-
PDF
- Split View
-
Views
-
Cite
Cite
Quentin Q. Fang, Tonya R. Mixson, Minerva Hughes, Brandy Dunham, Jody Sapp, Prevalence of the Agent of Human Granulocytic Ehrlichiosis in Ixodes scapularis (Acari: Ixodidae) in the Coastal Southeastern United States , Journal of Medical Entomology, Volume 39, Issue 2, 1 March 2002, Pages 251–255, https://doi.org/10.1603/0022-2585-39.2.251
Close - Share Icon Share
Abstract
Human granulocytic ehrlichiosis (HGE) is an emerging tick-borne disease recently recognized in the United States. The blacklegged tick, Ixodes scapularis Say, is the principle vector in the eastern United States. The disease has been commonly reported in the northeastern and upper midwestern states; however, suitable vectors and reservoir hosts exist in the southeast. To assay the prevalence of the HGE agent in vector ticks, we screened 818 individual I. scapularis from 15 locations in South Carolina, Georgia, and Florida using nested polymerase chain reaction, which targets the HGE agent 16S rRNA gene. Prevalence among locations ranged from 0 to 20%. The overall average prevalence of 15 sites was 1.6% (n = 818). Verification by sequencing the 16S rDNA from the positive samples showed 99.8-100% nucleotide identities with the sequences of the HGE agent in GenBank. These results were supported by the phylogenetic analysis using 16S rDNA sequences.
Human ehrlichiosis is an emerging tick-borne zoonotic infectious disease (Walker and Dumler 1996). Its symptoms mimic influenza and include fever, headache, myalgia, thrombocytopenia, and leukopenia. Three forms of ehrlichiosis, human monocytic ehrlichiosis (HME), human granulocytic ehrlichiosis (HGE), and Ehrlichia ewingii, have been reported in the United States (Anderson et al. 1991, Chen et al. 1994, Buller et al. 1999). We have previously reported the prevalence of the HME agent (E. chaffeensis) in the vector tick Amblyomma americanum (L.) from Georgia (Whitlock et al. 2000).
The precise taxonomic status of the causative agent of HGE has been argued by various workers who have treated it variously as Ehrlichia phagocytophila, "the HGE agent," E. equi, E. microti, Cytocetes microti, and C. phagocytophila (Chen et al. 1994, Dumler et al. 1995, Madigan et al. 1995, Asanovich et al. 1997, Walls et al. 1999, Chase et al. 2000). Recently, Bakken and Dumler (2001) and Dumler et al. (2000) confirmed that "the HGE agent" is Anaplasma (formerly Ehrlichia) phagocytophila. The pathogens of HGE and HME are maintained in wild mammals including deer and rodents and are transmitted to humans by the bite of infected ticks. The principal vector of HGE in the eastern United States is the blacklegged tick, Ixodes scapularis Say. Ixodes scapularis feeds on a wide range of hosts including deer, rodents, and, in the southeast, lizards (Keirans et al. 1996).
The initial cases of HGE were reported from Minnesota, Wisconsin, and northern California (Bakken et al. 1994). However, cases have now been reported from over 25 states in the United States (McQuiston et al. 1999). Human cases of HGE have been confirmed in the south, albeit in small numbers. The Centers for Disease Control and Prevention reported only one indirect fluorescent antibody diagnosed human case in both Georgia and Florida from 1995 to 1997 and none from South Carolina (McQuiston et al. 1999). However, South Carolina does not distinguish between HME and HGE (McQuiston et al. 1999); therefore, the lack of confirmed cases of HGE may be erroneous. In addition, many of the symptoms of HGE are nonspecific, which could lead to misdiagnosis and therefore underestimation.
One single case of HGE has been reported in Georgia (McQuiston et al. 1999), although the prevalence of the pathogen in vector tick populations is yet unknown. The primary goal of this study was to determine the prevalence of I. scapularis infected with the agent of HGE from the coastal plains of South Carolina, Georgia, and Florida using nested polymerase chain (PCR) techniques. PCR assays are becoming a common method to detect the agents of human ehrlichioses (Anderson et al. 1992b, CDC 1997). PCR and sequencing of the 16S rDNA has enabled researchers to identify, classify, and determine phylogenetic relationships of ehrlichiae (Anderson et al. 1991, 1992a; Chen et al. 1994, Wen et al. 1995). We used primers that target one such fragment specific for the HGE agent. We have verified these results by both replicating the experiments from original DNA template and then by sequencing HGE positive samples.
Materials and Methods
Tick Collection.
Ticks were collected from vegetation using a 1 by 1-m drag cloth. Adult I. scapularis were collected from Big Talbot Island, Little Talbot Island, King George Island, and Anastasia Island in Florida; Sapelo Island, Jekyll Island, George L. Smith State Park, Magnolia Springs State Park, and Skidaway Island in Georgia; Hunting Island, Edisto Island, Pinckney Island, Pritchard’s Island, and Santee State Park in South Carolina. All specimens were kept alive until transported to the laboratory where they were stored at -80°C.
