-
PDF
- Split View
-
Views
-
Cite
Cite
Q. Gao, N. W. Beebe, R. D. Cooper, Molecular Identification of the Malaria Vectors Anopheles anthropophagus and Anopheles sinensis (Diptera: Culicidae) in Central China Using Polymerase Chain Reaction and Appraisal of Their Position Within the Hyrcanus Group , Journal of Medical Entomology, Volume 41, Issue 1, 1 January 2004, Pages 5–11, https://doi.org/10.1603/0022-2585-41.1.5
Close - Share Icon Share
Abstract
In central China, Anopheles anthropophagus is considered the primary malaria vector and Anopheles sinensis is a secondary vector. Identification of these two cryptic species would facilitate studies on malaria transmission and the application of control measures. At present, the only reliable morphological markers occur in the egg stage, making this approach impractical for any large scale field studies. In this study, we report on the development of a polymerase chain reaction (PCR)-restriction fragment length polymorphism procedure involving the ribosomal DNA ITS2 region for discrimination of these species. The PCR-amplified product size of the ITS2 was 574 bp for An. anthropophagus and 594 bp for An. sinensis. Diagnostic restriction fragment length polymorphisms appeared with the restriction enzymes RsaI or HinfI. This diagnostic PCR was tested on mosquitoes collected from different locations throughout China. Specimens identified morphologically as An. anthropophagus in the adult and egg stage from one location in Quangdong Province were found to be An. sinensis, while specimens from Liaoning Province, which were variable in their egg morphology, were found to be An. anthropophagus. The presence of An. anthropophagus in Liaoning Province extends the range of this species north to 42°N. The ITS2 spacer sequence was used in a maximum parsimony phylogenetic reconstruction of six members of the Hyrcanus group, two members of the Lesteri subgroup, and one member of the Nigerrimus subgroup, with the resulting molecular groupings at odds with the current morphological groupings.
Malaria in central China is endemic, but unstable, and outbreaks involving millions of cases have occurred regularly since the 1950s (Li et al. 1995). As a result of these episodes, a well-organized and comprehensive vector control program was carried out in the 1980s and early 1990s (Li et al. 1995, Luo et al. 1996, Liu et al. 1996). By 1994, malaria had been significantly reduced, control levels had been met, and, as a result, vector control measures were withdrawn (Luo et al. 1996, Xili et al. 1996, Sleigh et al. 1998). However, in 1995, Henan Province experienced an outbreak of vivax malaria with infection rates as high as 29% in some counties (Sleigh et al. 1998).
The main malaria vector in the region was believed to be Anopheles sinensis Wied. Studies on this species in the early 1960s identified An. sinensis where the deck on the egg was wide, and a separate variety, An. sinensis "narrow egg variety," where the deck was quite narrow (Ma 1981). This was not the first reference to these two forms of An. sinensis, as both egg types had been collected in the Nanjing (Nanking) area as early as 1935 (cited in Reid 1953).
Further work on the vectors, conducted after the 1971–72 malaria epidemic, showed that An. sinensis "narrow egg variety" was more anthropophilic, endophagic, and endophilic than An. sinensis, which tended to feed predominantly outdoors on cattle. At this time, similarities were noted between the deck size of An. sinensis "narrow egg variety" and Anopheles lesteri Baisas and Wu, which also has eggs with a narrow deck. Consequently, An. sinensis "narrow egg variety" was considered a subspecies of An. lesteri and called Anopheles lesteri anthropophagus (Xu and Feng 1975). However, Ma (1981) elevated An. anthropophagus to species level based on its morphology, ecology, distribution, and vectorial capacity. The distribution of An. anthropophagus is thought to be widespread throughout central China south of 33°N. It occurs sympatrically with An. sinensis; however, its range is patchy compared with An. sinensis, which is more common and whose range is continuous throughout the region.
Sporozoite rates in An. anthropophagus have been recorded at 0.22%, 20 times higher than that found in An. sinensis (You et al. 1988). This, its anthropophilic behavior and its close association with malaria outbreaks, particularly Plasmodium falciparum, has resulted in this species now being established as the primary vector of malaria in central China (Li et al. 1995). Unfortunately, An. anthropophagus and An. sinensis are cryptic species, and egg morphology is the only reliable way of separating them. This method is impractical for the identification of the large numbers of field-collected specimens that are usually required for studies on vector distribution, ecology, behavior, and transmission as well as in the evaluation of control measures. Additionally, confusion will arise using egg deck width wherever An. lesteri and An. anthropophagus occur sympatrically, as was the case in earlier malaria vector studies (Ho et al. 1962).
