-
PDF
- Split View
-
Views
-
Cite
Cite
Susan R. Dean, Roger W. Meola, Factors Influencing Sperm Transfer and Insemination in Cat Fleas (Siphonaptera: Pulicidae) Fed on an Artificial Membrane System, Journal of Medical Entomology, Volume 39, Issue 3, 1 May 2002, Pages 475–479, https://doi.org/10.1603/0022-2585-39.3.475
Close - Share Icon Share
Abstract
Sperm transfer through the epididymis, a prerequisite for insemination of cat fleas, Ctenocephalides felis (Bouché), was stimulated by exposure of unfed male fleas to juvenile hormone III residues for 3 d at 25°C or exposure of unfed fleas to 37°C for 6 d. Sperm transfer was completed at least three times faster in unfed males held at 37°C than in those held at 25°C. Although percentage sperm transfer in fleas fed water or 0.15 M saline at 37°C was not significantly increased over that of unfed fleas, a significantly greater percentage of blood-fed males completed sperm transfer at 2, 3, and 6 d. At least two factors influenced insemination: exposure of fleas to host body temperature and amount of food consumption. When blood-fed males and females were paired and fed 0.15 M saline, 0% were inseminated at 25°C versus 35% at 37°C. Because percentage insemination did not increase in blood-fed males and females that were paired and fed 0.15 M saline at 37°C for an additional 48 h, continuous bloodfeeding appeared to be required for maximal rates of mating and insemination. Furthermore, no females were inseminated when blood-fed males and females were paired at 37°C and starved. Treatment of unfed fleas with juvenile hormone III did not substitute for bloodfeeding in stimulating mating and insemination; when blood-fed males were paired with JH III-treated females and vice versa and fed 0.15 M saline at 37°C, 0% were inseminated. However, when fleas were fed 0.15 M saline and exposed to 1,250 ppm juvenile hormone III or fed whole blood and exposed to 12.5, 125, or 1,250 ppm juvenile hormone III, percent insemination was significantly increased in comparison to the controls. Therefore, juvenile hormone secretion in blood-fed fleas may regulate mating success indirectly by stimulating sperm transfer.
In the cat flea, Ctenocephalides felis (Bouché), sperm transfer must take place before the male is capable of inseminating females. Sperm transfer is completed when the columnar epithelial cells that line the epididymis differentiate into flattened squamous cells thus forming a lumen through which sperm can leave the testes and enter the epididymis. In addition, sperm must travel from the epididymis to the ejaculatory duct and females must be receptive before insemination can take place. Sperm transfer and mating are usually associated with bloodfeeding. Akin (1984) reported that both occur in the cat flea within 24 h of feeding on a host animal. Bloodfeeding in fleas may be associated with several factors which, through nervous or endocrine processes, may initiate sperm transfer and mating behavior (Iqbal and Humphries 1970, Rothschild and Ford 1973, Prasad 1987, Hsu and Wu 2001). These factors include abdominal distention, the presence of a nutritional factor in blood, the act of bloodfeeding, and experience of a rise in temperature due to host blood or body temperature. In the cat flea, juvenile hormone stimulates sperm transfer (Dean and Meola 1997) and its secretion may be regulated by one or more of the aforementioned factors associated with bloodfeeding.
