-
PDF
- Split View
-
Views
-
Cite
Cite
Arun K. Tripathi, Veena Prajapati, Ateeque Ahmad, Kishan K. Aggarwal, Suman P. S. Khanuja, Piperitenone Oxide as Toxic, Repellent, and Reproduction Retardant Toward Malarial Vector Anopheles stephensi (Diptera: Anophelinae) , Journal of Medical Entomology, Volume 41, Issue 4, 1 July 2004, Pages 691–698, https://doi.org/10.1603/0022-2585-41.4.691
Close - Share Icon Share
Abstract
Anopheles stephensi (Liston) is a well-known vector of malarial parasite in tropical countries. The developing trend of resistance in mosquitoes toward synthetic mosquitocidal agents makes their management extremely difficult. Effectiveness of essential oils with aroma therapeutic values seems to be an emerging tool to combat this vector. Piperitenone oxide isolated from essential oil of a new genotype, Mentha spicata L. variety viridis, has been evaluated for larvicidal, ovicidal, oviposition-deterrent, developmental toxicity, and repellent properties against various stages of A. stephensi. The results indicated the higher efficacy of piperitenone oxide than the crude essential oil of M. spicata variety viridis in all the bioassay experiments. The lethal response of piperitenone oxide and the oil toward fourth instar larvae showed LD50 values of 61.64 and 82.95 μg/ml, respectively. Female adults of A. stephensi exposed to the oil laid ≈42 times less number of eggs at the dose of 60.0 μg/ml as compared with control, whereas exposure of piperitenone oxide at the same dose completely inhibited the oviposition. Furthermore, piperitenone oxide also completely inhibited egg hatching at the dose of 75.0 μg/ml in ovicidal assay. Developmental toxicity studies showed the significant developmental inhibition potential of the compound and oil. Additionally, piperitenone oxide was found to be highly toxic and repellent toward adults of A. stephensi as compared with oil.
Phytochemicals derived from various botanical sources have been proved to be useful in pharmaceuticals and pesticide industry (Benner 1993). A number of plant secondary metabolites have been reported to have detrimental (Syamala Devi and Vasudevan 1995) and repellent effects toward mosquito adults (Curtis et al. 1990). The essential oils of mints, belonging to family Labiatae, are widely used in flavoring, pharmaceuticals, medicines, and pesticides (Ansari et al. 2000). The commercially cultivated mints include Mentha arvensis, Mentha piperita, Mentha spicata, Mentha cardiaca, and Mentha citrata (Husain et al. 1988). The promising essential oils with biocidal activity include plants from genus Tagetes (Perich et al. 1995), Ocimum species (Bhatnagar et al. 1993), Cymbopogon species (Ansari and Razdan 1995), Pelargonium citrosum (Matsude et al. 1996), and M. piperita (Ansari et al. 2000). Essential oil of spearmint (M. spicata) has been reported as insecticidal against stored product insects (Tripathi et al. 2000), apart from its extensive use in various preparations ranging from medicine to food products. We come across with promising mosquitocidal potential of the essential oil of a new genotype of M. spicata variety viridis. The essential oil of this genotype was found to be highly rich in piperitenone oxide (71.15%) (Khanuja et al. 2001) and also found effective as fumigant against stored grain insects apart from antimicrobial properties (Khanuja et al. 2001). Piperitenone oxide has been reported as major constituent of the essential oils of Calamintha incana (Tumen et al. 1995), Mentha longifolia (Shah 1999), Lippia alnifolia Scau. (Matos et al. 2000), and M. spicata (Pino et al. 1998). However, this compound has not been reported for any bioactivities against mosquitoes.
For the first time, we report ovicidal, larvicidal, reproduction retardant, development inhibitor, repellent, and adulticidal effects of piperitenone oxide and essential oil of M. spicata variety viridis against malarial vector, Anopheles stephensi.
Materials and Methods
Essential Oil Extraction.
Fresh leaves (1 kg) of M. spicta variety viridis grown at the farm of this institute were collected and steam distilled for 4-5 h in clevenger-type apparatus to extract the oil. The yield of the oil was 0.1–0.2% of fresh leaves. The gas chromatography of the essential oil was done on a Varian Gas Chromatogram, model CX-3400, under the conditions: carrier gas hydrogen, injector (detector flame ionization detector, FID) temperatures, 220°C and 225°C, respectively, capillary column (Supelcowax–10, 30 m × 0.32 mm, film thickness 0.25 μm); and temperature programmed from 2 min at 40–270°C at 5°C/min. The area percentage was obtained on Varian 4400 integrator. The identity of the component was assigned by comparing their retention time with those of authentic samples.
