-
PDF
- Split View
-
Views
-
Cite
Cite
Kriangkrai Lerdthusnee, Nittaya Khlaimanee, Taweesak Monkanna, Noppadon Sangjun, Siriporn Mungviriya, Kenneth J. Linthicum, Stephen P. Frances, Thomas M. Kollars, Russell E. Coleman, Efficiency of Leptotrombidium Chiggers (Acari: Trombiculidae) at Transmitting Orientia tsutsugamushi to Laboratory Mice , Journal of Medical Entomology, Volume 39, Issue 3, 1 May 2002, Pages 521–525, https://doi.org/10.1603/0022-2585-39.3.521
Close - Share Icon Share
Abstract
Thirteen different laboratory colonies of Leptotrombidium chiggers [L. chiangraiensis Tanskul & Linthicum, L. deliense Walch and L. imphalum (Vercammen-Grandjean & Langston)] were evaluated for their ability to transmit Orientia tsutsugamushi (Hyashi) to mice. Of 4,372 transmission attempts using individual chiggers from all 13 colonies, 75% (n = 3,275) successfully infected mice. Transmission rates for the individual chigger colonies ranged from 7 to 80%. Increasing the number of chiggers that fed on a given mouse generally increased transmission rates. Transmission of O. tsutsugamushi to mice by different generations (F1-F11) of certain chigger colonies was stable; however, transmission rates varied greatly in other colonies. Transmission rates (both vertical and horizontal) of several L. chiangraiensis colonies and the L. deliense colony were the highest, suggesting that these colonies may be useful for the development of a chigger-challenge model that can be used to evaluate the efficacy of candidate scrub typhus vaccines or therapeutic agents in laboratory mice.
Scrub typhus is an acute febrile zoonotic disease resulting from infection with the gram-negative, intracellular bacteria Orientia (formerly Rickettsia) tsutsugamushi (Hyashi) (Tamura et al. 1995, Pradutkanchana et al. 1997). The disease is endemic in southeast Asia where it can account for 10–19% of patients admitted to hospitals with acute pyrexia of uncertain origin (Jatinandana 1971, Brown et al. 1984). Approximately 1 million cases occur each year and more than a billion people are at risk worldwide (Rosenberg 1997, Watt et al. 2000). Clinical manifestations of scrub typhus range from mild fever with few other symptoms to a fatal syndrome characterized by multiple-organ failure. Treatment with chloramphenicol or one of the tetracycline antibiotics typically results in rapid and complete recovery (Wisseman 1991). Scrub typhus is transmitted by several species of larval trombiculid mites, which are commonly known as chiggers (Tanskul et al. 1998). Because chiggers feed only once on a vertebrate host, transovarial transmission is thought to be the mechanism for maintenance of O. tsutsugamushi in the vector (Walker et al. 1975, Takahashi et al. 1994). Frances et al. (2000) demonstrated that O. tsutsugamushi could be transmitted to co-feeding mites and Takahashi et al. (1990) were able to infect chiggers fed on wild rodents; these observations suggested that transovarial transmission is not the sole method for maintenance of O. tsutsugamushi in vector populations. Traub et al. (1975) documented the first and only occurrence of horizontal transmission of O. tsustsugamushi.
Research on scrub typhus dwindled following the discovery that antibiotics rapidly cured scrub typhus (Rosenberg 1997). However, Watt et al. (1996) reported a form of scrub typhus in northern Thailand that responds poorly to conventional antibiotic therapy; subsequent in vitro and in vivo studies have supported this finding (Strickman et al. 1995, Watt et al. 1999). The presence of putative antibiotic-resistant strains of O. tsutsugamushi has led to a renewed interest in the identification of therapeutic agents that can be used to treat resistant strains (Watt et al. 1999). In addition, the U.S. military and the Republic of Korea have separate efforts to develop a scrub typhus vaccine.
One of the goals of the Armed Forces Research Institute of Medical Sciences (AFRIMS) in Bangkok, Thailand, is to develop a chigger-challenge animal model with which to evaluate the efficacy of candidate scrub typhus vaccines or therapeutic compounds. A total of 15 O. tsutsugamushi-infected chigger colonies is maintained at AFRIMS, including seven colonies of L. imphalum (Vercammen-Grandjean & Langston) (Li-1 to Li-7), five colonies of L. chiangraiensis Tanskul & Linthicum (Lc-1 to Lc-5), two colonies of L. fletcheri (Womersley & Heaslip) (Lf-1 and Lf-2), and a colony of L. deliense Walch (Ld-1). The goal of the study reported here was to evaluate the ability of mites from each colony (except L. fletcheri) to transmit O. tsutsugamushi.
Materials and Methods
Establishment of Chigger Colonies.