Although sperm transfer is completed by unfed fleas exposed to juvenile hormone residues for 3 d at 25°C or a temperature of 37°C for 6 d, insemination does not take place under these conditions (Dean and Meola 1997; unpublished data). Likewise, Zakson-Aiken et al. (1996) reported that unfed cat fleas remain unmated over a 30-d observation period. We have observed (unpublished data) that only cat fleas fed on protein-containing solutions, i.e., whole or hemolyzed blood, plasma or 3.5 and 7.0 g/dl albumin solutions were inseminated. In addition, we concluded that fat body reserves were depleted as an energy source for survival by the time that sperm transfer was completed in unfed fleas or those fed water or saline, and that they were unable to meet the energy requirements for mating. Cat flea matings last an average of 60 min and only 44% of first matings result in oviposition of viable eggs (Hsu and Wu 2000). Hsu and Wu (2001) demonstrated that unfed males do not mate with blood-fed females, presumably, because they have not completed sperm transfer. Hsu and Wu (2001) also observed that approximately five times as many blood-fed as unfed females mated with blood-fed males. Therefore, bloodfeeding may stimulate endogenous juvenile hormone secretion, which in turn may increase female receptivity to mating. Juvenile hormone secretion is the stimulus that brings females to a state of receptivity to mating in some flies and other insects (Wyatt and Davey 1996).
The objectives of this study were to determine which factors associated with bloodfeeding influenced sperm transfer and insemination and whether juvenile hormone treatment of male or female fleas substituted for bloodfeeding in stimulating mating and insemination.
Materials and Methods
Laboratory Insects.
Fleas were obtained from a colony originally supplied by Zoecon (Dallas, TX) in 1987 and maintained in culture at Texas A&M University. Adult fleas for the colony were fed on domestic cats housed in the Laboratory Animal Resources and Research facility on campus. Eggs collected daily were brought to our laboratory at the Center for Urban and Structural Entomology where they were placed on 30 g of larval rearing medium made from 6 g dried bovine blood, 23 g finely ground cat chow, and 200 mg powdered brewer's yeast in 310 mg of fine sand. Cocoons were sifted from the rearing medium and stored in plastic containers in an environmental chamber maintained at 25°C, 70–80% RH, and a photoperiod of 14:10 (L:D) h. Newly eclosed fleas of both sexes were collected by a vacuum separator (FleaData, Freeville, NY) and placed in plastic cages containing dog hair in groups of ≈100 each for experimental use. Groups of fleas of a single sex were collected by separating newly eclosed males and females using a vacuum separator viewed under a dissecting microscope and placing them in plastic cages in groups of ≈50. Five replicate cages of fleas were used in each experimental treatment. Adults were fed citrated cattle blood, 0.15 M saline or distilled water using an artificial membrane system previously described by Pullen and Meola (1995). Blood used in these experiments was collected aseptically from a herd of donor cattle that were maintained without insecticide or antihelminthic treatments.
Measurement of Percentage Sperm Transfer and Insemination.
A sample of 20 male and 20 female fleas was removed from each replicate experimental cage and dissected to determine percentage sperm transfer (sperm in the epididymis) and insemination (sperm in the spermatheca).
Sperm Transfer and Insemination in Fleas at 37°C.
To determine whether bloodfeeding accelerates the completion of sperm transfer and insemination, fleas were maintained on an artificial membrane system at 37°C for 6 d and starved or fed 7 ml of distilled water, 0.15 M saline or blood. Percentage sperm transfer and insemination were determined on days 2, 3, and 6.
Sperm Transfer and Insemination in Fleas Maintained at 25°C.
Fleas were held for 0, 3, 7, 10, or 18 d at 25°C to determine whether unfed male fleas completed sperm transfer and inseminated females at this temperature.
Pairing of Juvenile Hormone-Treated and Blood-fed Adults at 25°C.
To determine whether insemination of females would take place at 25°C when males were bloodfed and females exposed to juvenile hormone or vice versa, groups of males and females were either exposed to 1,000 ppm juvenile hormone III-treated hair at 25°C for 3 d, fed blood on an artificial membrane system at 37°C for 3 d or starved at 25°C for 3 d. A 200 μg/ml juvenile hormone III stock solution was prepared by taking an aliquot of a 1 mg/ml isooctane solution of technical juvenile hormone III (Sigma, St. Louis, MO), evaporating the isooctane solvent and redissolving it in acetone. To treat dog hair with 1,000 ppm of juvenile hormone, 5 ml of stock was applied to 1 g of dog hair in a petri dish. Treated dog hair was placed in a hood overnight to allow the acetone to evaporate. Approximately 0.5 g of hair was placed in each cage. Fleas of the opposite sex were combined in plastic cages filled with untreated dog hair at 25°C so that unfed or juvenile hormone-treated males were paired with blood-fed females and vice versa for 24 h. Male and female blood-fed fleas that were paired and held at 25°C served as controls.