Nucleic Acid Isolation.
The method of nucleic acid extraction was modified from Doyle and Doyle (1990) and followed modifications described in Whitlock et al. (2000). Individual ticks were ground with a sterile pestle in a 1.5-ml microcentrifuge tube containing 250 μl of lysis buffer (4.5 M guanidine thiocyanate, 25 mM sodium citrate, 0.5% lauryl sarcosine) and 250 μl of cetyltrimethylammonium-bromide (CTAB) buffer that contained 100 mM Tris HCl, pH 8.0; 1.4 M NaCl, 0.02 M EDTA, 2% (wt:vol) CTAB, 0.2% (vol:vol) 2-mercaptoethanol. DNA was dried and resuspended in 50 μl of 10 mM Tris-HCl (pH 8.0). The samples were then gel electrophoresed to verify the presence of high molecular weight DNA.
Polymerase Chain Reaction.
A nested PCR, which targets ehrlichial 16S rRNA gene, was used to detect the HGE agent in this study. The outer reaction of the nested PCR uses genomic DNA as template and the inner reaction uses PCR product from the outer reaction as a template. The outer primers, Ehr26F (5′-TTACACATGCAAGTCGRACG) and Ehr1430 (5′-AGTCACTRACCCAACCTTAAA), were designed in our laboratory by aligning available rickettsial 16S rDNA sequences from GenBank using Genetic Data Environment (GDE) software (Smith et al. 1994). This pair of primers targets a highly conserved region of the 16S rDNA sequence of Ehrlichia and related genera ≈1,400 bp in size. The inner primers, Ge2F (5′-AACGGATTATTCTTTATAGCTTGCT) and Ge9RC (5′-GGCACTATTAAAAGCAGCTCCAGG), were synthesized according to published sequences (Massung et al. 1998). The inner primers target a 546 bp fragment flanked by the outer primer pairs and are specific to the HGE agent only. PCR reactions were performed in a Perkin-Elmer 9600 Thermal Cycler with GeneAmp reagents (Perkin-Elmer, Foster City, CA). DNA was amplified in a 25-μl volume for 40 cycles. The outer reaction (Ehr26F and Ehr1430RC) consisted of denaturation at 94°C for 35 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min. The inner reaction (Ge2F and Ge9RC) consisted of denaturation at 94°C for 40 s, annealing at 58°C for 40 s, and extension at 72°C for 2 min. The PCR reaction mixture consisted of 1× DNA polymerase buffer (10 mM Tris-HCl, pH 8.5, 50 mM KCl), 2.5 mM MgCl2, 200 μM of each dNTP, 2.5 U/100 μl of DNA Polymerase, 0.2 μM of each primer, and 0.5 μl (≈0.1 μg-0.5 μg) of DNA template.
Negative controls (sterile water in place of template) and a positive control (DNA from cultured HGE agent supplied by W. L. Nicholson, CDC, Atlanta, GA) were run with each PCR reaction. PCR product (5 μl of the 25 μl reaction volume) was visualized on a 1% agarose gel containing a 1 kb standard DNA ladder.
Positive Verification.
All suspected positive samples were rescreened from the original template. Only those samples that produced identical results to the initial screening were counted as positives. These samples were then prepared for sequencing by low-melting gel purification using Wizard PCR preps purification kit (Promega, Madison, WI). Purified DNA was sequenced with an ABI 3100 Automated Sequencer at the Center of Agricultural Biotechnology, University of Maryland Biotechnology Institute. These sequences were transferred back to our laboratory where they were then aligned using GDE with rickettsial and ehrlichial 16S rDNA sequences downloaded from GenBank for verification. The aligned sequences were also analyzed cladistically using PAUP (Swofford 2001).
Results
A total of 818 individual I. scapularis, collected from 15 sites along the coastal region of South Carolina, Georgia, and Florida, was screened for the HGE agent using a nested PCR assay (Fig. 1). Outer PCR reactions failed to produce detectable products. However, the positive controls, which use the genomic DNA from cultured HGE agent cells as template, always produced the expected 1,400 bp fragment. The positive samples from nested inner reaction yielded a 546 bp product identical in size to the positive control (Fig. 2). All the positive samples remained positive when rescreened.
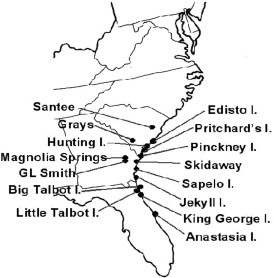
Fifteen collection sites in South Carolina, Georgia, and Florida from which I. scapularis were collected and screened.
