-
PDF
- Split View
-
Views
-
Cite
Cite
Brett H. Kirkland, Greg S. Westwood, Nemat O. Keyhani, Pathogenicity of Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae Tick Species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis, Journal of Medical Entomology, Volume 41, Issue 4, 1 July 2004, Pages 705–711, https://doi.org/10.1603/0022-2585-41.4.705
Close - Share Icon Share
Abstract
Nymphal and adult ticks from three different tick species, Dermacentor variabilis Say, Ixodes scapularis Say, and Rhipicephalus sanguineus Latrielle, were treated with conidia and blastospores of the entomopathogenic fungi Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae Metschnikoff. Dose-response experiments indicated that a critical concentration of fungal spores is required for infection and mortality. Over a 28-d time course, fungal suspensions of either B. bassiana or M. anisopliae at 108 conidia/ml resulted in 50–70% mortality in adult I. scapularis and R. sanguineus, but <20% mortality in D. variabilis ticks. R. sanguineus nymphs were highly susceptible to both entomopathogenic fungi, displaying >60% mortality within 14 d postinfection and >90% mortality within 21–28 d postinfection. D. variabilis nymphs also were more susceptible than their corresponding adults, displaying mortalities ranging from 20 to 40% 28 d postinfection. I. scapularis nymphs, however, seemed to be slightly less susceptible than adults (45% mortality, 28 d postinfection). The addition of nutrients to fungal cell suspensions did not have any noticeable effects on mortality toward any of the tick species tested. Significant mortality against D. variabilis adults (≈65%) was noted only when B. bassiana fungal cells with growth media carryover were used as the inoculum against the ticks. Entomopathogenic fungi such as B. bassiana and M. anisopliae may have the potential for controlling populations of I. scapularis and R. sanguineus, and under certain conditions D. variabilis. Our results indicate that inoculum conditions can greatly affect successful virulence and subsequent mortality.
Ticks are considered major vectors of animal diseases and second only to mosquitoes as vectors of human infectious diseases (Sonenshine 1993, Parola and Raoult 2001). Obligate hematophagous arthropods, ticks are distributed worldwide and parasitize almost every class of vertebrates. Human tick-borne diseases range from bacterial to viral, protozoal, and even fungal. Ixodes scapularis Say, the blacklegged tick, is an important vector for Lyme disease, babesiosis, and human granulocytic ehrlichiosis, resulting from infections by Borrelia burgdorferi Johnson, Schmid, Hyde, Steigerwald & Bremmer; Babesia microti Franca; and Erhlichia sp., respectively (Keirans et al. 1996, Walker 1998, Piesman et al. 1999). Dermacentor and Rhipicephalus tick species are known to carry Erhlichia, Francisella (tularensis), and Rickettsia, as well as other bacterial parasites (Higuchi et al. 1999, Singh-Behl et al. 2003). The latter two bacterial species are responsible for tularemia, and a range of spotted and tick bite fevers (Rocky Mountain, Mediterranean, and African tick bite), respectively. Because the incidence and variety of diseases associated with tick bites seem to be on the rise, many researchers are increasingly considering the phenomena as an important emerging infectious disease threat (Walker 1998, Singh-Behl et al. 2003).
Effective reduction and control of tick populations remains difficult, although several methods exist for tick control (Pegram et al. 2000, Eisler et al. 2003). Chemical acaricides such as organophosphates, carbamates, and pyrethroids display reasonable efficacy for the control of tick populations, but they are often toxic to humans as well as other nontarget organisms and can lead to environmental damage. As an alternative, much study has been directed toward biological control methods for ticks (George 2000, Kaaya 2000a, Samish 2000). These include the promotion of natural predators such as beetles, spiders, and ants; the use of tick parasites ranging from insects to nematodes, and even the mass release of sterilized males. A further method for tick biocontrol is the selection of bacterial and fungal pathogens of ticks. In this regard, the utility of the bacterium, Bacillus thuringiensis, and of the entomopathogenic fungus Metarhizium anisopliae Metschnikoff has been investigated (Zhioua et al. 1997, Kaaya 2000b, Gindin et al. 2002). Metarhizium isolates have been shown to be pathogenic toward engorged I. scapularis larvae and females. In addition, use of M. anisopliae for controlling I. scapularis under field conditions seems promising (Benjamin et al. 2002).
