-
PDF
- Split View
-
Views
-
Cite
Cite
Douglas D. Colwell, Micheal O’Connor, Scanning Electron Microscopy of Sarcophagid (Diptera) Larvae Recovered from a Case of Human Cutaneous Myiasis, Journal of Medical Entomology, Volume 37, Issue 6, 1 November 2000, Pages 854–859, https://doi.org/10.1603/0022-2585-37.6.854
Close - Share Icon Share
Abstract
Scanning electron microscope observations were made on second instars of unindentified sarcophagid maggots recovered from the foot of a 2-mo-old child. The child had a 3-d history of irritation and swelling of the left foot, and larvae were present in the skin on the plantar surfaces. The lesions were not furuncular, but erythema and a serous exudate were evident and swelling of the infested toes was noted. Larvae were removed manually from the lesions and fixed in formalin. External features, examined by scanning electron microscopy (SEM), were typical for muscomorph larvae and shared features common to other sarcophagids. Details of the cephalic, thoracic, and terminal abdominal sensory structures and variation in the structure of spines from various body regions are described. Comparisons of SEM observations on the cephalic, thoracic, and posterior abdominal regions with other published information did not yield information that allowed the accurate identification of the larvae from this infestation.
Cutaneous myiasis by larvae of the family Sarcophagidae (Diptera) is an infrequent occurrence in western Canada. In most reported cases, larvae were isolated in boil-like lesions, or furuncules, that usually are localized on the head, neck, and chest regions. Infants <1 yr of age were the usual victims. The flies responsible for this type of myiasis were most often members of the genus Wohlfahrtia (Ford 1936, Haufe and Nelson 1957). A singular case of aural myiasis in humans was reported in Alberta (Curtis 1956). The causative organism was identified as Neobellieria (=Sarcophaga) citellivora (Shewell), a species that causes a lethal cutaneous myiasis in ground squirrels (Michener 1993). The female flies of this species apparently larviposit on the unbroken skin of young ground squirrels. Lesions produced by the larvae rapidly enlarge to the point where the skin is breached. Once this occurs, death of the host usually results within 7 d of the initial infestation.
Other cases of myiasis ascribed to sarcophagids are of the intestinal variety or result from invasion of traumatic injuries (Ali-Khan and Ali-Khan 1974). Intestinal myiasis in the above case was the result of contaminated food, whereas in the traumatic injury case the flies were attracted to odors associated with the wound.
The current report describes a case of human cutaneous myiasis caused by sarcophagid larvae. No adults were reared from the specimens collected and therefore identification was not possible. Scanning electron microscopy was used to describe features of the second instars for possible species determination in the future.
Case Report
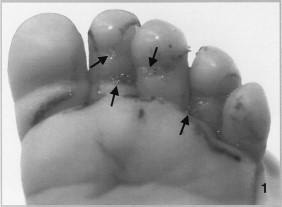
A 2-mo-old infant was admitted to the Medicine Hat Regional Hospital, Medicine Hat, Alberta, Canada, on 24 June with a 2-d history of irritability and a 24-h history of redness, swelling and oozing from the plantar surface of the left foot. The following day the foot was reexamined, and slight swelling was noted and numerous small maggots were observed in the swollen, reddened areas between the toes and at the joints (Fig. 1). The infant had been placed outdoors in the shade of a tree for several hours ≈3 d before the initial visit to the hospital. The infant’s feet normally were protected by clothing; however, in this instance none was present. No other risk factors could be identified. Twenty-three larvae were removed by forceps and another larva was removed when an occlusive dressing was placed over the foot.
Scanning Electron Microscope Observations
Larvae were fixed in10% neutral buffered formalin immediately after removal from the foot. For scanning electron microscope (SEM) observations, larvae were cut into anterior and posterior portions with the anterior portions composed of the cephalic segment, the three thoracic segments, and one or two abdominal segments. After an overnight wash in distilled water the specimens were dehydrated in a graded series of ethanol. Dehydrated specimens were critical point dried and mounted on aluminum stubs using colloidal silver paste. Mounted specimens were observed and photographed using an Hitachi S750 SEM (Tokyo, Japan).