The identification of cryptic species is a major problem for many of the world’s malaria taxon (Baimai et al. 1984). To resolve this problem, DNA-based markers are now frequently being used as a reliable means of separating species. One region of the mosquito genome that has produced useful polymerase chain reaction (PCR)-based diagnostic markers is the ribosomal DNA (rDNA) gene family (Collins and Paskewitz 1996). Most of these approaches use species-specific sequence variation within the rDNA internal transcribed spacers (ITS1 and ITS2). One useful method has been to identify restriction sites or diagnostic sequence insertions/deletions by using a simple PCR-restriction fragment length polymorphism (RFLP) procedure. This approach permits an initial crude assessment of the sequence variation in these regions by revealing the presence/absence of restriction sites and/or the presence of sequence insertions and deletions, and has been useful in resolving cryptic species groups and subgroups (Beebe and Saul 1995). However, if the species-specific sequence variation is small, which can often be the case, then allele-specific PCR can be developed by using PCR primers designed to specific polymorphisms within these regions (Cornel et al. 1996, Walton et al. 1999).
Molecular technologies, such as allozyme electrophoresis (Ye and Zhu 1989) and restriction pattern differences of the genomic DNA (Li et al. 1990, Li et al. 1991), have been approached as a means of separating An. anthropophagus and An. sinensis. However, constant low temperature storage of the field material is crucial to maintain good quality protein and DNA required for these techniques, and this can often be difficult to achieve in the field. To alleviate the mosquito storage problem, an allele-specific PCR assay was developed to identify An. sinensis and An. anthropophagus, but it was only capable of identifying 78.9% of field-collected material (Ma et al. 1998). These authors suggested that the unidentified samples contained other yet unknown cryptic species.
In this work, we have outlined a diagnostic procedure that uses universally conserved primers to amplify the rDNA ITS2 and then a restriction digest that produces RFLP profiles that distinguish the malaria vectors An. sinensis and An. anthropophagus. The procedure was then tested on field-collected material from a variety of collection sites in China. Implications to the current eggshell morphology are discussed. We then compared these ITS2 sequences with other members from the Hyrcanus group, Nigerrimus subgroup, and Lesteri subgroup to investigate the current informal groupings of these mosquitoes with the ITS2 genetic groupings.
Materials and Methods
Material Examined
Most of the specimens used in this study came from seven sites in China (Fig. 1). Material from Jiangsu Province (site 1, n = 40), Sichuan Province (site 2, n = 20), and Guangxi Province (site 3, n = 40 and site 4, n = 40) are from established colonies reliably identified as An. anthropophagus. Field material was collected from Hubei Province (site 2, n = 20), Guangdong Province (site 5, n = 8), and two sites in Liaoning Province (site 6, n = 46 and site 7, n = 25). All material was identified as An. anthropophagus based on eggshell morphology and deck width or on adult females using wing and leg morphology, as in the keys of Ma (1981). An. sinensis came from an established colony maintained at site 1 (n = 20). The species Anopheles nigerrimus Giles used in the cladistic analysis was collected from East Timor.
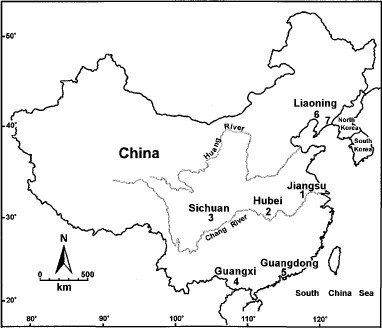
Map of China with the collection sites for An. anthropophagus and An. sinensis.
DNA Extraction, ITS2 Amplification, PCR Product Digestion, and Visualization
DNA was extracted from whole mosquitoes using the technique of Patricia Roman (Black and Munstermann 1996). PCR amplification of the ITS2 was carried out in 25 μl volumes using a thermal cycler (MJ Research, Boston, MA, or Eppendorf Mastercycler, Hamburg, Germany). The final PCR mixture contained 1× Taq buffer II (GeneAmp; Roche, Branchburg, NJ), 1.5 mM MgCl, 0.125 mM of each dNTP, 50 ng of each primer, 5% DMSO, 1 U of Taq polymerase (AmpliTaq Gold; Roche), and 5.0–10.0 ng (1.0 μl) of extracted genomic DNA.