Beauveria bassiana (Bals.) Vuill. is an entomopathogenic fungus with demonstrated virulence toward a large range of insect hosts. B. bassiana has been found to be naturally associated with ticks and several laboratory studies have highlighted the potential for B. bassiana in tick biocontrol. Beauveria-mediated virulence has been demonstrated toward livestock ticks, including Rhipicephalus appendiculatus Neumann, Amblyomma variegatum F., and Boophilus decoloratus Koch (Kaaya and Munyinyi 1995, Kaaya et al. 1996). In this study, we investigate the virulence of B. bassiana and M. anisopliae toward adults and nymphs from several important human disease carrier tick species, including Dermacentor variabilis Say, I. scapularis, and Rhipicephalus sanguineus Latrielle.
Materials and Methods
Adult and nymphal ticks including D. variabilis, I. scapularis, and R. sanguineus were obtained from the Department of Entomology, Oklahoma State University Tick Rearing Facility. In preliminary experiments, several strains of B. bassiana and M. anisopliae were tested for virulence versus adult R. sanguineus. Strains B. bassiana (ATCC 90517) and M. anisopliae (ATCC 20500) displayed the highest relative virulence and were chosen for further study, with the former isolate having been demonstrated to express high levels of several secreted cuticle-degrading enzymes (Gupta et al. 1992). Fungal strains were grown on Sabouraud dextrose + 1% yeast extract or Potato dextrose (PD) media on either agar plates or in liquid broth. Plates were incubated at 26°C for 10–12 d and conidia were harvested by flooding the plate with sterile distilled water (dH2O) containing 0.01% Tween 20. Conidial suspensions were filtered through glass wool, and final spore concentrations were determined by direct count by using a hemocytometer. Liquid broth cultures were inoculated (1:20) with conidia harvested from plates to a final concentration of 5 × 106 conidia per milliliter. Cultures were grown for 3–4 d at 26°C with aeration. Cultures were filtered (two times) through glass wool to remove mycelia, and the concentration of blastospores (and conidia) was determined by direct count. Cells in the flow through were harvested by centrifugation (10,000 × g, 15 min, 4°C), washed twice with sterile dH2O + 0.01% Tween 20, and resuspended to a concentration of 108 spores per milliliter. Serial dilutions were made into sterile dH2O + 0.01% Tween 20 as indicated. The cell culture supernatant (spent media) was filtered through a 0.22-μm sterilization membrane and added back to harvested cells as indicated.
Fungal virulence toward ticks was determined using suspensions of varying spore concentrations of fungal cells harvested from plates or from liquid broth. Ticks were sprayed or submerged for ≈30 s in spore suspensions and blotted dry with either paper towels or a cotton swab. Sterile dH2O containing 0.01% Tween 20 was applied to ticks as a control. Each trial for both adults and nymphs included 20–50 ticks, and trials at each concentration replicated at least three times. Ticks were placed in conical tubes or microtiter plate chambers with Styrofoam plugs and placed in a humidity chamber (≈90% RH) with a 12-h day (27°C)/night (25°C) cycle.
Ticks were periodically examined microscopically for fungal growth and mortality was recorded every 2–3 d. Data were analyzed by PROC MIXED in SAS by using a linear mixed model. The least significant difference (LSD) test was conducted for comparisons between treatments and control inoculations, and, where indicated, for comparisons between treatments (Kuel 2000).
Infected adults were examined by scanning electron microscopy (SEM) throughout the time course of the experiment. Adult ticks were fixed in Trump's media (2% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.2) at 4°C, washed with dH2O, and then dehydrated in a graded series of ethanol to absolute ethanol, before treatment with hexamethyldisilazane for 30 min. Mounted samples were subsequently sputter-coated with 30-nm gold/palladium and observed using a Hitachi S-570.
Results
Infection assays using fungal cell suspensions of M. anisopliae and B. bassiana washed into sterile dH2O containing 0.01% Tween 20 resulted in significant mortality toward R. sanguineus (≈70%, 28 d postinfection) and I. scapularis (65% mortality) in a dose-dependent manner, but only limited virulence against D. variabilis (≈10%) (Fig. 1). Because B. bassiana species are known to produce at least two different potential pathogenic cell types depending upon the growth conditions of the organism, both blastospores (produced predominantly in rich-broth liquid media, 70–80% containing 20–30% conidia) and conidia (isolated from Sabouraud agar plates, >95%) were prepared and used as inocula on the ticks. Only small differences in virulence were observed between the B. bassiana blastospores and conidia or between B. bassiana and M. anisopliae. Experiments were conducted to determine the dose dependence of fungal virulence against the tested tick species. These data indicated that a critical threshold of fungal cells (108/ml) was required for mortality (>50%) in adult R. sanguineus and I. scapularis (Fig. 1). Mortality within a control group, inoculated with sterile dH2O containing 0.01% Tween 20 was <5 ± 2%.
