-
PDF
- Split View
-
Views
-
Cite
Cite
Nobuko Tuno, Kaori Miki, Noboru Minakawa, Andrew Githeko, Guiyun Yan, Masahiro Takagi, Diving Ability of Anopheles gambiae (Diptera: Culicidae) Larvae , Journal of Medical Entomology, Volume 41, Issue 4, 1 July 2004, Pages 810–812, https://doi.org/10.1603/0022-2585-41.4.810
Close - Share Icon Share
Abstract
Anopheles gambiae Giles larvae usually live near the surface of shallow and temporary aquatic habitats. How deep the larvae can dive and how long they can submerge may be related to feeding efficiency and predator avoidance. This study examined diving behavior of An. gambiae larvae in the laboratory. We recorded diving depths and larval mortality of second and fourth instars in clean water and muddy water by using deep water (32-cm) and shallow water (20-cm) columns. In deep water columns with clean water, we found that 2% of second instars and 6% of fourth instars died from diving, whereas 3% of second instars and 11% of fourth instars died in muddy water. The fourth instars dived deeper in muddy water than in clean water. The mortality rates of the fourth instars subjected to diving stimulations were significantly higher than those in the shallow water columns. Therefore, larval diving behavior may offer the benefits of predator avoidance and food acquisition but also incur energetic costs and increased mortality.
Most habitats of Anopheles gambiae Giles and Anopheles arabiensis Patton are small, temporary, sunlit, turbid pools of water, whereas Anopheles funestus Giles is often found in permanent water bodies (Gillies and De Meillon 1968, Minakawa et al. 1999, Gimnig et al. 2001). An. gambiae larvae generally live near the surface of aquatic habitats, but they dive and remain at the bottom for some time if some mechanical disturbance takes place. While at the bottom, they often adopt a C-shaped posture, resembling dead insect carcasses. They also can move along the bottom, turning over particle after particle (N.T., unpublished data). These observations suggest that the diving ability of anopheline larvae (i.e., depths and duration of diving) may be related to predator avoidance and feeding.
Romoser and Lucas (1999) studied mosquito pupal diving behavior. They showed that diving behavior varied dramatically among mosquito genera: Culex pipiens L. and Anopheles stephensi Liston make short-duration, shallow dives, whereas Aedes aegypti (L.), Aedes albopictus (Skuse), and Aedes triseriatus (Say) make longer duration dives, typically to a depth at which they become neutrally or negatively buoyant. They suggested that pupal diving behavior helps avoid predation and the mechanical shock of a direct hit by a raindrop and prevents them from being washed away from their container habitats by overflowing water. In this article, we report a study on the diving ability of An. gambiae larvae and the effects of water condition and larval age on larval diving ability.
Materials and Methods
This study was conducted using An. gambiae larvae from a laboratory colony maintained in the Vector Biology Control and Research Center, Kenya Medical Research Institute, Kisumu, western Kenya. This strain was derived from wild mosquitoes collected in the vicinity of the Institute. All experiments were conducted in 500-ml graduated cylinders (34 cm height, 5 cm in inner diameter at room temperature [26 ± 2°C).
The diving ability of the second and fourth instars was examined under two water conditions to determine the effect of turbidity on larval diving behavior under two different water depths. Two water conditions were dechlorinated tap water (hereafter referred to as "clean water"), and mixture of local black cotton soil with dechorinated water (hereafter referred to as "muddy water"). The muddy water had ≈2–3-mm thick sediment at the bottom of the muddy water column and the turbidity was 170-400 Formazin turbidity units. In the deep water columns, individual larva was discharged into the 500-ml graduated cylinder with 32-cm-deep water from a plastic cup by using a pipette with 0.5 ml of water. In most cases, the discharging acts provoke a dive. When they did not dive, we discharged another 0.5 ml of water with a pipette at the surface and repeated until they started to dive (three stimulations were sufficient). Caution was taken not to touch the larvae and to maintain similar stimulation intensity for all larvae. The depth each larva reached and whether it returned to the surface were recorded. Those individuals that did not return to the surface immediately also were observed for survivorship within 6 h. In total, we measured the diving depths and mortalities for 100 second and 100 fourth instars in each of the two water conditions (clean and muddy water); thus, a total 400 larvae were used. In the shallow water columns, second and fourth instars were individually placed in the same 500-ml graduated cylinders that were filled with 20-cm-deep clean or muddy water. Their survivorship was examined within 6 h. A total of 400 mosquito larvae were used in the shallow water column group.
Fisher's exact test was used to test the difference in larval mortality between the deep water and shallow water columns and between second and fourth instars under clean and muddy water conditions. The effects of water conditions and larval age on diving depth were analyzed using the two-way analysis of variance (ANOVA) and two-tailed t-tests.
