia individuals collected at the three sampling locations in La Habra Heights. All specimens collected by sweep net at these sites were female. The seasonal activity of the two species is shown in Fig. 2. The dominant species, F. conspicua, was collected from early April through December, with a distinct peak in activity in June and July. From mid-June through mid-July, collections ranging from 500 to 850 flies/20 min (up to >40 flies per minute) were documented. Flies were persistent and flew near the collectors, particularly the head, waist, and feet. F. benjamini was never abundant, but it also was most common in June and July in La Habra Heights, and the correlation coefficient (r) between its activity and that of conspicua was 0.797. However, F. benjamini seemed to be relatively more common in cooler months. Between May and October 2003 in La Habra Heights (horse riding area), 2.5% of 3,087 canyon flies were F. benjamini, whereas between November 2003 and April 2004, 55.9% of 34 flies were F. benjamini.
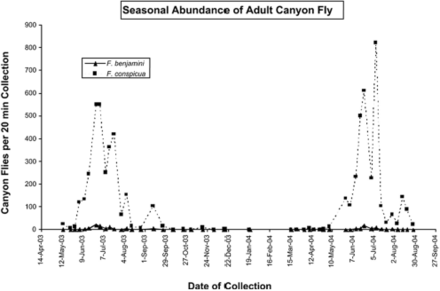
Seasonal abundance (flies attracted to and collected in 20 min by a person using a sweep net) of Fannia spp. at the Las Palomas riding ring area in the city of La Habra Heights, CA.
Collections from people in Woodcrest also were predominantly female F. conspicua. Activity of this species was highest (up to ≈40 flies per 5 min collecting period) early and late in the day between late April and early June, with another apparent activity peak in September and October. No F. conspicua were taken between January and March. In contrast, canyon flies taken in February and March were F. benjamini, which was never as common as F. conspicua became later in the year. Occasional specimens of F. benjamini were taken in Woodcrest in August and October.
We, both ≈72-kg males, did not differ in the number of "canyon flies" collected in the 10 matched, 5-min time intervals using a paired t-test on the differences (t = 0.68, df = 9, P = 0.51). Individual 1 collected an average (±SE) of 65 ± 34 females per 5-min period, whereas individual 2 collected an average of 48 ± 17 females per 5-min period.
Adult Fly Survival.
Proportional survival of adult female F. conspicua collected in the field on 24 July 2003 and maintained in the laboratory is shown in Fig. 3. A survival analysis was performed on the numbers dying per time interval by treatment (MINITAB Release 14.12; Minitab, Inc., University Park, MD). The mean times ± SD to death for the treatments were 1.1 ± 0.1 d for water only, 7.8 ± 6.1 d for 10% sugar water plus raw liver, 12.4 ± 4.7 d for 10% sugar water plus liquid milk, 14.9 ± 5.6 d for 10% sugar water, and 43.2 ± 37.6 d for water plus a mixture of dry milk and dry sugar. All flies given water only were dead by 4 d. Flies given 10% sugar water diets (alone or with milk or liver) survived for up to 24–26 d, and some flies lived up to 128–130 d on the water plus dry milk and dry sugar diet in the laboratory. In pairwise comparisons of the survival curves, the differences were very highly significant statistically (χ 2> 315.8, df = 1, P < 0.000) for all comparisons except 10% sugar water versus 10% sugar water plus milk (χ 2 = 2.61, df = 1, P = 0.107), 10% sugar water versus 10% sugar water plus liver (χ 2 = 0.73, df = 1, P = 0.393) and 10% sugar water plus milk versus 10% sugar water plus liver (χ 2 = 5.64, df = 1, P = 0.018). Whereas the 10% sugar water plus milk did differ statistically from 10% sugar water plus liver, the differences were relatively subtle, and probably based on differences in the shape of the curve early in the trial (mortality in the first several days was nil in the liquid milk treatment, but was higher in the liver treatment). Adult longevity clearly was extremely low with water only, intermediate with liquid food sources, and high with liquid water plus dry milk and dry sugar.
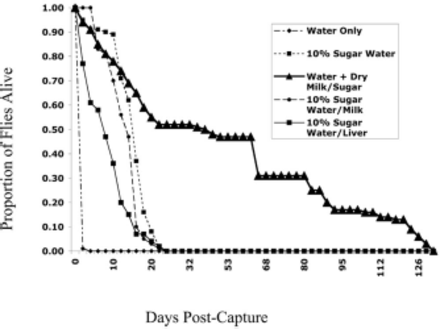
Survival of cohorts (100 females each) of field-collected adult F. conspicua held in the laboratory with different food and water sources.