Primers for the ITS2 region were ITS2A (5′-TGTGAACTGCAGGACACAT) and ITS2B (5′-TATGCTTAAATTCAGGGGGT): these were designed to the 5.8S and 28S subunits, respectively. The cycling regime used involved an initial denaturation of 94°C for 10 min, then 35 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min with minimal transition times.
Undigested ITS2 PCR products were run on agarose gel (1.0%) at 100 V for 40 min (containing 0.3 μg/ml of EtBr) and visualized at 312 nm. Digests were performed using 5.0 μl of the PCR product, 1 μl of the appropriate digest enzyme buffer, 4 μl of double distilled water, and 2 U of enzyme (initially made up as a 2× stock). PCR products were digested for 2 h at 37°C, and the DNA fragments were run on a 3% agarose gel (100 V for 40 min) containing EtBr as above and visualized at 312 nm.
ITS2 Polymorphism Identification
To identify the presence of polymorphic ITS2 copies within the rDNA array (intragenomic), 5 μl of the ITS2 PCR product was run for 2 h at 200 V on a 10% acrylamide gel. The gel rig was kept cool by partial immersion in an ice water bath. The gel was then stained with 0.5 μg/ml ethidium bromide for 3 min and visualized at 312 nm. Intragenomic polymorphisms were identified by the presence of heteroduplexes, which were observed as slower moving bands in the acrylamide gel caused by mispairing of the double-stranded DNA duplexes retarding DNA migration.
Sequencing
ITS2 PCR products were cloned into either pGem-T vector or XL10 Gold Kan (as per Stratagene PCR-Script Amp cloning kit, La Jolla, CA), following the manufacturer’s recommendations. Positive colonies containing single copies of the ITS2 fragment were selected using a sterile pipette tip and then dipped into the PCR reaction mixture and cycled through 1 cycle of 94°C for 4 min, followed by 24 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Amplified products were purified using the Qiagen protocol (QIAquick PCR purification kit, Victoria, Australia). Sequencing was performed using ABI Big Dye Terminator kit (PE Biosystems, Foster City, CA), according to the manufacturer’s recommendations, and using the ITS2A, M13 forward, and reverse sequencing primers. The reaction mixture was cycled through 25 cycles of 94°C for 30 s, 50°C for 15 s, and 60°C for 4 min. Products were cleaned with two isopropanol washes and air dried under vacuum. Sequencing was performed using an ABI 377.
Mosquito isolates selected for cloning and sequencing included one individual of An. anthropophagus from the Jaingsu colony (four clones sequenced), one field-collected isolate from Guangdong (four clones sequenced), and one colony isolate of An. sinensis from the Jaingsu (three clones sequenced). An. nigerrimus Giles of the Nigerrimus subgroup was collected from East Timor in 2001 (two clones sequenced). Consensus sequences for each species were deposited into Genbank.
Cladistic Analysis
The ITS2 DNA sequences from different members of the Hyrcanus group and Lesteri subgroup were downloaded from Genbank. These species were An. hyrcanus (AF261149), An. kweiyangensis (AF261150), An. liangshanensis (AF146750), An. yatsushiroensis (AF146749), and two species from the Lesteri subgroup, An. lesteri Baisas and Hu (AF145464) and An. crawfordi Reid (AF261949). A sequence alignment was generated using the above sequences and our sequences of An. sinensis, An. anthropophagus, and An. nigerrimus. Many of the sequences obtained from Genbank did not contain 5.8 and 28S gene regions, and consequently the final sequence alignment was restricted to only the ITS2 region (≈440 bp) and excluded ≈90 bp of the 5.8S.
Sequence alignment was performed using the PILEUP algorithm in GCG package (Genetics Computer Group, version 8, 1994), and several gap-weight and gap-length-weight values were tested and then examined visually to determine the most favorable (Philips et al. 2000). The gap-weight value and the gap-length-weight value were set to 2.0 and 0.1, respectively. A maximum parsimony analysis was then performed with the branch-and-bound option using the PAUP 4.0b4a package (Swofford 2002). Default settings were used, and a transition:transversion ratio (ti:tv) of 1:1 was selected and 1,000 bootstrap replicates were used to assess branch support.