Views of cephalic and thoracic segments of a representative larva, at a low magnification, showed the prominent mouth hooks, an array of cuticular ridges associated with the mouth (oral grooves) and well-developed sensilla (Fig. 2). Bands of spines were located on the anterior edges of both ventral and dorsal surfaces of the thoracic segments. The numbers of spines were higher on these surfaces than on the lateral surfaces. Prothoracic spiracles were present with typical digit-like protrusions.
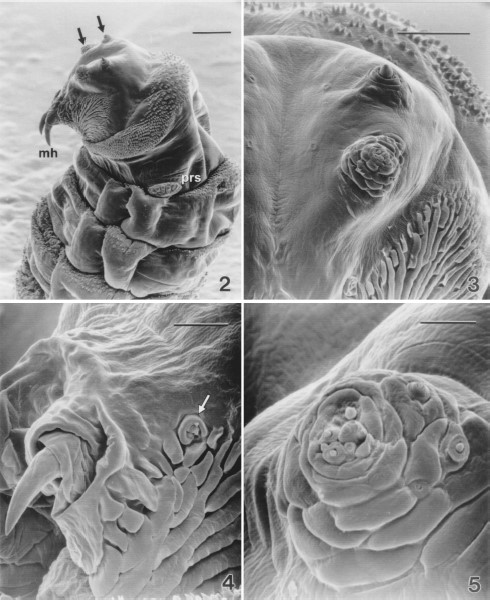
Low magnification scanning electron micrograph of the cephalic segment and the thoracic segments of a second-instar sarcophagid removed from between an infant's toes. (2) Cephalic sensilla (arrows), mouth hooks (mh), prothoracic spiracles (prs), and distribution of spines on these segments are evident (Bar = 100 μm). (3) Antennal (upper) and maxillary (lower) sensory complexes (Bar=50μm). (4) Detail of a mouth hook and the oral ridges. Mandibular sensory complex is evident (arrow) (Bar = 25 μm). (5) Maxillary sensory complex of a second-instar sarcophagid (Bar = 10 μm).
Details of the cephalic sensory complexes showed paired sensory pits that were seen on either side of the mid-line (arrows, Fig. 3). Lateral to the pits were the antenno-maxillary lobes each with two compound sensilla, the maxillary complex and the antennal complex. The latter were composed of a large central cone-shaped sensillum surrounded by a cuticular ring. Several small pit sensilla were associated with the ring.
Near the mouth hooks and the cuticular oral grooves were the mandibular sensory complexes (Fig. 4) composed of a C-shaped cuticular ridge surrounding three sensilla. A small peg sensillum was located near the opening of the cuticular ridge. A small pit sensillum was located within the opening of the ridge. The larger pit sensillum was surrounded by a cuticular ridge bearing two to three cuticular spines.
The maxillary sensory complex (Fig. 5) was composed of numerous papillae within a cental cluster surrounded by concentric cuticular ridges. Associated with the surrounding ridges were several additional sensory papillae.
The prothoracic spiracles were located at the posterior-lateral edge of the first thoracic segment. These were characterized by the presence of digit-like protrusions, each with a slit-like opening (Fig. 6). Each spiracle had seven to eight of these protrusions.
Cuticular sensillae were distributed across the ventral surface of thoracic and abdominal segments. The thoracic sensillae (Fig. 7) were typically of two types; trichoid sensilla, characterized by three setae and the papilla sensilla. There tended to be two types of papillae characterized by size of the papilla and the presence or absence of a cuticular ridge surrounding the papilla. Abdominal segments typically had papillae and pit sensillae.