Fannia eggs were observed when they were deposited in clear view on the mesh screen of the containers, or more rarely on the liver, milk-soaked tissues, or wicks. Statistical comparisons were not conducted because of the differential mortality in the treatments and the fact that specific oviposition materials were not supplied. Still, flies laid no eggs when provided water only. Despite their much longer average life span, females given water, dry sugar, and dry milk deposited a total of only ≈20 eggs. Flies in the remaining treatments deposited ≈60 eggs (10% sugar water), 80 eggs (10% sugar water and liquid milk), and >400 eggs (10% sugar water and liver).
Mating Behavior and Developmental Sites.
Loose swarms of F. conspicua males were observed in April-June in Woodcrest and in July in La Habra Heights. Swarms in Woodcrest were seen primarily early (before 1000 hours) and late (after 1600 hours) in the day, and the La Habra Heights males were observed swarming in late morning (1100–1200 hours). Swarming sites contained from one up to ≈40 individuals. Individual males in the swarms would hover briefly and pursue other males entering their area and then seemed to return to the approximate original location. In Woodcrest males repeatedly gathered near the peak of a single-story house roof at heights of 3 to 4 m. Swarms also were regularly seen at lower heights (0.5–2 m) above a hillside covered by Aptenia. The same locations were used on multiple days and for at least several weeks. Males rarely also were taken while sweep-netting near the collector in Woodcrest, and on one occasion mating with a captured female occurred almost immediately in the sweep net. In La Habra Heights, swarming males were observed at heights of 1 to 2 m in the opening of a shed and near a line of short pine trees, both in the vicinity of Aptenia-covered slopes.
The emergence traps initially failed to collect Fannia when deployed in La Habra Heights (April-June 2004) for 2–6 wk at a time over a variety of potential habitats. These included leaf debris beneath trees, soil in the bottom of a wet drainage, damp soil on a grassy bank below a horse watering trough, soil that had been plowed to bury seasonal grasses for fire control, and soil that had recent gopher activity. After observing males swarming directly above an Aptenia-covered slope in Woodcrest, an emergence trap was deployed there in early June 2004, and it collected four to six flies per day for the next 3 d. To eliminate any possibility that flies might have burrowed through small openings at the base of the trap, the trap was removed and the Aptenia torn away from the area previously covered by the trap. The top 5–7 cm of soil was removed and placed into plastic bags for transport to the laboratory, where it was placed into buckets sealed with a clear plastic lid coated with sticky material, except for small air exchange openings (1 mm in diameter). Multiple F. conspicua emerged from the soil as late as 7 wk after collection.
Subsequently eight more traps were deployed over Aptenia-covered slopes in La Habra Heights near the horse riding area. Four of these were placed over a recently covered (≈1 to 2 yr) slope, whereas the remaining four were placed over Aptenia that had been there for several years. Traps on the recently covered slope yielded occasional F. conspicua, whereas the traps placed over the older Aptenia growth often had 1–5 F. conspicua in the collecting heads when checked the following week.
The older mats of Aptenia were ≈10–20 cm in depth. They had newer growth on top and older leaves in various stages of decay beneath. When the top layers were lifted back, the older leaves were observed to have numerous Fannia eggs on them (Fig. 4). Eggs were laid singly and in depressions or folds in the vegetation.

Eggs of F. conspicua deposited in the field on older leaf of A. cordifolia.
The first F. conspicua emergence from samples taken 6 August was observed 52 d later. The sample with only vegetation (sample 1, Aptenia plus some pine needles) produced 78 flies from 52 to 67 d postcollection (Fig. 5A). Weighted mean time of emergence (through 67 d) for this group of flies was 56.8 d for males and 59.8 d for females. After drying and then being wetted again beginning 106 d after initial collection, two more flies (males) emerged 145 d after initial soil collection. The sample with Aptenia plus soil (sample 2) produced 59 flies from 52 to 80 d postcollection (Fig. 5B). Weighted mean time of emergence was 53.6 d for males and 58.3 d for females. The soil sample wetted from date of collection (sample 3) yielded no flies. The sample with soil wetted after 31 d (sample 4) produced 27 flies ≈1 mo later, with males emerging after an average of 59.8 d and females after 63.3 d (Fig. 5C). The soil sample wetted after 64 d (sample 5) produced a single male and a single female 149 d after initial collection (85 d after wetting). Soil samples taken from beneath the Aptenia on 30 July (the week before emergence samples were taken) ranged from 0.8 to 10.1% moisture. Periodic visual examination of the samples indicated that the larvae of F. conspicua tended to feed on the moist and decaying surface of Aptenia leaves and stems, grazing on surface tissue and associated microflora.