Results
The ITS2 PCR products for An. sinensis and An. anthropophagus ran at ≈594 and 574 bp, respectively. Restriction enzyme analysis of the ITS2 PCR products using RsaI and HinfI produced diagnostic RFLPs (Fig. 2). After digestion with RsaI, specimens of An. anthropophagus from sites 1, 2, 3, 4, 6, and 7 produced a distinct band at 500 bp and less distinct bands under 100 bp. When digested with HinfI, this material produced a distinct band at 400 bp and some indistinct bands at under 100 bp. The An. sinensis specimens from Jiangsu (site 1) and four of the eight specimens from Guangdong (site 5, identified morphologically as An. anthropophagus) produced two strong bands at 330 and 200 bp when digested with RsaI and a strong band at 570 bp when digested with HinfI (Fig. 2).
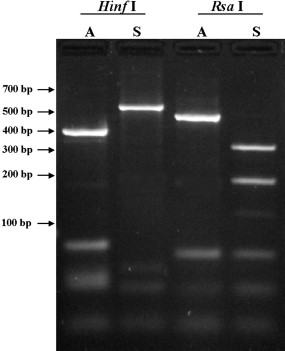
A 3.0% agarose gel containing ITS2 PCR products of An. sinensis (S) and An. anthropophagus (A) digests with HinfI (lanes 1 and 2) and RsaI (lane 3 and 4).
The PCR products were assessed for intragenomic polymorphisms (polymorphisms within copies in the rDNA array) by acrylamide gel electrophoresis to determine whether the PCR products could be directly sequenced. Acrylamide gel electrophoresis is sensitive to DNA secondary structure, and mispaired DNA duplexes (heteroduplexes) will migrate more slowly through the gel, resulting in the appearance of multiple bands. All isolates from all locations showed very minor shifts in the acrylamide gel, indicating the presence of small sequence polymorphisms in copies within the rDNA array (Beebe et al. 2001). Thus, cloning was required before sequencing. Clones selected were sequenced in both directions for complementary reads, and one sequence from each species was placed in Genbank (AF543860-1). These sequences included regions of the 5.8S and 28S rDNA gene, that when removed, revealed the ITS2 to be 476 bp for An. sinensis and 456 bp for An. anthropophagus. The ITS2 sequence for the species An. nigerrimus from the Nigerrimus subgroup was also submitted to Genbank (AF543862).
The An. anthropophagus ITS2 sequence from the colony material was found to be the same as the field site material from Hubei and Liaoning. Sequences from the An. sinensis colony were the same as those of the Guangdong specimens. The sequence for An. anthropophagus showed 76.2% similarity to An. sinensis with 113 substitutions and 39 insertions and deletions. The GC content for An. sinensis and An. anthropophagus was 50.5% and 46.0%, respectively. Genbank database comparisons identified two other ITS2 sequences for An. anthropophagus (AF84172, AJ004941) and one other sequence for An. sinensis (AJ004942). Intraspecific variation was 0.4% to 1.5% among An. anthropophagus sequences and <0.4% among An. sinensis sequences.
From preliminary evaluation of the PILEUP sequence alignment, it was evident that An. lesteri of the Lesteri subgroup showed higher sequence similarity to An. sinensis (92%) than to An. crawfordi (≈65%) from the Lesteri subgroup. Moreover, An. lesteri demonstrated more similarity to the unplaced members of the Hyrcanus group (92–77%) than to An. crawfordi. The most distant species was An. nigerrimus, with 59% similarity to An. sinensis and 76% similarity to An. crawfordi. The maximum parsimony analyses of the sequence alignment resolved a single unrooted tree (Fig. 3), which contained 499 total characters. Of these, 198 characters were parsimony informative, 107 variable characters were parsimony uninformative, and 194 characters were constant. The tree had a length of 547, a consistency index (CI) of 0.8245, a retention index (RI) of 0.6852, and a rescaled consistency index (RC) of 0.5650.