Fractionation, Isolation, and Purification of Piperitenone Oxide.
The essential oil (20 g) of M. spicata variety viridis was chromatographed over 200 g silica gel, and a total of 14 fractions (500 ml each) was collected. Fractions 1 and 2 eluted in hexane, fractions 3 and 4 in hexane:ethylacetate (9.8:0.2), fractions 5 and 6 in hexane:ethylacetate (9.5:0.5), fractions 7 and 8 in hexane:ethylacetate (9:1), fractions 9 and 10 in hexane:ethylacetate (8:2), fractions 11 and 12 in hexane:ethylacetate (1:1), and fractions 13 and 14 in ethylacetate. Fractions 5 and 6, after column chromatographed with hexane:ethylacetate, gave piperitonene oxide of 96% purity, as monitored on Gas Chromatogram.
Insect Culture.
Egg, larval, and adult stages were obtained from laboratory strain of A. stephensi maintained at ambient rearing conditions in the Bioprospection Group, GRB Division of this institute. All the bioassays were conducted at 25 ± 3°C, 60.0 ± 5% RH, and 12:12 (L:D).
Larvicidal.
The larvae of A. stephensi mosquitoes were bred in chlorine-free tap water and fed a 5% yeast suspension. Essential oil of M. spicta variety viridis and piperitenone oxide was evaluated at the level of 20.0, 40.0, 60.0, 80.0, 100.0, and 120.0 μg/ml in tap water. Tween-80 was used as emulsifier at a concentration of 0.001%. Tap water mixed with Tween-80 was used as control. Standard WHO test (WHO 1981) was employed with slight modification in the test procedure. A single fourth-stage larva was put into each of 20 vials containing 5.0 ml of the test solution of each concentration. Observation on larval mortality was recorded after 24 h. Larvae were considered dead, when they did not react to touching with a needle.
Ovicidal.
The eggs of A. stephensi mosquitoes were obtained from laboratory-bred culture. Twenty eggs were assigned to six treatments (5.0, 10.0, 25.0, 50.0, 75.0, and 100.0 μg/ml in tap water) separately of M. spicta variety viridis oil and piperitenone oxide and were put into each of 10 vials containing 5.0 ml of the test solution of each treatment. Treatments were replicated 10 times and included tap water mixed with 0.001% of Tween-80 as control. Percentage of egg viability was calculated by dividing the number of larvae that emerged from the eggs 7 d after treatment by the total number of eggs laid.
Oviposition Deterrence.
The effect of M. spicta variety viridis oil and piperitenone oxide on oviposition and egg hatching by A. stephensi was studied by introducing 20 gravid females (fed on rabbit blood) and unlimited number of males from a laboratory colony in 25 × 25 × 25-cm oviposition cages under choice conditions. The cages contained six 100-ml glass dishes with 10.0, 20.0, 30.0, 40.0, and 60.0 μg/ml concentrations of the compound and oil. Each cage had a control glass dish having only tap water with 0.001% of Tween-80. The test was replicated four times. The number of eggs was counted for 7 d. The laid eggs were observed for hatching and subsequent survival unto second larval instar only. Oviposition inhibition percentage was calculated according to Mulla et al. 1974.
Developmental Toxicity.
The effect of M. spicta variety viridis oil and piperitenone oxide on developmental toxicity was observed at of 5.0, 10.0, 20.0, 40.0, 60.0, and 80.0 μg/ml concentrations prepared in water containing 0.001% Tween-80. Ten second instar larvae were transferred from the culture into 100-ml glass dishes containing test solutions (50.0 ml). Each treatment was replicated 10 times. Water containing 0.001% Tween-80 only was used as control. Larvae were fed ad libitum and kept on rearing conditions. Larval mortalities were counted at 2, 4, and 10 d after treatment. Larvae were considered either alive (clearly moving normally) or dead (no movement and no response to gentle probing). Development of the remaining live larvae was monitored daily till adult emergence. Percentage of successful pupation and adult emergence was calculated from the data recorded.
