-
PDF
- Split View
-
Views
-
Cite
Cite
Palmira Guevara, Delia Pinto-Santini, Agustina Rojas, Gladys Crisante, Nestor AÑez, José Luis Ramirez, Green Fluorescent Protein-Tagged Leishmania in Phlebotomine Sand Flies , Journal of Medical Entomology, Volume 38, Issue 1, 1 January 2001, Pages 39–43, https://doi.org/10.1603/0022-2585-38.1.39
Close - Share Icon Share
Abstract
In this work we have used for the first time green fluorescent protein (GFP) tagged cells of the human parasite Leishmania donovani to observe its development in the gut of phlebotomine sand flies. Low numbers of GFP-tagged L. donovani were more easily detected than nontagged Leishmania, suggesting that GFP-tagged Leishmania could be used to efficiently study the biology of Leishmania in their vectors, and open the possibility of using nonaxenic flies. Using this method, we found that GFP-tagged L. donovani, the ethiological agent of Old World Kala-azar, were able to establish an infection within the gut of Lutzomyia species, which are vectors of New World Leishmania. The GFP-tagged parasites divide successfully in the gut of colonized and in wild caught Lu. longipalpis (Lutz & Neiva, 1912), Lu. ovallesis (Ortiz, 1952), and Lu. youngi (Feliciangeli & Murillo, 1985). In the case of Lulongipalpis the labeled parasite exhibited a normal anterior development as the one observed in its natural vector.
Phlebotomine sand flies serve as the vectors for human leishmaniasis caused by several species of Leishmania. The typical infection is cutaneous although the parasite may invade subcutaneous or deeper tissues causing disfigurement. Species of Leishmania causing this type of lesion belong to the subgenera Leishmania and Viannia, and are transmitted by sand flies of the genera Phlebotomus and Lutzomyia in the Old and New World, respectively. The most serious disease, Kala-azar, or visceral leishmaniasis involves organs such as liver, spleen, lymph nodes, and bone marrow of the mammalian hosts, including humans (Killick-Kendrick 1979). L. donovani is the causative agent of Kala-azar, whereas L. infantum (Lainson and Shaw 1987) causes visceral leishmaniasis of the Mediterranean basin, which mainly affects dogs. Both species are transmitted by sand flies of the genus Phlebotomus. In the New World there is controversy about the identity of the Leishmania species causing visceral leishmaniasis. Some authors suggest that L. infantum was introduced to the Americas by European settlers and their dogs during colonization, and that local Diptera of the genus Lutzomyia became vectors of the disease (Momen et al. 1993). Other authors have implicated autochthonous species such as L. chagasi (Lainson and Shaw 1987), and lately L. colombiensis (Kreutzer et al. 1991), as causative agents for leishmaniasis in the New World. Transmission of leishmaniasis occurs when infected female sand flies take a blood meal and the parasite transforms from amastigote to promastigote and multiplies within the sand fly gut. The production of infective Leishmania within the sand fly gut is thought to depend on a restricted association between Leishmania and sand fly species. This association has allowed the identification of specific developmental patterns of Leishmania in their sand fly vectors (Lainson and Shaw 1987). This includes suprapylarian (anterior gut) development for species of the subgenus Leishmania, and peripylarian (spread through the gut) development for Viannia subgenus species (Lainson and Shaw 1987, Molyneux and Killick-Kendrick 1987).
The use of green fluorescent protein (GFP) as molecular tool has proven very useful in many biological experiments (Tsien 1998). In this study we transfected L. donovani cells with molecular constructs expressing GFP, and then used GFP-tagged L. donovani cells to infect colonized Lu. longipalpis and wild caught specimens of Lu. ovallesi and Lu. youngi. The purpose of this experiment was to determine whether L. donovani, an Old World parasite, was able to divide and establish a suprapilaryan gut development in New World sand fly species.
Materials and Methods
GFP Construct.
Recombinant pXGFP5 (S65T)-R-promoter used to transfect Leishmania cells was constructed by harnessing GFP5 (S65T) version (Siemering et al. 1996) to the ribosomal gene promoter of L. amazonensis (Uliana et al. 1996) in tandem with the neomycin marker (G418) in the BamHI/XbaI of the polylinker in plasmid pX (Fig 1; LeBowitz et al. 1990).
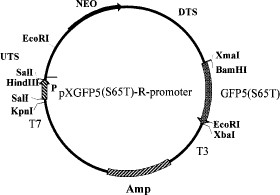
Plasmid pXGFP5 (S65T)-R-promoter restriction map. P, Leishmania amazonensis ribosomal promoter; UTS and DTS, upstream and downstream transcription sequences of L. major dehydrofolate reductase gene; NEO, coding region for neomycin resistance gene; GFP5 (S65T) Serine 65 threonine mutant of Aqueoria victoria Green fluorescence protein; Amp, ampicillin resistant gene from E. coli.