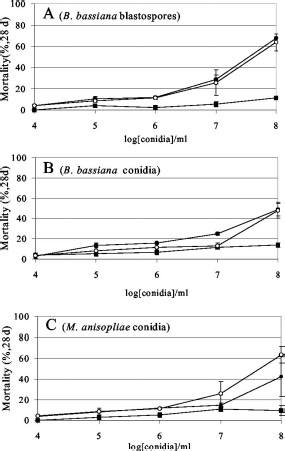
Percentage of mortality 28 dpost infection of adult R. sanguineus (●), I scapularis (○), and D. variabilis (■) inoculated with B. bassiana blastospores (A), B. bassiana conidia (B), and M. anisopliae conidia (C) as a function of spore concentration. Values given are means of three experiments ± SE.
A time course of the mortality measured every 7 d using 108 fungal cells per milliliter as inoculum indicated that for the susceptible species (R. sanguineus and I. scapularis), significant mortality required at least 14 d of infection, with ≈50% of the overall mortality occurring 14–21 d postinfection (Fig. 2). Fungal mycelial outgrowth was visible 21 d postinfection on (dead) ticks (Fig. 4).
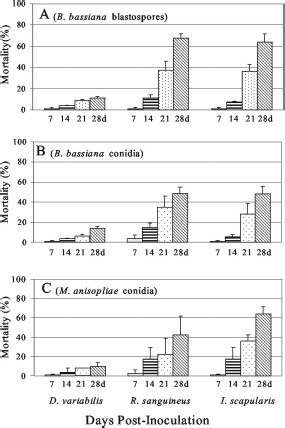
Weekly mortality rates for adult R. sanguineus, I. scapularis, and D. variabilis, inoculated with B. bassiana blastospores (A), B. bassiana conidia (B), and M. anisopliae conidia (C) by using 108 fungal cells per milliliter. Values given are means of three experiments ± SE.
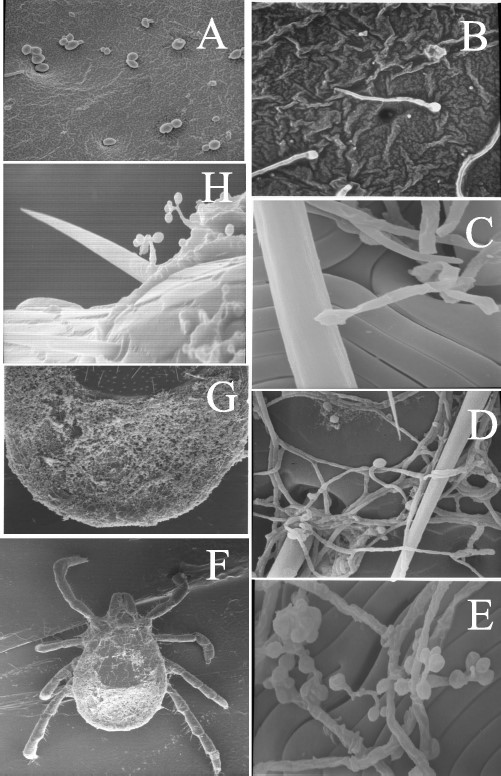
Electron micrographs of the B. bassiana conidia-mediated infection process. Conidia bound to tick cuticle, 1 h postinfection (A, R. sanguineus, 1,400×). Conidial germination, 18–24 h postinfection (B, I. scapularis, 1,300×;C, D. variaibilis, 3,600×). Proliferation and mycelial formation across the tick cuticle surface 7–14 dpostinfection (D, I. scapularis, 2,000×; E, D. variabilis, 3,400×). Tick cadaver 21–28 d postinfection, illustrating extensive growth particularly in the posterior region of the tick (F, R. sanguineus, 15×; G, R. sanguineus, 30×). Conidiogenesis, outgrowth of conidia from tick cadaver 28 d postinfection (H., D. variabilis, 1,800×).
R. sanguineus nymphs were much more susceptible to fungal infection and subsequent mortality than their respective adults (using B. bassiana conidia, χ2 = 37.03, df = 1, P < 0.001; using blastospores, χ2 = 17.62, df = 1, P < 0.001). Both B. bassiana (108 cells per milliliter) and M. anisopliae resulted in >60% mortality within 14 d, and almost 100% mortality within 21 d postinfection (Fig. 3). Although there was large variation within samples, D. variabilis nymphs also seemed to be more susceptible to fungal infection by B. bassiana conidia (but not blastospores) than their respective adults (using B. bassiana conidia, χ2 = 9.52, df = 1, P = 0.002; using blastospores; χ2 = 2.53, df = 1, P = 0.1114); however, mortality remained low (15–45%, 28 d postinfection). In contrast, I. scapularis nymphs did not seem to be any more susceptible to the fungi than conspecific adults (Figs. 2 and 3).