Vapor Toxicity.
The essential oil of M. spicata variety viridis and piperitenone oxide was dissolved in acetone to make desired concentrations. Aliquot (0.50 ml) of the test solution was dispensed over a cardboard sheet of size (22 × 35 mm) and thickness (2.5 mm) equal to commercially available mosquito mats, so that amount of M. spicata variety viridis and piperitenone oxide received per mat was 50.0, 100.0, 150.0, 200.0, and 250.0 mg and 5.0, 10.0, 20.0, 30.0, 40.0, and 50.0 mg, respectively. Solvent was allowed to evaporate at room temperature. The treated cardboard sheet was placed on a mosquito mat machine, and machine was kept on for 15 min. The cardboard used in control was dispensed with acetone only. Vapor toxicity was evaluated in a special designed apparatus as per Vartak and Sharma 1993 with slight modifications. For the design of experimental apparatus (Fig. 1), perspex glass sheet was used. A chamber A with dimension of 60 × 60 × 60 cm was made. This chamber was provided with sliding door S1 (30 × 30 cm) on one side and a tunnel hole (9 × 9 cm) on the other side. A small-sized chamber B (20 × 20 × 20 cm) was designed separately, having copper wire mesh sheet on top (M), sliding door S2 (19 × 16 cm) on one side, and another sliding door S3 on the other side, where a tunnel T of size (9 × 9 cm and 5 cm long) was fixed. In the chamber B, sliding door S3 was closed, and fifty 6- to 8-d-old females of A. stephensi were released in chamber B through sliding door S2. After release of mosquitoes, S2 was closed. The chamber B was placed in big chamber A, in which treated cardboard mat along with mat machine was kept at the corner. Observations on adult mortality at varying dosages were recorded at 1 h after the treatment. The experiment was repeated thrice. Before start of every experiment, all the chambers were thoroughly washed with soap water and detergent, and dried properly. Data recorded on adult mortality were analyzed statistically for LC50 and LC95 values.
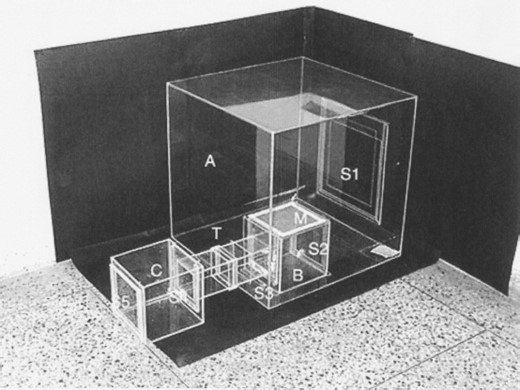
Designer apparatus for evaluation of vapor toxicity and repellency against mosquito adults. (A) Test chamber; (B) small chamber with top of copper wire mesh (M); (C) chamber with tunnel for repellency studies; (S1, S2, S3, S4, and S5) sliding doors; and (T) tunnel.
Repellency.
The essential oil of M. spicata variety viridis and piperitenone oxide was dissolved in acetone to make desired concentrations. Repellency was evaluated using the apparatus (Fig. 1), as mentioned in vapor toxicity assay, but with further attachments. Another chamber C was constructed (20 × 20 × 20 cm), having sliding door S4 on one side and a tunnel T (9 × 9 cm and 15 cm long) with a collar. The chamber C was connected with chamber B through tunnel. For repellency studies, the criterion was migration of mosquitoes from chamber B to chamber C through the connecting tunnel after 1 h for assessment of true repellency. A rabbit (anesthetized) was placed on the copper wire mesh surface of chamber B. Thirty adult female mosquitoes were released in chamber B, and sliding door S2 was closed, whereas sliding door S3 was opened for any migration of mosquitoes in chamber C.
Statistical Analysis.
Probit analysis (Finney 1971) was used to analyze lethal doses (LC50/LD50 and LC95/LD95) of the oil and its pure constituent. Linear regression was used to describe the relationship between dosages and mortality (SPSS 1999).
Results
Oil Extraction and Identification of Piperitenone Oxide.