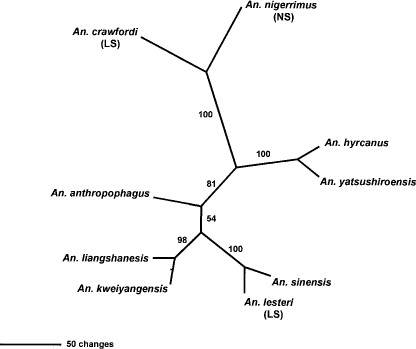
The single most parsimonious tree (unrooted) of the ITS2 sequence alignment for species members in the Hyrcanus group, Lesteri subgroup (LS), and the Nigerrimus subgroup (NG). Branch lengths indicate distances or sequence changes in the alignment. The tree length was 547, CI = 0.8245, RI = 0.6852, and RC = 0.5650. Numbers on branches are branch support indices from 1,000 bootstrap replicates.
Discussion
Throughout parts of central China, An. anthropophagus is regarded as the primary malaria vector and An. sinensis the secondary vector. These are closely related species that are difficult to separate in the adult stage using morphological characters. Both these species appear genetically dissimilar and can be identified by PCR-RFLP analysis of the ITS2, digested with either RsaI or HinfI.
While mosquitoes collected from Guangdong had been identified as An. anthropophagus by adult and egg morphology, molecular analysis of this material identified four (of eight) specimens as An. sinensis. This observation was subsequently confirmed by sequence analysis. Moreover, the material from Liaoning (sites 6 and 7) showed some degree of polymorphism in the egg deck width. This characteristic has been classified as wide, moderate, narrow, or very narrow (Reid 1968, Harrison 1972) and has been used to separate species in the Hyrcanus group. In this study, according to deck width, some specimens from Liaoning appeared similar to An. anthropophagus (narrow deck), while others appeared as An. sinensis (wide deck). However, all specimens from Liaoning were typed as An. anthropophagus using the PCR-RFLP procedure. These findings suggest that egg deck width may not be as reliable in separating An. anthropophagus and An. sinensis as previously thought.
Recent studies along the North/South Korean border identified An. sinensis as the most abundant mosquito biting humans, making up 79.3–95.8% (>4000 specimens) of the mosquitoes collected. An. lesteri was the second most common anopheline, but made up only 1.5–5.9% of the mosquitoes collected (Strickman et al. 2000). These authors recognized the difficulty of separating adults of An. sinensis and An. lesteri and questioned the possible relationship of Korean An. lesteri and Chinese An. anthropophagus. The human biting behavior of An. sinensis noted by these authors is atypical of that observed for this species in China, where it is primarily a cattle feeder. The results of this current study have extended the distribution of An. anthropophagus 1,200 km to the north of its previously known range (sites 6 and 7, Fig. 1, and 42°N, 120°E). This location is directly north of the Korean Peninsula, and it is possible that this species may occur there, but, as was found with the Guangdong material in this current study, morphological characters cannot be used to identify the possible existence of An. anthropophagus. Recent molecular analysis of material from the Republic of Korea identified only An. sinensis, An. lesteri, and An. yatsushiroensis, although only a small amount of material was examined (Ma et al. 2000).
The PCR method described in this work has some advantages over other published methods of species identification. The method of Li et al. (1990), which was based on genomic DNA restriction digests, is limited because it requires high quality DNA (both in storage and extraction) for the generation of consistent genomic DNA RFLPs. The allozyme method of Ye and Zhu (1989) requires the mosquitoes to be kept frozen (below −50°C) at all times until processed. When performing field work, especially on remote locations, both of these methods present impractical storage constraints. The allele-specific PCR method described by Ma et al. (1998) has many advantages over the other two methods; however, the authors found their PCR could only identify 78.9% of their field-collected material, and their explanation for this was the presence of other cryptic species.