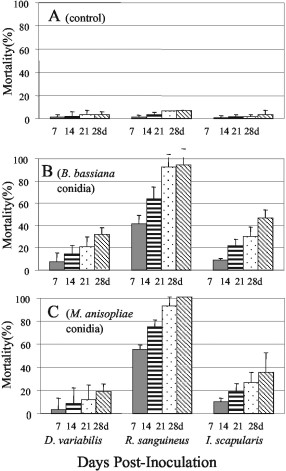
Weekly mortality rates for R. sanguineus, I. scapularis, and D. variabilis nymphs inoculated with buffer controls (A), B. bassiana conidia (B), and M. anisopliae conidia (C) by using 108 fungal cells per milliliter. Values given are means of three experiments ± SE.
Significant mortality (≈65%) was observed in adult D. variabilis infection assays only when B. bassiana cells were applied to the ticks directly from the broth culture, i.e., with culture media carryover. Virulence decreased by washing the blastospores, but could be restored by suspension in spent media (Table 1). The data indicated significant differences (P < 0.001) for comparisons between the treatment (unwashed blastospores or washed blastospores supplemented with spent broth) and control inoculations with dH2O or Sabouraud broth, inoculations using 107 conidia per milliliter or 108 conidia per milliliter (washed into dH2O), and washed blastospores. This effect was not due to availability of nutrients because fungal cells supplemented with Sabouraud media (1:10) did not result in any significant increase in mortality (Table 1). These data indicate that secreted factors found in spent culture broth seem to enable virulence toward D. variabilis.
Effect of inoculum composition on B. bassiana-induced mortality against R. sanguineus, D. variabilis, and I. scapularis
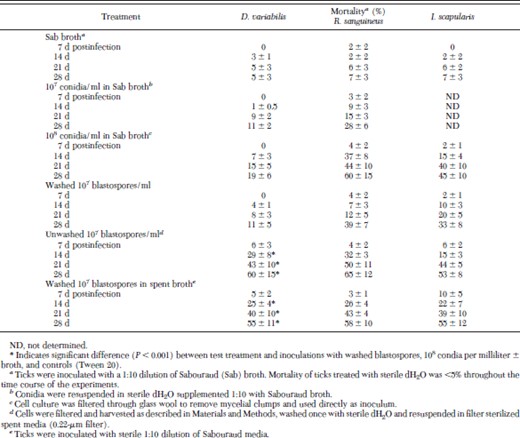
Effect of inoculum composition on B. bassiana-induced mortality against R. sanguineus, D. variabilis, and I. scapularis

Ticks were examined throughout the 28-d time course of the infections by scanning electron microscopy (Fig. 4). Comparable concentrations of conidia were visible 1–12 h postinfection on all three ticks species tested, although the distribution of conidia was not uniform across the body of the ticks. Conidial germination and proliferation were much more evident on both I. scapularis and R. sanguineus ticks during the first week postinoculation, than on D. variabilis ticks, although a wide variation was observed. Germination of most of the bound conidia was visible within 24–48 h postinfection. Hyphal growth was evident 2–14 d postinfection, although clear instances of appressoria or penetration events were difficult to distinguish. Extensive fungal growth was visible on several distinct regions of the tick anatomy including the anal and genital grooves and apertures, and on the alloscutum. Patches of fungal growth also could be seen on the capitulum, especially around the mouthparts, and scattered around the idiosoma, particularly within the various lateral and marginal grooves along the surface contours of the organism. In several instances, bacteria or other fungi, clearly distinguishable from the inoculated organism (B. bassiana) could be seen on the ticks. B. bassiana cells were observed on living D. variabilis ticks throughout the time course of the experiments performed, including at the 28-d time point. Conidial binding, germination, and even mycelial growth was apparent on the surface of D. variabilis ticks, indicating that the observed low mortality rate may be due to inhibition of critical events required for penetration of the cuticle.
Discussion
Because entomopathogenic fungi seem not to harm vertebrates or most beneficial insects, much attention has been directed toward development of strains of these organisms for biocontrol of harmful nuisance pest species (McCoy 1990, Leathers et al. 1993). The relative technical ease of spore mass production, coupled to advances in storage and application formulations, has led to numerous commercial uses of B. bassiana and M. anisopliae strains for control of insect pests in agricultural, ecological, and domestic settings in a number of countries (Ferron 1981, Roberts and Humber 1981, McCoy 1990, Leathers et al. 1993).