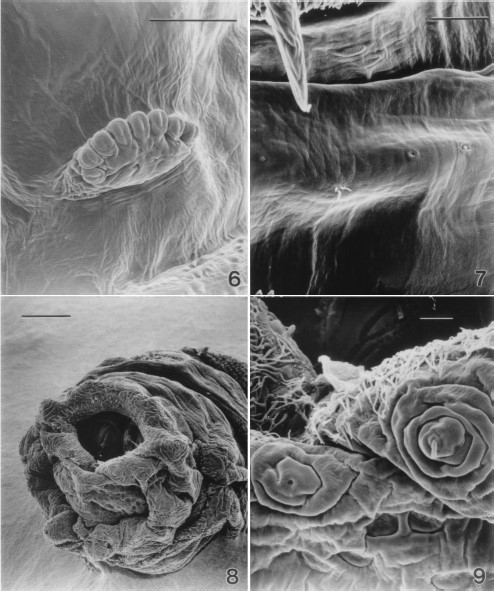
Scanning electron micrograph of the prothoracic spiracles of a second-instar sarcophagid removed from between an infant's toes. (6) Six spiracular openings (Bar = 50 μm). (7) Three cuticular sensilla on the ventral surface of a thoracic segment of a second-instar sarcophagid. From left to right, a trichoid sensillum with three setae, a campaniform, and a coeloconic sensillum are present (Bar = 25 μm). (8) Low magnification scanning electron micrograph of the terminal abdominal segments of a second-instar sarcophagid. Spiracular plates can be seen within the stigmal cavity, anal papillae are evident as are papillae with sensillae surrounding the stigmal cavity (Bar = 100 μm). (9) Terminal abdominal segment of a second-instar sarcophagid (Bar = 10 μm).
Present on the posterior abdominal segment were the large, paired anal papillae, numerous sensillae and the posterior spiracles (Fig. 8). The posterior spiracles were enclosed within the deep stigmal cavity. The edge of the stigmal cavity was adorned with a collar of cuticular filaments (Figs. 8 and 9). Sensillae were located at the apex of papillae distributed around the stigmal cavity. Two types of sensillae were noted; a pit type and a papillae type (Fig. 9).
Shape, density, and orientation of the cuticular spines varied along the length of the body and also among dorsal, ventral, and lateral surfaces. Spine densities tended to be greater on the dorsal and ventral surfaces than on the lateral surfaces. However, on the first thoracic segment the dorsal surface had fewer spines than the ventral surface (Figs. 2 and 10 and 11). In regions with lower spine densities the shape tended to be broader than in the other regions where densities were greater (Figs. 10–12). Spines on abdominal segments were longer and more robust than on the anterior segments. Generally, spine points were oriented caudally; however, on the next to the last abdominal segment, an additional band of spines, with a cephalad orientation, was found on the posterior edge (Fig. 13).
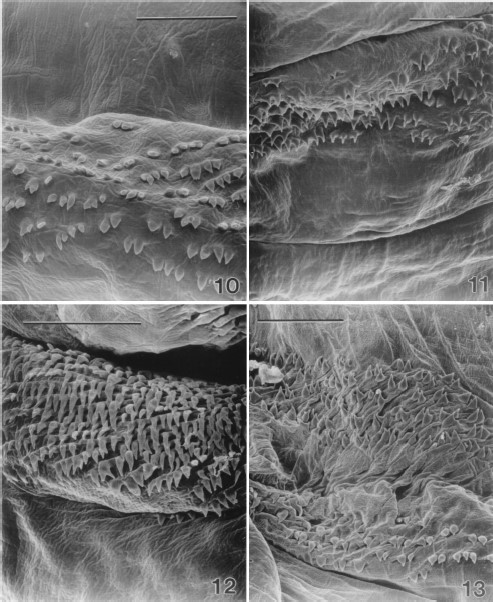
Scanning electron micrograph of spines on the dorsal surface of a thoracic segment of a second-instar sarcophagid removed from between an infant's toes. (10) Caudally oriented spines are sparse and taper broadly to a fine point. Most have a single point although occasional spines will have multiple points (Bar=50μm). (11) Spines on the ventral surface of a thoracic segment of a second-instar sarcophagid. Spines are arranged in bands at the anterior edge of each segment and are more dense than on the dorsal surface. Caudally oriented spines taper rapidly to a fine point. Most have a single point although occasional spines will have multiple points. (Bar=50μm). (12) Spines on the dorsal surface of an abdominal segment of a second-instar sarcophagid. Caudally oriented spines are more robust and longer than those on the anterior segments and are arranged in dense bands at the anterior edge of each segment. They taper rapidly to a fine point although occasional spines will have double points. (Bar=50μm). (13) Spines on the ventral surface of the seventh abdominal segment of a second-instar sarcophagid. Spines in the lower portion of the image are oriented cephalad and are located at the posterior edge of the segment. Spines in the upper portion of the image are oriented caudally and are on the anterior portion of the segment. (Bar= 50 μm).