Pairing of Juvenile Hormone III-Treated and Blood-Fed Adults at 37°C.
To determine whether insemination of females would take place at 37°C when males were bloodfed and females were exposed to juvenile hormone or vice versa, groups of males and females were exposed to 1,000 ppm juvenile hormone III-treated hair for 3 d at 25°C or fed blood for 3 d at 37°C. Fleas of the opposite sex were combined in plastic cages filled with untreated dog hair and fed 0.15 M saline for 24 h. Blood-fed males and females that were paired and fed blood for 24 h at 37°C served as controls. In addition, bloodfed males and females were paired and either starved for 24 h or fed 0.15 M saline for 24 or 72 h at 37°C. Blood-fed males and females were also paired and fed 0.15 M saline for 72 h at 25°C.
Insemination of Saline or Blood-Fed Fleas Maintained on Juvenile Hormone III-Treated Hair.
To determine whether juvenile hormone III exposure increased percentage insemination in fed fleas, fleas were placed in plastic cages filled with dog hair treated with juvenile hormone III and fed 0.15 M saline or whole cattle blood. Ten cages were filled with dog hair treated with 0 (control), 1.25, 12.5, 125, or 1,250 ppm juvenile hormone III. Cages containing fleas on dog hair treated with the same concentration of juvenile hormone III were split into two groups, one group was fed saline and the other was fed blood. Percentage sperm transfer and insemination were determined on days 3 and 6.
Results
Sperm Transfer and Insemination in Fleas at 37°C.
Although sperm transfer took place regardless of whether fleas were starved or fed water, 0.15 M saline or blood, a significantly greater percentage of blood-fed fleas completed sperm transfer on days 2, 3, and 6 (Table 1). Only fleas fed cattle blood were inseminated on days 2, 3, and 6.
Percentage sperm transfer and insemination of fleas maintained on an artificial membrane system at 37°C
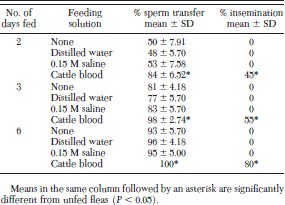
Percentage sperm transfer and insemination of fleas maintained on an artificial membrane system at 37°C

Sperm Transfer and Insemination in Fleas at 25°C.
Sperm transfer in unfed fleas held at 25°C was not significantly increased compared with newly eclosed males until day 7 (Table 2). Sperm transfer increased steadily thereafter through day 18. None of the females caged with the males were inseminated by day 18 and almost all fleas were dead by day 21.


Pairing of Juvenile Hormone III-Treated and Blood-Fed Adults at 25°C.
Because the males did not complete sperm transfer, blood-fed females that were paired with unfed males were not inseminated (Table 3). Furthermore, no females were inseminated when blood-fed males were paired with unfed females, although males had completed sperm transfer. Likewise, pairing 1,000 ppm juvenile hormone III-treated male fleas with blood-fed females and vice versa resulted in 0% insemination. In addition, when blood-fed males and females were paired for 24 h at 25°C, 0% were inseminated.
Percentage insemination of cat fleas initially segregated by sex and exposed to 1,000 ppm juvenile hormone IIItreated dog hair or bloodfed for 3 d before recombining the sexes in cages for 24 h at 25°C
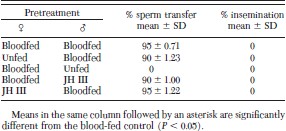
Percentage insemination of cat fleas initially segregated by sex and exposed to 1,000 ppm juvenile hormone IIItreated dog hair or bloodfed for 3 d before recombining the sexes in cages for 24 h at 25°C

Pairing of Juvenile Hormone III-Treated and Blood-Fed Adults at 37°C.