Cell Transfection.
L. donovani LSB-51.1 (MHOM/SD/00/Khartoum) promastigote cells were electroporated with 50 μg of pXGFP5 (S65T)-R-promoter construct as described by Kapler et al. (1990) with the following modifications. Two pulses of 450V, 125 μF at 10-s intervals were applied to 0.4 ml of 108 cells per milliliter in 0.4-cm cuvettes (Rudenko et al. 1990) followed by incubation for 24 h in M199 (without Neomycin). After 2 wk incubation in M199 in the presence of 50 μg/ml G418, the cultures were examined under a Zeiss (Jena, Germany) Axioscope DIC light microscope fitted with an HBO 50 fluorescent lamp (350-490 nm wavelength) and MC80 camera.
Feeding Lutzomyia with GFP-Leishmania.
Colonized Lu. longipalpis or wild-caught Lu. ovallesi and Lu. youngi were induced to feed on defibrinated rabbit blood containing GFP-tagged L. donovani promastigotes. The feeding system consisted of a hamster cheek pouch attached to a glass feeder warmed by a circulating water bath (Bastien 1990). The GFP-tagged promastigotes were diluted with inactivated rabbit blood to give an estimated 3.5 × 105 viable tagged promastigotes per ml. Each engorged fly was estimated to have ingested ≈100 GFP-tagged promastigotes. As a control, nonfluorescent L. donovani were used to infect sand flies. Fed sand flies were separated and maintained in an insect cage (20 by 20 by 20 cm) and provided with a solution of sucrose (50:50, vol:vol) with Geneticin G418 (7.5 μg/ml) and kept at 25oC. Flies were dissected 3, 4, or 5 d after infection, and the fresh alimentary canals were examined for the presence of parasites using light and fluorescence microscopy. A schematic view of a Phlebotomine sand fly is presented in Fig 2.
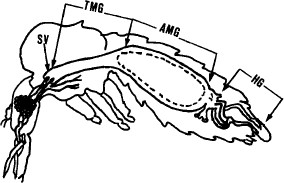
Transversal view of a Phlebotomine sand fly. SV, stomodeal valve; TMG, thoracic midgut; AMG, abdominal migut; HG, hindgut.
Results
Figure 3 A, shows cultured GFP-Leishmania promastigotes under fluorescent microscopy expressing GFP5 (S65T) gene from the ribosomal promoter. A strong green color was spread throughout the cytoplasm including the flagella.
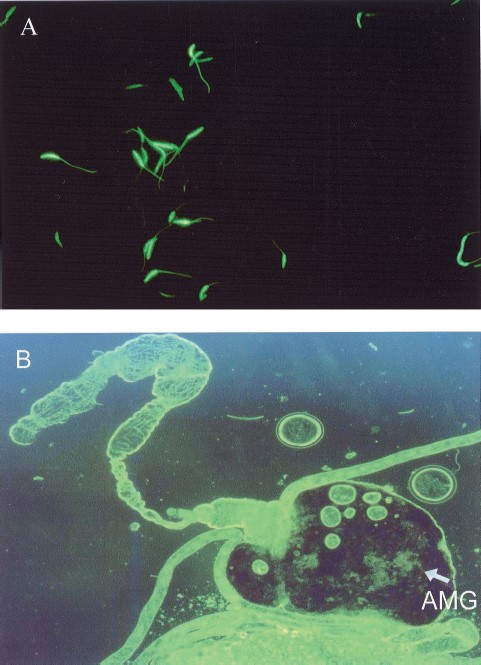
Fluorescence microscopy of GFP-Leishmania. (A) Promastigote cells in culture observed at 400× magnification. (B) View of the alimentary tract of Lu. longipalpis under a fluorescence microscope at 50× magnification, the arrow points to bundles of dividing GFP-Leishmania inside the abdominal midgut AMG.
GFP-tagged Leishmania parasites ingested by Lutzomyia sand flies were easily seen as typical promastigotes and dividing forms in the gut of the dissected, infected flies. On days 3 and 4 after infection the parasites were restricted to the abdominal midgut (Fig. 3B, 4A, 4B and Table 1). However, on day 5 in the case of Lu. longipalpis, green fluorescent promastigotes were observed in the thoracic midgut (Fig. 4 D) with most, clustered at the stomodeal valve (Fig. 4 C and D). In no case were fluorescent parasites observed colonizing the hindgut.
Localization of GFP-Leishmania within sandfly guts (species of Lutzomyia assayed, days after infection, and origin of flies).
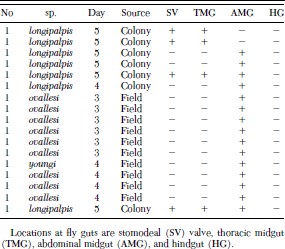
Localization of GFP-Leishmania within sandfly guts (species of Lutzomyia assayed, days after infection, and origin of flies).