Gas chromatography (GC) analysis of the M. spicata variety viridis oil revealed piperitenone oxide (71.15%) as major constituent along with limonene (1.36%), carvone (5.78%), and β-caryophyllene (2.35%) as minor constituents. Piperitonene oxide (Fig. 2) is a viscous liquid with the spectral data: mass spectrometry, MS m/z (rel. int.): 166 [M]+ (calculation for C10H14O2), 148 (20), 138 (80), 137 (30), 91 (30), 79(40), 67 (100), 41 (60); 1H-NMR (CDCl3) 300 MHz: 1.52 (S, 3H, H-10), 1.86 (S, 3H, H-9), 2.13 (S, 3H, H-8,), 2.42 (m, 4H, H-4, 5), 3.25 (S, 1H, H-2); 13C-NMR (CDCl3) 75 MHz: 197.1 (C-1), 62.1 (C-2), 62.3 (C-3), 28.0 (C-4, 5), 127.5 (C-6), 147.8 (C-7), 22.8 (C-8)*, 22.6 (C-9)*, 21.3 (C-10) (peak values of C-8 and C-9 are not confirmed therefore assignments may be interchanged).
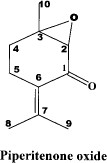
Larvicidal.
Piperitenone oxide and M. spicata variety viridis oil showed LD50 values of 61.64 and 82.95 μg/ml, repectively (Fig. 3). Thus, piperitenone oxide was 1.34-fold more toxic than the oil itself toward fourth instar larvae of A. stephensi. At the dose of 100 μg/ml, the piperitenone oxide gave 100.0% mortality, whereas M. spicata variety viridis oil resulted into 66.15% larval mortality only at the same dose. Furthermore, regression analysis of the data also showed significant (F = 96.93, df = 6, P < 0.01; F = 185.45, df = 6, P < 0.01) toxicity toward fourth instar larvae exposed to piperitenone oxide and M. spicata variety viridis oil, respectively (Fig. 3).
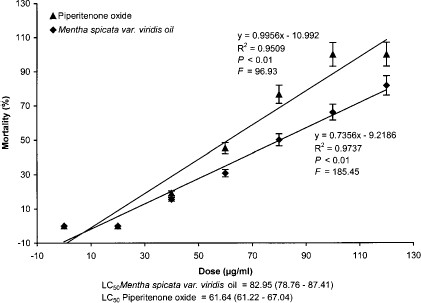
Dose-response relationship of the larval mortality of A. stephensi exposed to piperitenone oxide and M. spicata variety viridis oil.
Ovicidal.
Linear regression analysis performed on the data showed that piperitenone oxide and M. spicata variety viridis oil significantly (F = 36.23, df = 6, P < 0.01; F = 521.34, df = 6, P < 0.01, respectively) reduced the viability of eggs exposed (Fig. 4). Piperitenone oxide completely inhibited egg hatching at dose of 75.0 μg/ml, whereas M. spicata variety viridis oil could inhibit 78.82% egg hatching only at the same dose. The lethal inhibition dose of egg hatching (IH50) was 25.77 and 45.83 μg/ml for piperitenone oxide and M. spicata variety viridis oil, respectively.
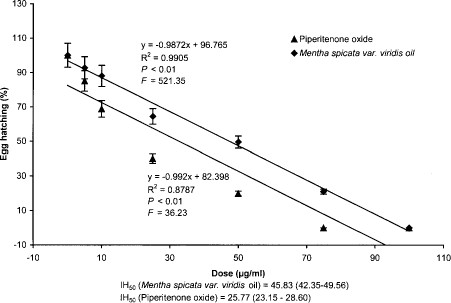
Relationship of percentage of egg hatching of A. stephensi eggs to doses of piperitenone oxide and M. spicata variety viridis oil.
Oviposition Deterrence.
Piperitenone oxide and M. spicata variety viridis oil both significantly (F = 48.60, df = 6, P < 0.01; F = 246.20, df = 6, P < 0.01, respectively) reduced egg laying (oviposition) by A. stephensi as concentration increased (Table 1). At the dose of 60 μg/ml, piperitenone oxide-exposed A. stephensi female adults laid no eggs, whereas M. spicata variety viridis oil-exposed adults laid 41.55 times less number of eggs as compared with control. Thus, both the compound and oil reduced oviposition by A. stephensi adults significantly (F = 58.51, df = 6, P < 0.01; F = 240.60, df = 6, P < 0.01, respectively), as evidenced from percentage of oviposition-deterrence values (Table 1). Similarly, both the compound and oil significantly (F = 10.70, df = 6, P < 0.01; F = 68.30, df = 6, P < 0.01, respectively) reduced the viability of eggs laid. Furthermore, both the compound and oil significantly (F = 3.46, df = 6, P < 0.01; F = 8.99, df = 6, P < 0.01, respectively) suppressed survival of larvae emerged from laid eggs (Table 1). Piperitenone oxide and M. spicata variety viridis oil both caused 48.04 and 13.0% egg hatching at dose of 20.0 μg/ml, but the emerged larvae could not survive up to second instar at the same dose.