When females treated with 1,000 ppm juvenile hormone III were paired with blood-fed males and vice versa and fed 0.15 M saline for 24 h at 37°C, 0% were inseminated, although males had completed sperm transfer (Table 4). When male and female blood-fed fleas were paired at 37°C and starved, none were inseminated. However, when blood-fed males and females were paired and fed either saline or blood at 37°C for 24 h, percentage insemination was 35 and 45%, respectively; percentage insemination did not increase when blood-fed fleas were fed saline an additional 48 h at 37°C. Blood-fed fleas that were fed saline for 72 h at 25°C were not inseminated.
Percentage insemination of cat fleas initially segregated by sex and fed cattle blood, 0.15 M saline or exposed to 1,000 ppm juvenile hormone III-treated dog hair for 3 d before recombining the sexes in cages
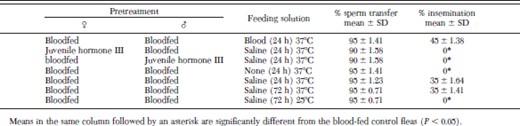
Percentage insemination of cat fleas initially segregated by sex and fed cattle blood, 0.15 M saline or exposed to 1,000 ppm juvenile hormone III-treated dog hair for 3 d before recombining the sexes in cages

Insemination of Saline and Blood-Fed Fleas Maintained on Juvenile Hormone III-Treated Hair.
When fleas were fed 0.15 M saline and maintained on 1,250 ppm juvenile hormone III-treated dog hair, percentage insemination was significantly greater than in the controls on days 3 and 6 (Table 5). When fleas were fed cattle blood and maintained on 12.5 or 1,250 ppm juvenile hormone III-treated dog hair, percentage insemination was significantly greater than in the controls on days 3 and 6.
Percentage insemination of fleas maintained on JH III-treated dog hair and fed 0.15 M saline or whole blood on an artificial membrane system at 37°C for 6 d
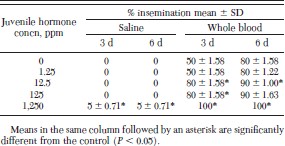
Percentage insemination of fleas maintained on JH III-treated dog hair and fed 0.15 M saline or whole blood on an artificial membrane system at 37°C for 6 d

Statistics.
Means were compared with the control using a 2-sample, unpaired t-test (P > 0.05).
Discussion
Sperm transfer is an age-dependent process at 25°C. Delayed sperm transfer and the emergence of males from their cocoons several days later than females may prevent males and females of the same cohort from mating. Sperm transfer was accelerated by exposing fleas to a temperature approximating that of a host animal. It was completed in unfed fleas at least three times faster at 37°C than at an ambient temperature of 25°C. Because fleas completed sperm transfer at the same rate regardless of whether they were starved or fed water or saline at 37°C, we concluded that sperm transfer was not dependent upon the act of feeding or the amount of abdominal distention. However, since sperm transfer was significantly increased when fleas were fed blood versus water or saline and flea weight gain is significantly greater on blood (unpublished data), we cannot rule out the possibility that the amount of food consumption influences sperm transfer. Similarly, Bai and Prasad (1979) reported that ≈50% of the rat fleas X. astia Roths. and X. cheopis Roths. completed sperm transfer when fed on saline versus 75–100% of those fed blood over a similar time interval.
Cat fleas also must be exposed to a temperature approximating that of a host animal (≈37°C) for mating and insemination to take place. When male and female blood-fed fleas were paired and fed 0.15 M saline for 72 h at 25°C or for 24 h at 37°C, 0% were inseminated at 25°C versus 35% at 37°C. Hsu and Wu (2001) reported that temperature is a critical factor in the mating behavior of the cat flea, and that cat fleas will only mate within a 34–42°C temperature range. Likewise, Iqbal and Humphries (1970) reported that the rat flea, Nosopsyllus fasciatus (Bosc.) will only mate within a temperature range of 30–35°C.