Results
We observed both active and passive dives. An active dive involved twisting the body in a zig-zag manner, whereas a passive dive resembled a free-fall, in which the body was held straight and the larva sank to the bottom. Usually, the larvae actively swam downward (active diving) to break the adhesion with water surface and then changed to passive mode; however, passive and active modes were both observed at every depth. Some larvae attempted to ascend to the surface, but they were unable to sustain their activities and began to sink and eventually drowned. In the clean water columns (32-cm depth), among the 100 second instars tested, 10 (10%) reached to the bottom of the water column and two (2%) died (Table 1). The proportion of the second instars that reached the bottom and mortality rates in the clean water columns were similar to those in the muddy water columns (9% reached the bottom, 3% mortality rate). For the fourth instars, 16% reached to the bottom and 6% died in the clean water columns, similar to those in the muddy water columns (21% reached the bottom and 11% died in the muddy water columns, Table 1; Fisher's exact test, P = 0.31). In the shallow water group, higher proportion of mosquito larvae dived to the bottom of the 20-cm water column for both clean water and muddy water, but no mortality was observed (Table 1). Diving stress increased larval mortality in fourth instars in the 32-cm depth water columns, compared with the 20-cm depth water columns (Fisher's exact test: clean water, P = 0.03; muddy water, P < 0.001).
Mortality of the second and fourth instars of An. gambiae s.s. larvae in clean and muddy water column
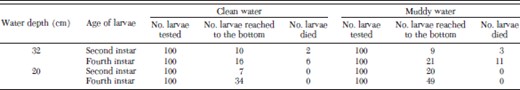
Mortality of the second and fourth instars of An. gambiae s.s. larvae in clean and muddy water column

Table 2 shows the average diving depths after the drowned individuals were excluded from the analyses. ANOVA revealed that water turbidity and larval age significantly affected the diving depth (larval age: F = 7.990; df = 1, 374; P < 0.01 and turbidity: F = 9.465; df = 1, 374; P < 0.01). The fourth instars dived significantly deeper (mean ± SD, 16.90 ± 7.80) in the muddy water columns than in the clean water columns (12.83 ± 8.25) (P = 0.001; Table 2). However, the diving depth of the second instars was similar between muddy and clean water columns (P = 0.404). The water turbidity affected the diving depth of second and fourth instars differently. In clean water column, the diving depth of second and forth instars was similar (P = 0.530), whereas in muddy water the forth instars dived deeper than the second instars (P < 0.001).
Diving depth of survivors of second and fourth instars of An. gambiae in clean and muddy water column
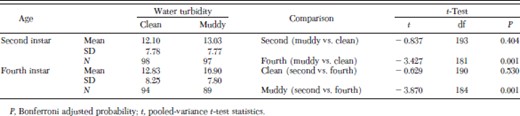
Diving depth of survivors of second and fourth instars of An. gambiae in clean and muddy water column

Discussion
This study has demonstrated that the fourth instars of An. gambiae dived significantly deeper in muddy water columns than in clean water columns. The mortality rates of fourth instars in 32-cm depth water columns were significantly higher than those in shallower depth (20-cm) water columns, regardless of water turbidity. The vertical movement of larvae and pupae is affected by buoyancy. The relative buoyancy density of anopheline larvae is >1.0, but <1 for pupae because pupae contain space for wing and proboscis development and considerable air is trapped inside the pupae (Romoser 1975, Clements 1999). Thus, mosquito larvae can sink passively to the bottom of the water column after detaching themselves from the water surface, whereas pupae consume considerable energy to dive (Lucas and Romoser 2001). In our study, we observed that An. gambiae larvae moved actively to detach from the water surface and then often passively sank. Unlike pupa, mosquito larvae do not have specific organs to stock air. As a consequence, a larva diving deeper will consume more energy to swim back to the water surface. Mosquito larvae would drowned if they did have the energy or ability to swim back to the air-water interface.
Gimnig et al. (2001) reported that water bodies inhabited by both of An. gambiae and An. arabiensis were shallower (mean depth 9.7 cm) than those that did not contain An. gambiae complex larvae (mean depth 22.9 cm). Water depth of larval habitats generally correlates with other biotic or abiotic factors such as habitat persistence, temperature, algae content, and predator presence (Minakawa et al. 1999). In this study, we demonstrated that water depth directly affected the mortality rate of An. gambiae when larvae were forced to submerge. It is not known whether female anophelines can detect habitat depth and thereby avoid laying eggs in deep habitats, because water depth may be correlated with many variables that directly or indirectly affect female oviposition behavior. Fourth instars dived deeper in muddy water than in clean water, whereas the second instars did not show such a difference. Perhaps An. gambiae larvae are adapted to reach to the muddy bottom where they could avoid predation or acquire food. Such adaptation might be more apparent for older larvae but not for younger ones. Therefore, larval diving behavior may not only offer the benefits of predator avoidance and food acquirement but also incur energy expenditure and increasing mortality.
Acknowledgements
We thank S. Juma and T. Otieno for technical assistance. We are grateful to Edward D. Walker and two anonymous reviewers for constructive criticism on the manuscript. This work is supported by National Institutes of Health grant R01 (AI) 50243 and KAKENHI15770012.
References Cited