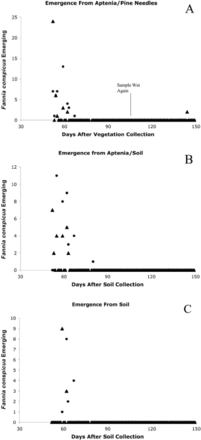
Emergence of adult F. conspicua from soil or vegetation samples collected in early August under A. cordifolia in La Habra Heights, CA. (A) Aptenia–pine needle mixture (vegetation only) misted from day 0–80 after collection, allowed to dry, and then remisted beginning at day 106. (B) Aptenia–soil mixture misted from day of collection. (C) Soil with some Aptenia leaves and roots misted beginning at day 31 after collection. Females, ●; males, ▲.
Discussion
These are the first substantial field life history studies with members of the F. benjamini complex. In general, the muscid subfamily Fanniinae is temperate in distribution, but the benjamini group has a preponderance of Neotropical members (Chillcott 1960). Two other members of the genus, Fannia canicularis (L.) and F. femoralis (Stein), are common in southern California but are intolerant of high temperatures (above ≈27–30°C) (Meyer and Mullens 1988). It seems that F. conspicua and particularly F. benjamini share this trait, because their numbers drop with the onset of very hot summer weather, and they seem to be far more common in coastal mountain range habitats than in warmer inland areas. The current study suggests F. benjamini may be more sensitive to high temperatures than is F. conspicua, but more studies on temperature relationships are warranted. The distinct seasonal peak abundance of "canyon flies", especially F. conspicua, may reflect temperature variation, seasonal rainfall (see below) or both. Winkler and Wagner (1961) reportedly worked with F. benjamini in the laboratory using adults collected primarily in the San Bernardino Mountain foothills (Lytle Creek area). They observed, but did not document, adult activity from early spring through fall; early season activity was midday, whereas summer activity was early or late in the day. However, they did not address how the flies were identified; they may not have been aware of closely related species, and some species were added to the complex subsequently (Turner 1976).
Chilcott (1960) noted that Fannia spp. commonly feed on natural plant sugar sources such as honeydew and plant sap. We are unaware of quantitative studies on prevalence of sugar feeding in nature, but the laboratory data show that sugar or food of some kind is essential for adult survival beyond a very few days. Winkler and Wagner (1961) supplied adult F. benjamini with either no food, sugar, or protein hydrolysate. Flies with no food died in <2 d, and they reported (but did not quantify) that flies with access to sugar lived longer than flies with protein access. Similarly, Garcia and Radovsky (1962) observed that flies fed only blood in the laboratory lived only ≈2 d, whereas flies fed sugar solution lived ≈2 wk. Our data show that flies with no food die very quickly. The best survival (up to 128–130 d) was by flies given access to water and to dry milk and sugar.
Although egg production was not carefully quantified, flies with access only to sugar laid very few eggs. In contrast, flies with access to raw liver laid many eggs, although they died in <25–27 d. Some of these were laid on the liver itself, but most were deposited on the mesh container screen, which all other fly food treatment groups also had. Poorbaugh (1969) fed adult F. benjamini on liquid blood of various species, supplemented with honey-soaked pads. Garcia and Radovsky (1962) observed that F. benjamini frequently fed on blood from tabanid wounds in the field and on mouse blood in the laboratory. Garcia and Radovsky (1962) also dissected wild-caught females over time (gonotrophic status unknown at capture) that were held with or without blood as a protein source; both groups had access to sugar solution. Some (two of five) sugar-fed females had eggs, but 12 of 13 blood-fed females had eggs, suggesting F. benjamini requires a protein source for oogenesis. A liquid form of protein probably is necessary for proper egg development in F. conspicua, but further studies are needed. Although a few males were collected in Woodcrest by sweep netting near the collector, the majority of flies collected in this way at all sites consistently were female. The attraction of almost exclusively females to a vertebrate also suggests vertebrates may be a source of materials required for oogenesis and would explain the powerful attraction of female canyon flies to carbon dioxide (Gerry and Mullens 2006).
Observations on mating behavior of F. conspicua in the field (small male swarms often near cover such as low tree boughs) are consistent with other members of the subfamily (Chillcott 1960). It was the presence of males near the succulent ground cover A. cordifolia that prompted examination of soil under Aptenia (or Aptenia itself) as a larval development site.