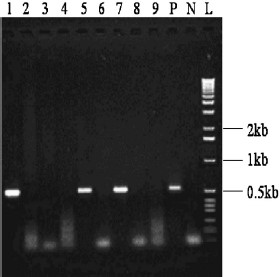
One percent agarose gel illustrating the 546 bp product characteristic of the HGE agent after nested PCR amplification. Lanes 1–9: tick samples. Tick samples in lanes 1, 5, and 7 are positive, all from Jekyll Island, GA (voucher numbers 324, 325, and 328).Lane P: positive control. Lane N: negative control. Lane L: 1 kb standard marker.
Of the 818 ticks screened, 13 were positive. Positives were from four of the 15 collection sites (Table 1). Of the 13 positives, five were from Hunting Island, SC (n = 121), one from Edisto Island, SC (n = 143), one from Sapelo Island, GA (n = 61), and six from Jekyll Island, GA (n = 30). All ticks screened from the Florida collection sites were negative. The overall occurrence for all sites was 1.6%. The occurrence rate from individual sites ranged from 0.0 to 20.0%. Variations of HGE agent frequency among sites were significant (G = 19.01, df = 14, P = 0.0005).
Infection prevalence calculated using the number of individual I. scapularis that produced the 546 bp16S rRNA gene fragment characteristic of the agent of HGE after PCR amplification
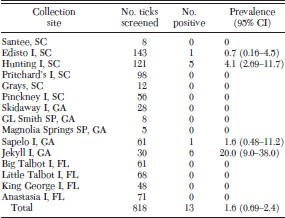
Infection prevalence calculated using the number of individual I. scapularis that produced the 546 bp16S rRNA gene fragment characteristic of the agent of HGE after PCR amplification

Sequence verification of the positive samples showed that our positive PCR products had 99.8-100% nucleotide identities with the HGE agent 16S rDNA sequences in GenBank. A neighbor-joining tree generated by PAUP supported the above conclusion. The sequences from our samples were most closely related to published HGE sequences and E. phagocytophila than any other species in the genus Ehrlichia (data not shown).
Discussion
This study confirmed the presence of the HGE agent in I. scapularis from the coastal regions of the southeastern United States, although the infected ticks were not distributed evenly among the collection sites. The overall prevalence rates of the HGE agent in southern I. scapularis were low compared with the prevalence rates of HGE in northeastern I. scapularis, which ranges from 4 to 53% (Telford et al. 1996, Schwartz et al. 1997, Varde et al. 1998, Curran et al. 2000). Interestingly, our study indicated clearly the small geographic foci of infections; for example, Jekyll Island where the infection rate was 20%. The higher prevalence rate at Jekyll Island may have indicated a more stable foci of infection as well as or the lower migration of infected reservoir hosts into areas in which the disease does not occur.
The low infection rates of HGE agent in ticks from the southeastern United States may be explained by several reasons. First, our observations may not reflect the true over all infection rates in southeast due to limited sampling size and locations. In contrast, more thorough investigations have occurred in the Northeast and Midwest because prior research suggests that the agent primarily occurs in these areas (McQuiston et al. 1999). Further survey for the infection rates in tick vectors and mammalian reservoir hosts is needed in the southeastern United States. Second, the differences in tick infection rates may be due to the differences in host association between northern and southern tick populations. The northern populations of I. scapularis are thought to feed primarily on mammals. In contrast, southern I. scapularis parasites a broader range of hosts including lizards, mammals, and birds (Keirans et al. 1996). Previous studies have shown that the causative HGE agent exists in the southeast in mammalian reservoir hosts (Magnarelli et al. 1999). With a higher number of nonmammalian hosts available, it may be less likely that I. scapularis will find a mammalian reservoir host that is infected with the HGE agent. Third, it is possible that the low prevalence rate of HGE agent in blacklegged ticks in the South is related to the low infection rate of HGE agent in mammals. However, the knowledge of infection rates of the HGE agent in mammals in the south is lacking.
Although we report relatively low prevalence rates of the causative HGE agent in South Carolina, Georgia, and Florida, there is an increased risk of exposure to humans. All but one of the sites collected are State Parks and/or popular tourist areas. Because HGE is an emerging disease, many people are unaware of the symptoms and risks. There is a need for both increased awareness and surveillance of ehrlichiosis, especially in the southeastern United States. Many states do not consider HGE or HME a notifiable disease (McQuiston et al. 1999), and given that the symptoms mimic those of influenza, it is highly likely that reported cases are a gross underestimate of true infections.
Acknowledgements
We are grateful to William L. Nicholson (CDC, Atlanta, GA) for supplying the HGE agent cell culture for our use as a positive control. We thank John E. Whitlock and Chris H. Gorham for laboratory assistance, Lance A. Durden and James E. Keirans for tick identification and helpful discussion, and Oscar J. Pung and Kenyon Mobley for reading the early draft. We extend our thanks to the two anonymous reviewers and the co-editor of this journal for manuscript improvements. This research was funded by grants from the Office of Research and Sponsored Services and the Faculty Research Committee at Georgia Southern University and partially by the NIH grant (AI)40729-01A2.
References Cited