Discussion
Adults were not reared from larvae collected from this infestation, and identification was not possible because several features of the infestation were unlike previous descriptions and there are no reliable keys for larval sarcophagids. The surface structures observed were compared with other published data so that keys for larvae can be developed eventually.
Cephalic sensillae were similar to those shown by Schmidt (1993) for third-instar Arachnidomyia aldrichii (Parker). The labial organ as reported by Schmidt (1993) was not observed in the current study, possibly because of the conformation of larvae at the time of fixation. The overall similarity of these structures makes it unlikely that they will be useful for species determination.
Structure of the prothoracic spiracles may prove useful in identification of the larvae. There was wide variation among the various genera of this family in the number of digit-like lobes that comprise the prothoracic spiracles (see Cantrell 1981), although each genus appeared distinct. Table 1 presents a comparison of the number of lobes on prothoracic spiracles reported from sarcophagids that parasitize mammalian hosts as well as several from species that parasitize insects or live in carrion. Unfortunately there must be a more detailed examination of this feature before it will be of much utility in species identification because there is overlap in the number of lobes among several species.
Summary of the number of lobes reported on the anterior spiracles of various sarcophagid species
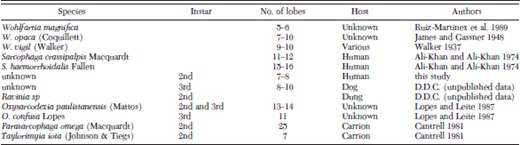
Summary of the number of lobes reported on the anterior spiracles of various sarcophagid species

Detailed examination of the spines has not been the focus of published reports, but there appears to be instances where spine structure varies between species (Leite and Lopes 1987). However, more detailed study of the specific groups of sarcophagids would be necessary to discern any patterns and to potentially develop useful keys. An evaluation of the influence of host and nutritional state on spine structure also would be necessary, particularly in light of a study of spine morphological variation in the calliphorid Cochliomyia hominovorax (Mangan and Welch 1990). These authors showed that spine structure was influenced by larval environment and therefore was not of any taxonomic or systematic value.
Features of the larvae in our study were compared with those of Wohlfahrtia magnifica (Shiner) Ruiz-Martinez et al. (1989, 1990). Despite superficial similarities, there was little basis for comparison because detailed examination of spines was not reported by the above authors. One conspicuous difference was in the number of lobes on the anterior spiracles (Table 1); W. magnifica having fewer than was observed for the larvae in this study.
Acknowledgements
The authors thank Christine Himsl-Rayner for expert technical assistance, Sheila Torgunrud and Erin Cadieu for preparation of the digital images, and clinical colleagues Gerald A. Vaz and William P. Taylor.
References Cited
Author notes
Department of Laboratory Medicine, Palliser Health Authority, Medicine Hat Regional Hospital, 666 - 5th Street S.W., Medicine Hat, AB, T1A 4H6, Canada.