In addition to temperature, the amount of food consumption by fleas may influence mating and insemination. Hinkle (1992) reported that blood consumption by cat fleas fed on an in vitro system averaged 2.28 μl/d, almost three times less than the average blood consumption by fleas fed on a host animal, 6.97 μl/d. Likewise, percentage insemination was decreased when fleas were fed on an artificial membrane system versus on a host animal. Our results showed that when cat fleas were fed blood on an artificial membrane system for 2 d, 84% of males completed sperm transfer and 45% of females were inseminated. By the third day of bloodfeeding, the percentage insemination increased to 55%. In contrast, Akin (1984) found that when cat fleas fed on a host animal, most males completed sperm transfer within 2 h of the initiation of bloodfeeding and ≈70% of females were inseminated by 8 h. Thomas et al. (1997) reported that first matings between cat fleas take place at an earlier time on a host than on an in vitro system. In addition, our results showed that when fleas were separated by sex and fed blood for 3 d on an artificial membrane system, none were inseminated when they were paired at 37°C and starved. In contrast, Hsu and Wu (2001) reported that some fleas mated under these same conditions when they were initially fed on cats for 3 d.
The decreased insemination rates that we observed in comparison with those of fleas fed on a host animal may contribute to the lower fecundity of cat fleas fed on an artificial membrane system. Cat fleas fed on a host animal lay an average of 24–25 eggs per female per day (Hinkle 1992). In contrast, fleas fed cattle blood on an artificial membrane system lay an average of two to three eggs per female per day (Wade and Georgi 1988, Hinkle 1992, Pullen and Meola 1995). Zakson-Aiken et al. (1996) demonstrated that unmated females in cages that are fed blood on an artificial membrane system lay fewer eggs than females in cages to which males have been added. In our results, a greater percentage of fleas that were exposed to 12.5 or 1,250 ppm juvenile hormone III-treated hair were inseminated in comparison with controls on days 3 and 6, and, in an earlier study, we showed that the fecundity of fleas treated with these concentrations of juvenile hormone was also significantly increased (Meola et al. 2001).
Evidently, fleas must feed continuously on blood to achieve maximal rates of mating and insemination. Although ≈40% of blood-fed females were inseminated regardless of whether they were fed blood or saline for 24 h after pairing them with blood-fed males, percentage insemination did not increase when saline-fed fleas were fed saline for an additional 48 h. We hypothesized that saline-fed fleas depleted their fat body reserves and were unable to meet the energy requirements for mating and insemination. Hinkle (1992) demonstrated that blood-fed cat fleas that are starved for 12 h lose all body weight gained from bloodfeeding and Akin (1984) reported that almost all cat fleas died within 24 h of removal from a host animal. An alternate explanation for the observed absence of insemination in saline-fed fleas was that their abdomens were not sufficiently expanded. Hsu and Wu (2001) observed that blood-fed males were unable to mate with unfed 4-d-old females even though they mated with some 1-d-old females. They postulated that failure to mate was caused by abdominal shrinkage in females due to starvation.
Juvenile hormone III-treatment of fleas did not substitute for blood-feeding in stimulating mating and insemination in cat fleas. Therefore, juvenile hormone does not directly influence mating as it does in certain other insects (Schal et al. 1997). However, it may increase percentage insemination in saline and blood-fed fleas indirectly by accelerating sperm transfer in males.
Acknowledgements
This research was funded by Texas Agricultural Experiment Station Project 6526.
In conducting the research described in this report, the investigators adhered to the "Guide for the Care and Use of Laboratory Animals," as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facilities are fully accredited by the American Association of Laboratory Animal Care.
References Cited