Effect of Mentha spicata variety viridis oil and piperitenone oxide on fecundity and fertility of Anopheles stephensi
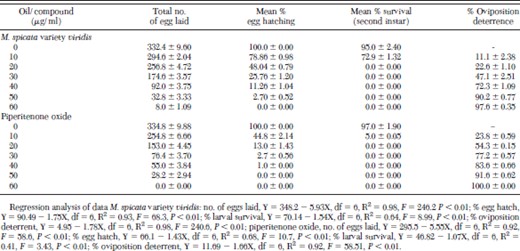
Effect of Mentha spicata variety viridis oil and piperitenone oxide on fecundity and fertility of Anopheles stephensi

Developmental Toxicity.
Exposure of piperitenone oxide and M. spicata variety viridis oil to second instar larvae caused deleterious effects on further survival and development into pupa and adult (Table 2). Dosage-mortality data showed dose-dependent relationship with larval mortality as the days progressed. At the dose of 40.0 μg/ml, piperitenone oxide completely inhibited the larval survival at day 10, whereas M. spicata variety viridis oil caused 82.0% mortality at the same dose (Table 2). Interestingly, at 10.0 μg/ml, piperitenone oxide-exposed second instar larvae completely failed to transform into adults. Similar results were obtained with M. spicata variety viridis oil at dose of 20.0 μg/ml. The lethal developmental inhibition values of both oil and compound are given in Table 3. As the days progressed, the toxicity of both the test chemicals increased in terms of larval mortality. Lethal toxicity (LD50) toward 10-d larvae was 3.7 times more than 2-d-old larvae exposed to piperitenone oxide. However, in case of M. spicata variety viridis oil, it was only 2.4 times (Table 3). Effect of piperitenone oxide and M. spicata variety viridis oil on the survival of pupae and adult emergence was not dose dependent (Table 2).
Developmental toxicity of Mentha spicata variety viridis oil and piperitenone oxide exposed to second larvae of Anopheles stephensi
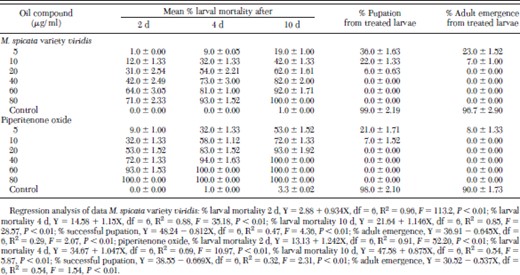
Developmental toxicity of Mentha spicata variety viridis oil and piperitenone oxide exposed to second larvae of Anopheles stephensi

Lethal response of Mentha spicata variety viridis oil and piperitenone oxide fed to larvae of Anopheles stephensi at days 2, 4, and 10
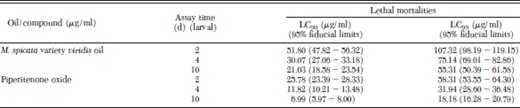
Lethal response of Mentha spicata variety viridis oil and piperitenone oxide fed to larvae of Anopheles stephensi at days 2, 4, and 10

Vapor Toxicity.
Piperitenone oxide was highly toxic toward the adults of A. stephensi in vapor toxicity assay, as it provided complete adult mortality at 5 times less dose rate as compared with M. spicata variety viridis oil (Fig. 5). At the dose of 50.0 mg/ml, the oil gave no mortality, whereas piperitenone oxide caused complete mortality. LC50 values of piperitenone oxide and M. spicata variety viridis oil were found to be 19.9 and 156.2 mg/ml, respectively. Thus, the lethal toxicity of piperitenone oxide was 7.8 times more than that of M. spicata variety viridis oil (Fig. 5).