As a group, Fannia spp. use a very wide array of organic substrates for development, ranging from fungi and rotting plant material to decaying animals or manure (Chillcott 1960). The older stands of hillside A. cordifolia, which seem to be a key type of development site for F. conspicua, represent a leaf litter or possibly fungi-mold type habitat, but it is unclear exactly what immature F. conspicua are feeding on. Egg deposition in small depressions also was noted in the laboratory by Winkler and Wagner (1961). Poorbaugh (1969) observed that larvae of F. benjamini fed on the surface of moist (but not wet) microbial films on fecal or alfalfa pellets in the laboratory and did not enter the substrate. This agrees with our observations on feeding by larvae of F. conspicua. The pattern of depositing eggs singly in depressions of old Aptenia leaves was seen frequently in the field and agrees with the single egg deposition patterns seen in the laboratory.
At least two other local species, F. canicularis and F. femoralis, tolerate rather low moisture levels (40–47% moisture) in the poultry manure in which they develop (Mullens et al. 2002). The moisture of soil collected in La Habra Heights in July (near the end of peak adult abundance) beneath A. cordifolia was exceedingly low (≤10%). Despite this, live immatures of F. conspicua were present. With or without supplemental moisture, adult flies emerged 7–21 wk after sample collection in early August, and males tended to emerge a bit earlier than females. Protandry in F. benjamini in the laboratory also was noted by Poorbaugh (1969), who documented emergence from rabbit feces in 25–63 d (20–26°C). Winkler and Wagner (1961) reported emergence in "somewhat >15 d" at temperatures of 20–25°C.
Chillcott (1960) and Poorbaugh (1969) discuss the possibility of a prepupal diapause in "canyon flies", and Poorbaugh (1969) documented extended development (prolonged larval or pupal stage) of F. benjamini in rearing dishes that were allowed to dry out. Such a phenomenon could influence the seasonal occurrence of adult F. conspicua and possibly F. benjamini as well; the rather prolonged fly emergence from our laboratory-held samples does suggest stasis in dry conditions. Very small numbers of adults emerged as long as 5 mo after collection from samples first wetted 1 to 2 mo after collection. F. conspicua developed to maturity when given only vegetation, so soil is not absolutely necessary. Emerging flies were presumably collected very soon in the trap collecting heads, and no food (e.g., protein) sources beyond the vegetation itself were available in the rearing pans. Thus the late-emerging flies are assumed to have been present as immatures in the samples from the time of collection.
Without knowing more about how temperature, moisture, and food quality influence "canyon fly" development, it is difficult to ascertain how many generations they have per year. Some adult activity year-round suggests that some emergence may occur most of the year. Larvae hatching from eggs laid between May and July in an area such as La Habra Heights enter a soil habitat that, although protected against temperature extremes, is very dry. Seasonal rains typically do not occur until December-February, when temperatures are cool and development may be prolonged. Multiple generations of "canyon fly" per year certainly are possible; emergence under suitable laboratory conditions probably requires a minimum of ≈3 to 4 wk (Poorbaugh 1969). However, given the marked moisture and temperature fluctuations in nature (low or high temperatures and dry conditions are assumed to slow development), we think it is likely that there are three to five generations per year. This deserves more study.
Long-term residents of La Habra Heights report that numbers of adult "canyon flies" have been drastically higher since the early 1990s, which fits with the present observations on emergence from A. cordifolia and soil beneath it. This exotic (South African) ground cover was planted extensively for erosion and fire control in southern California beginning in the mid-1980s. One can assume it takes several years to accumulate organic debris and develop a significant layer of dead leaf litter in new plantings, and emergence trap data and direct observations suggest this is necessary for "canyon fly" oviposition and development. Large areas of A. cordifolia exist, and the present studies show that an area of only 0.25 m2 can produce 50–100 adult flies. Both "canyon fly" species (F. conspicua and especially F. benjamini, whose immature habitat we did not find) must use other types of larval habitats with which they evolved. People have unwittingly supplied F. conspicua with a highly suitable but unnatural habitat; the natural habitat could be similar (e.g., leaf litter in well-drained slopes), especially if it receives some supplemental moisture via irrigation. We did not evaluate whether removal of A. cordifolia could reduce subsequent adult Fannia numbers, but it is a logical next step. If so, the benefits of reduced "canyon fly" nuisance would need to be balanced against benefits of A. cordifolia as an attractive ground cover useful for erosion and fire control.
Acknowledgements
The observations and cooperation of several La Habra Heights residents, notably Carol Meisenbacher, Steve Blagden, Shelley Andros and Francis Schultz are appreciated. We also appreciate the assistance of Tamim Nawaey and Coralie Szijj in sorting and identifying collected specimens. Financial support for this study was provided by the city of La Habra Heights.
References Cited