Rodents were routinely collected during studies on the epidemiology of scrub typhus in Chiangrai (Tanskul and Linthicum 1997, 1999; Tanskul et al. 1998) and Nonthaburi (Tanskul et al. 1994, Frances et al. 1999) provinces in Thailand. Chiggers collected from the rodents were maintained individually until it was determined if the host rodent was infected with O. tsutsugamushi. Once a rodent was confirmed as O. tsutsugamushi positive, chiggers collected from that rodent were reared individually and used to establish colonies (Tanskul et al. 1998). Samples of larvae of the F1 and subsequent generations were tested for the presence of O. tsutsugamushi to establish transovarial and filial infection rates. The seven L. imphalum and five L. chiangraiensis colonies originated from specimens collected in Chiangrai Province in northern Thailand, whereas the L. deliense colony originated from Nonthaburi Province in central Thailand. All procedures involving animals were approved by the AFRIMS Institutional Animal Care and Use Committee (IACUC).
Laboratory Transmission from Chiggers to Mice.
Chiggers from a given colony were placed individually or in pools of 2–50 on the ear of an anesthetized O. tsutsugamushi-naive ICR mouse. The mouse was placed in a restraining cage (10 cm long by 3 cm diameter) to prevent self-grooming. Food was provided ad libitum. The restraining cage was placed over a pan of water to prevent escape of infected chiggers. Mice were held in the restraining cages for 3–4 d, then anesthetized, removed from the restraining cage, and all chiggers removed from each mouse by hand. The mouse was then placed in a polycarbonate cage and checked daily for 30 d for signs and symptoms (rough coat, inactivity, inappetence) of O. tsutsugamushi infection. Ill mice were euthanized and peritoneal scrapings examined microscopically for the presence of O. tsutsugamushi by Giemsa stain and direct-fluorescent antibody technique (Roberts et al. 1975). Mice that did not develop clinical scrub typhus at the end of the 30 d observation period were euthanized and their sera evaluated for O. tsutsugamushi antibody using an indirect immunoperoxidase test (Tanskul et al. 1998). The determination of transmission of O. tsutsugamushi by chiggers was based on either the detection of O. tsutsugamushi in mouse peritoneal scrapings or presence of specific antibody against O. tsutsugamushi.
Effect of Chigger Generation on O. tsutsugamushi Transmission to Mice.
The 13 colonies of O. tsutsugamushi-infected chiggers evaluated in this study have been maintained at AFRIMS for from 3 to 11 generations. Transmission rates of O. tsutsugamushi by different generations of 12 of the colonies of Leptotrombidium chiggers were determined using the procedures described above.
Statistics.
We used chi-square analysis to determine if the number of mice infected with O. tsutsugamushi by a given colony of chiggers was significantly different from the number of mice infected by chiggers from all of the colonies. Chi-square analysis with Bonferroni adjustment and Fisher exact test with Bonferroni adjustment (where n < 8) were used to analyze generational differences and colony line differences within a species.
Results
Orientia tsutsugamushi transmission rates for individual chiggers from each of the 13 colonies are provided in Table 1; 74.9% (n = 3,275) of the 4,371 transmission attempts using chiggers from all 13 colonies successfully infected mice. Transmission rates in colonies Li-1, Li-3, Li-4, and Li-7 were not significantly different from transmission rates of all colonies. Transmission rates in colonies Ld-1, Lc-1, Lc-2, Lc-5, and Li-2 were significantly (χ2 test, P < 0.05) higher than rates with all colonies, while rates in colonies Lc-3, Lc-4, Li-5 and Li-6 were significantly lower (Table 1).
Transmission efficacy of 13 colonies of Leptotrombidium chiggers for O. tsutsugamushi
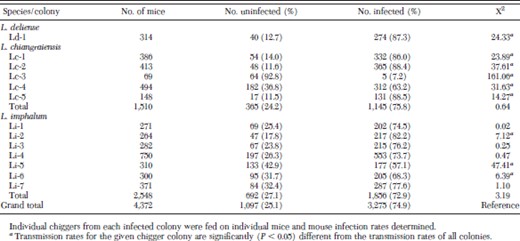
Transmission efficacy of 13 colonies of Leptotrombidium chiggers for O. tsutsugamushi

The effect of the number of chiggers placed on the host on O. tsutsugamushi transmission rates is shown in Table 2. A total of 225 feeds was conducted with two or more chiggers. In general, increasing the number of chiggers fed on a mouse increased transmission rates; however, in some instances increasing the number of chiggers actually yielded lower transmission rates (Table 2).
Effect of the number of Leptotrombidium chiggers placed on each mouse on transmission of O. tsutsugamushi
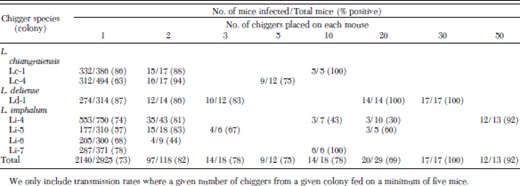
Effect of the number of Leptotrombidium chiggers placed on each mouse on transmission of O. tsutsugamushi

Transmission rates for different generations of 12 of the Leptotrombidium chigger colonies are provided in Table 3. Transmission rates for each of these colonies were never identical from one generation to the next; however, certain colonies (Lc-1, Lc-2, Lc-5, Ld-1, and Li-7) had transmission rates that remained relatively stable from generation to generation (Table 3). In contrast, colonies Lc-4, Li-1, Li-2, Li-3, and Li-4 had transmission rates that differed significantly (χ2 test with Bonferroni adjustment and Fisher exact test with Bonferroni adjustment, P < 0.05) from one generation to the next (Table 3).