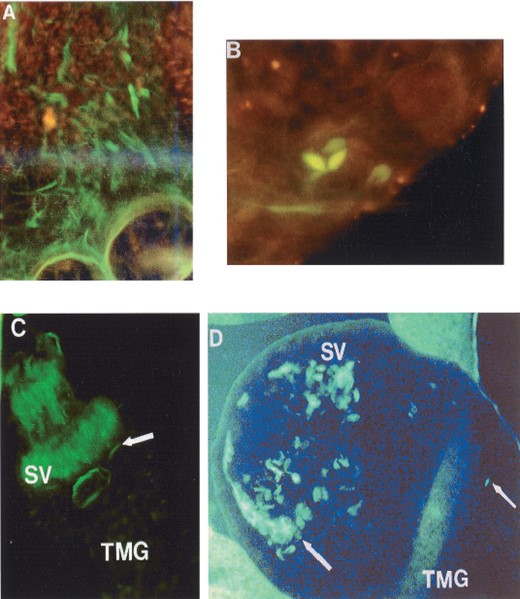
Fluorescence microscopy of GFP-Leishmania. (A and B) Dividing GFP-Leishmania promastigote inside the abdominal midgut (AMG), respectively, of Lu.longipalpis (400× magnification). (C and D) Clusters of GFP-Leishmania in the stomodeal valve (SV) and thoracic midgut (TMG) of Lu.longipalpis (400× magnification).
To compare the behavior of transfected and nontransfected Leishmania in the gut of experimentally infected sand flies, we infected colonized Lu. longipalpis with nonfluorescent parasites and demonstrated that the course of the infection was similar to their fluorescent counterparts. An expert technician using normal light microscopy was able to detect only two (11.7%) infected flies from a total of 17 lightly infected flies detected using the fluorescent microscope.
Discussion
Here we used GFP-tagged L. donovani cells to infect specimens of Lu. longipalpis from a closed laboratory colony; in addition, because no other organism present within the insect guts is detected by fluorescent microscopy, it was possible to use wild-caught specimens of Lu. ovallensi and Lu. youngi.
The use of GFP-tagged parasites made it possible to easily observe the development of L. donovani within the gut of unnatural sand fly hosts, including wild flies of the New World. The abundant number of fluorescent flagellates, including dividing forms and typical promastigotes, observed in the abdominal midgut, thoracic midgut, and around the stomodeal valve of infected Lu. longipalpis flies suggests normal development of L. donovani in a phlebotomine sand fly different from its original vectors in the Old World. In the case of Lu. ovallesi and Lu. youngi we did not score GFP-L. donovani cells in the thoracic midgut or around the stomodeal valve (Table 1). Confirmation of this result by examination a larger number of infected flies would imply that only in Lu. longipalpis viscerotropic Leishmania can complete their developmental cycle. This result is consistent with the fact that in New World Lu. longipalpis is the only demonstrated vector of visceral leishmaniasis.
The use of GFP-tagged Leishmania opens the way for further studies on the developmental patterns of Leishmania in their sand fly vectors, as well as to perform epidemiological studies such as determining vectorial capacity without the use of colonized sand flies, which are not an easily obtained product. This fact not only saves effort but also renders Leishmania researchers with conditions closer to the natural for the sand flies. Another advantage observed in our experiments was the possibility of following the infection in those flies with low numbers of flagellates in their guts. Thus, when common light microscope was used to detect this kind of infection, a trained technician missed >80% of the infected flies detected by fluorescent microscopy. Although this result cannot be compared with the more rigorous search done in field experimentation, it may contribute to explain the low indexes of naturally infected flies reported in endemic areas (Añez et al. 1994).
Considering that GFP-tagged L. donovani behaved in Lutzomyia sand flies in a way consistent with the developmental pattern of this parasite in its natural vector (Lainson and Shaw 1987), and that the presence of GFP did not affect this behavior, it is possible to conclude that despite the low numbers of fly used, Lu. longipalpis and other New World Lutzomyia species may potentially become vectors of Kala-azar, and support the view that American viscerotropic Leishmania may be of exotic origin and be transmitted by local sand flies, as has been suggested for L. infantum (Killick-Kendrick 1979, Momen et al. 1993). Finally, the use of this new tool may permit the study of the effect of co-infections in sand flies and also may demonstrate the presence of infective forms in atypical places as suggested by Killick-Kendrick (1979) regarding the possible invasion of salivary glands of the infected flies.
Acknowledgements
This work was supported by grants CONICIT S1-95000524, S1-95000889 and S1-98002681 to J.L.R., P6 and N.A., respectively. Thanks to J. LeBowitz for plasmid pX, J. Hseloff for GFP5(S65T) mutant, and K. Stuart for L. donovani isolate 51.1
References Cited