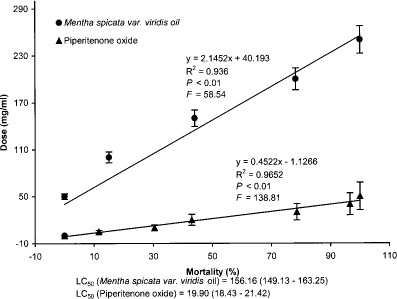
Dose-response relationship between percentage of adult mortality of A. stephensi exposed to vapors of piperitenone oxide and M. spicata variety viridis oil.
Repellency.
Exposure of piperitenone oxide to A. stephensi adults showed complete repellency at 5 times less dose to that of M. spicata variety viridis oil after 1-h duration (Fig. 6). At the dose of 10.0 mg/ml, piperitenone oxide provided complete repellency, whereas M. spicata variety viridis oil could achieve a repellency of 18.6% only (Fig. 6).
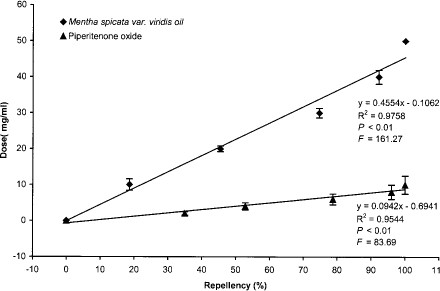
Dose response of piperitenone oxide and M. spicata variety viridis oil vapors on percentage of repellency to adults of A. stephensi.
Discussion
Protection against mosquitoes is generally obtained by the use of synthetic pyrethroids formulated in the form of mosquito coil, mat, incense stick, and electronic assemblies or by using repellent such as dimethyl phthalate, diethyl toluamide, and dimethyl carbate. With the culminating problems associated with the development of resistance (Curtis and Pasteur 1981) in mosquitoes and health problems associated with the views of conventional pesticides, natural products (Arnason et al. 1989), especially essential oil, may provide useful future alternative means for mosquito control. A considerable number of plant derivatives have shown potential toxicity toward mosquitoes (Elhag et al. 1996, 1999; Hassanali et al. 1990). Some of these plant materials demonstrated broad spectrum activity involving short-term, long-term, and IGRy effects. The results of the current study revealed that piperitenone oxide induces similar range of activity against various life stages of A. stephensi. The larval and cumulative mortalities relationship was dose dependent, and significant morphogenetic aberration was recorded in the larvae and/or in pupae before adult emergence.
Larvicidal, ovicidal, oviposition-deterrent, and developmental toxicities assays revealed that both the oil and its purified constituent piperitenone were at par. But in vapor toxicity and repellent assays, piperitenone oxide showed many fold better activity than the crude oil of M. spicata variety viridis.
Piperitenone oxide was obtained in fractions 5 and 6 when the oil was fractionated on the column chromatogram. We evaluated all the fractions (1–14) individually as well as in various combinations for adulticidal and repellent properties. Surprisingly, it was found that none of the fractions or their combinations were found active, except fractions 5 and 6. This eliminates any possible synergistic action of other minor constituent with piperitenone oxide.
Piperitenone oxide showed vapor toxicity toward A. stephensi adults at very low doses, with LC50 values of 19.9 mg/ml. Similarly, this compound provided complete repellency at 1-h duration at the dose of 10 mg/ml. This makes the possible exploitation of the compound as vapor toxicant under household conditions.
The vapor phase-mediated repellency of an essential oil or its pure constituents fades with time because of diffusion and continuous dilution of the vapors in the local conventional air currents, resulting in its gradual reduction and elimination from the vicinity. Therefore, formulations need application of suitable techniques in view of faster diffusion of volatile oils from liquid base than from semisolid preparations (Oyedele et al. 2000).
Because the essential oil of M. spicata variety viridis is used as medicament, cosmetics, and many pharmaceutical preparations (Khanuja et al. 2001), its use as mosquitocidal agent will be quite safe. Further studies are required for its vapor toxicity against mammalian system to exploit commercially.
Acknowledgements
We are thankful to Council of Scientific and Industrial Research, New Delhi, and Department of Biotechnology, Government of India, New Delhi, for providing partial financial assistance for the present investigation.
References Cited