The use of these organisms in the biocontrol of arthropod pests, such as ticks, has only recently gained impetus. The pathogenicity of entompathogenic fungi to different developmental stages of R. sanguineus has been investigated (Samish et al. 2001). In these studies, M. anisopliae was the most virulent isolate, with B. bassiana, M. flavoviride, and Paecilomyces fumosoroseus strains resulting in significantly lower mortality under the conditions tested. The virulence of M. anisopliae also has been tested against unfed and engorged adult R. appendiculatus. Fungal concentrations as low as 106 spores per milliliter induced 35% (unfed) and 80% (engorged) mortality in these ticks, respectively (Kaaya and Hassan 2000). Similarly, almost 100% mortality has been shown using M. anisopliae at high conidia concentrations (4 × 109 spores per milliliter) against unfed adult I. scapularis, whereas only 107 spores per milliliter induced comparable mortality in engorged adult females (Zhioua et al. 1997, Benjamin et al. 2002). It is likely that increased exposure, and hence susceptibility, occurs during engorgement due to the extension of the cuticle. These data indicate that lower infectious fungal concentrations may be effective in controlling infestations on feeding animals. Some caution, however, should be taken in extrapolating from laboratory results to effective on-host biocontrol. Although, M. anisopliae and B. bassiana induced high mortality in ticks confined in bags on Zebu cattle ears (Kaaya and Hassan 2000), a preliminary report using M. anisopliae versus Boophilus microplus (Canestrini) ticks (unconfined) on stabled bulls, did not result in a reduction in the total number of ticks that continued to parasitize the animals (Correia et al. 1998).
The effects of M. anisopliae also have been determined on questing I. scapularis adults (Benjamin et al. 2002). In the field, a mortality of slightly >50% was noted among ticks collected from vegetation plots sprayed with an aqueous formulation of M. anisopliae. Here, too, the authors note that their results may contain an upward bias in the mortality rate due to two factors. First, collected ticks were placed in vials under optimum conditions for fungal growth and some may not have died under field conditions; and second, ticks were collectively housed in a single vial/plot, possibly resulting in horizontal transfer of infection from a subpopulation of infected ticks to uninfected ticks.
Other factors that may influence the practical application of pathogenic fungi in tick biocontrol include formulations (aqueous versus oil), fungal growth conditions, and number of applications. For M. anisopliae aqueous formulations resulted in almost 65% mortality in potted grass tetrapacks, whereas oil formulations under identical conditions resulted in >80% mortality (Kaaya 2000b). Furthermore, the results of this study should be interpreted with the understanding that other B. bassiana and M. anisopliae isolates may be more or less pathogenic toward ticks and that passage through tick species may select for more virulent or species-specific isolates.
Our results demonstrated dose-dependent mortality toward unfed adult and nymphal R. sanguineus and I. scapularis, with limited mortality versus D. variabilis ticks by using the entomopathogenic fungi B. bassiana and M. anisopliae, harvested from either agar plates or liquid media and washed into sterile dH2O containing 0.01% Tween 20. The detergent was used to increase the recovery of fungal cells from agar plates, decrease aggregation of the isolated conidia, and ensure a more even distribution of the cells during application. Surface mycosis was observed on treated ticks and both B. bassiana and M. anisopliae could be recovered from infected specimens, although other fungal species were occasionally observed during fungal isolation from tick cadavers. A critical spore concentration was required for effective mortality, presumably to overcome tick defenses during penetration of the tick's cuticle and proliferation inside the host. Significant mortality against D. variabilis was observed only when B. bassiana blastospores with growth media carryover (i.e., supplemented with spent media) was used as the inoculum. Importantly, addition of fresh media to harvested cells did not induce any increase in mortality under the conditions tested. The most likely explanation for our observations is that B. bassiana secretes important virulence factors that can enhance or enable pathogenesis. Growth in various media, including Sabouraud/yeast extract, is known to result in secretion of hydrolytic and proteolytic enzymes, as well as various mycotoxins such as beauvericin and oosporein, and to result in the acidification of the media (Kucera and Samsinakova 1968; Gupta et al. 1992; Mazet et al. 1994; St Leger et al. 1997, 1998). Further study of inoculum conditions to identify important virulence enhancing components may, therefore, lead to improvements in application formulation of these agents.
Acknowledgements
We thank H Aldrich and D. Williams for help with the microscopy, Y. Joo (University of Florida, IFAS, Department of Statistics) for assistance with statistical analysis, and L. O. Ingram for critical comments during manuscript preparation. This paper is Florida Agricultural Experimental Station Journal series number R-09912.
References Cited