Transmission of O. tsutsugamushi by different generations of 12 colonies of Leptotrombidium chiggers.
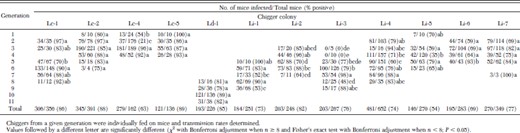
Transmission of O. tsutsugamushi by different generations of 12 colonies of Leptotrombidium chiggers.

Discussion
Pathogenicity of O. tsutsugamushi for laboratory mice is influenced by at least three factors: route of inoculation, antigenic strain, and natural resistance of the host (Groves and Kelly 1989). In contrast to other hematophagous arthropods such as mosquitoes, fleas and lice, chiggers do not normally ingest blood (Traub and Wisseman 1968). Following attachment to the vertebrate host, chiggers inject a digestive fluid that causes disintegration of cellular contents. Chiggers feed upon serum exudates and the products of cell lysis at the site of attachment (Traub and Wisseman 1968, Traub et al. 1975). Although the exact mechanism by which chiggers inoculate O. tsutsugamushi has not been documented, we assume that the pathogen is inoculated into the extra-cellular fluid rather than directly into the blood.
Although the role of chigger saliva on O. tsutsugamushi transmission has not been evaluated, the wealth of data from other vector/pathogen systems suggests that chigger transmission of O. tsutsugamushi may be significantly different than intravenous inoculation. These data suggest that chigger-challenge may be the best way to test infection of experimental animals with O. tsutsugamushi. In one of the few studies assessing transmission of scrub typhus to humans using laboratory-infected chiggers, Shirai et al. (1982b) suggested that vector-transmitted O. tsutsugamushi infection was the preferable method of assessing protection from scrub typhus.
In this study, efficiency of transmission of O. tsutsugamushi varied among the 13 colonies of infected chiggers, with rates ranging from 7 to 88%. Recently, Frances et al. (2001) reported that two lines of L. deliense varied in their ability to transmit O. tsutsugamushi to mice, with F1 and F2 generations transmitting the parasite less efficiently than did the parental generation. Tanskul et al. (1998) reported that 100% of naive mice became infected following infestation by O. tsutsugamushi-infected L. chiangraiensis and L. imphalum. Significantly lower infection rates were reported by Rapmund et al. (1972), who found that 7% (3/42) of L. arenicola Traub transmitted O. tsutsugamushi to mice, with similar rates reported for L. fletcheri (reported as L. akamushi) (Rapmund et al. 1969). In contrast, Shirai et al. (1982a) found that 85–99% of mice fed on by individual L. arenicola or L. fletcheri became infected. Shirai et al. (1982b) reported on the successful infection of three human volunteers using laboratory colonies of L. fletcheri and L. arenicola.
Since chigger transmission of O. tsutsugamushi to mice can be efficient (Shirai et al. 1982a, 1982b; Tanskul et al. 1998), transmission rates obtained using individual chiggers may reflect actual chigger infection rates. Although we did not assess chigger infection rates or efficiency of transovarial transmission in any of the 13 colonies, Tanskul et al. (1998) reported transovarial transmission rates of 93–100% in parental and F1 generations of L. chiangraiensis and L. imphalum. Similar transovarial transmission rates have been reported for other species (Rapmund et al. 1969, 1972; Traub and Wisseman 1968; Roberts and Robinson 1977; Takahashi et al. 1988). Our results show that transmission rates by different generations of chiggers are stable (colonies Lc-1, Lc-2, and Lc-5) or can vary (colonies Lc-4 and Li-3). Kollars et al. (2001) showed O. tsutsugamushi infection rates in eggs produced by individual chiggers from L. imphalum colonies Li-1 thru Li-7 ranged from 8 to 45%. These rates are significantly lower than our results would suggest. Clearly, it will be necessary to evaluate both vertical and horizontal transmission rates to determine whether the variation in chigger to mouse transmission we observed reflects actual chigger infection rates or results from variation in transmission efficiency.
Acknowledgements
Funding for this project was provided by the Military Infectious Diseases Research Program of the U.S. Army Medical Research and Materiel Command, Fort Detrick, MD.
In conducting the research described in this report, the investigators adhered to the "Guide for the Care and Use of Laboratory Animals" as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facilities are fully accredited by the American Association of Laboratory Animal Care.
References Cited