The failure of allele-specific PCR to produce PCR products may indeed be caused by the presence of other cryptic species, or quite possibly by the presence of intraspecific genetic variants that contain sequence polymorphisms within the primer regions. As these species-specific primers are usually designed to the polymorphic regions of the ITS2, there is the likelihood that these regions may also show intraspecific polymorphisms that could inhibit primer annealing and cause PCR amplification failure. Such anomalies may require the investigators to go back and redesign the primer design strategy. The PCR procedure described in this work has advantages over the procedure of Ma et al. (1998), as it uses universal primers for amplification of the ITS2, which should ensure that all specimens tested deliver a PCR product. Subsequent restriction digestion of these products should assist in resolving anomalies, such as poor quality DNA, the presence of other cryptic species, or genetic variants. One would imagine that morphological inconsistencies with other species would be expected in large, closely related mosquito groups like the Hyrcanus group. Thus, in the early stages of studies into the molecular identification of cryptic mosquito groups, the use of conserved primers presents clear advantages in resolving such anomalies. As the whole ITS2 region is amplified, cryptic mosquito groups can be genetically dissected using a variety of methods such as restriction digest and/or heteroduplex analysis, thus avoiding the heavy reliance on DNA sequencing, which frequently requires cloning before sequencing. This type of approach was employed in a genetic study of the Anopheles bancroftii group from Australia and Papua New Guinea and revealed independently evolving sympatric genotypes (possible species), of which one genotype displayed wing fringe polymorphisms that were once though to be diagnostic characters (Beebe et al. 2001).
The PCR-RFLP profiles generated by this method appear consistent over a large geographic distribution through China and add confidence that the profiles generated are species specific. Also, a closer look at the Genbank sequences suggests that this PCR-RFLP procedure should also discriminate An. lesteri from An. anthropophagus and An. sinensis, as inspection of the An. lesteri ITS2 sequence indicated the presence of a species-diagnostic HinfI restriction site ≈200 bp into the 5′ region (≈98 bp into AF145464). This restriction site should produce species-diagnostic bands at ≈200 and 330 bp.
The grouping that resulted from the phylogenetic reconstruction of nine species spanning the Hyrcanus group and Lesteri and Nigerrimus subgroups were at odds with the morphological groupings of Reid (1953) and Harrison (1972). Reid (1953) placed An. lesteri and An. crawfordi together in the Lesteri subgroup because of the strong morphological similarities shown in their larval and pupal stages. The grouping from the molecular data shows An. lesteri to have closer genetic affinities with An. sinensis (90.6% similarity) and little identity to An. crawfordi (64% similarity). However, both Harrison (1972) and Reid (1953) group An. crawfordi and An. lesteri together and An. sinensis out on its own. Both An. anthropophagus and An. sinensis have the same adult morphology, although they show only 74.5% similarity and appear on different branches on the tree in Fig. 3. Conversely, the adult morphology of An. lesteri places it away from An. sinensis and into the Lesteri subgroup, yet these species show over 90% genetic similarity at the ITS2 sequence level.
It is important to note that the ITS2 sequence variation among these few members of the Hyrcanus group is considerable (8.0% to 60%). The many insertion and deletion elements will probably prove too variable to use for phylogenetic reconstruction and will make the selection of any outgroups difficult. Thus, it would be prudent to look for another marker to investigate the evolution of this group. Nonetheless, this insight into the genetic relationships of members of the Hyrcanus group suggests that the molecular data from the ITS2 are at odds with the current morphological grouping. The ability to separate species using overt morphological characters is an indispensable tool to have in the field; however, these morphological characters should be developed from species whose genetic identity has been confirmed by DNA-based techniques.
The Hyrcanus group is a large group of closely related species, whose members range throughout the southern Palaearctic and Oriental regions that extend from Spain east through into China, north into Mongolia and Russia, and south down the Indonesian archipelago to East Timor (Reid 1953). The group contains 28 species, 5 in the Lesteri subgroup, and 4 in the Nigerrimus subgroup, with the remaining 19 species unplaced within the group (Harbach 1994, Nguyen et al. 2000). Sixteen of these species have been identified within China (Ma 1981). Attempts have been made to bring some order to this group (Reid 1953, 1968, Harrison 1972, Harrison and Scanlon 1975). However, as Nguyen et al. (2000) points out, this is an extremely complex group with a huge range. Species have recently been described at a country level without consideration for the possible range of other species. Thus, some species may be nominal and eventually synonymized. The current groupings (Harbach 1994) are from Harrison (1972), who pointed out that any attempt to study the taxonomy of this group must be made on all stages of the life cycle. However, it appears that alpha taxonomy will never fully resolve this group, and a combination of morphological and molecular markers will be required.
Acknowledgements
This investigation received financial support from the United Nations Development Program (UNDP)/WORLD BANK/World Health Organization (WHO) Special Program for Research and Training in Tropical Diseases (TDR).
References Cited



