-
PDF
- Split View
-
Views
-
Cite
Cite
Amaiur Esnaola, Aitor Larrañaga, Jorge González-Esteban, Arturo Elosegi, Joxerra Aihartza, Using biological traits to assess diet selection: the case of the Pyrenean Desman, Journal of Mammalogy, Volume 104, Issue 6, December 2023, Pages 1205–1215, https://doi.org/10.1093/jmammal/gyad061
Close - Share Icon Share
Abstract
Traditionally, researchers have assessed diet selection by comparing consumed versus available taxa. However, taxonomic assignment is probably irrelevant for predators, who likely base their selection on characteristics including prey size, habitat, or behavior. Here, we use an aquatic insectivore, the threatened Pyrenean Desman (Galemys pyrenaicus), as a model species to assess whether biological traits help unravel the criteria driving food and habitat preferences. We reanalyzed data from a previous taxonomy-based study of prey selection in two contrasting streams, one with excellent conservation status and the other affected by diversion for hydropower and forestry. Available and consumed prey were characterized according to nine biological traits, and diet selection was estimated by comparing availability—measured from Surber net samples, and consumption—analyzed by metabarcoding desman feces. Traits offered a biologically coherent image of diet and almost identical selection patterns in both streams, depicting a highly specialized rheophilic predator. Desmans positively selected prey with a preference for fast flow and boulder substrate, indicating their preferred riffle habitat. On the other hand, they positively selected prey with larger but not the largest potential size, living in the water column or the litter, and not inside sediments. They also chose agile prey, swimmers or prey attached to the substrate, prey with high body flexibility, and prey living exposed and clustered in groups. Overall, our results offer a picture of desman diet preference and point to biological traits as being better than taxonomic identity to describe the diet preference of consumers.
Tradicionalmente, los investigadores han estimado la selección de dieta comparando los taxones ingeridos con los disponibles. Sin embargo, la asignación taxonómica probablemente sea irrelevante para los depredadores, que probablemente basan su selección en características tales como el tamaño de presa, su hábitat o su comportamiento. Aquí, utilizamos como especie modelo un insectívoro acuático, el amenazado desmán ibérico (Galemys pyrenaicus), para evaluar si los rasgos biológicos ayudan a desvelar los criterios que rigen las preferencias de alimentos y de hábitat. Reanalizamos los datos de un estudio previo basado en taxonomía sobre la selección de presas de desmán en dos ríos diferentes, uno en excelente estado de conservación y el otro afectado por la derivación de agua para producción de energía hidroeléctrica y por actividades forestales. Se caracterizaron las presas disponibles y consumidas en función de nueve rasgos biológicos y se estimó la selección de dieta comparando la disponibilidad, medida a partir de muestras de redes Surber, y el consumo, determinado mediante metabarcoding de las heces del desmán. Los rasgos biológicos ofrecieron una imagen biológicamente coherente de la dieta y unos patrones de selección casi idénticos en ambos ríos, representando a un depredador reófilo altamente especializado. Los desmanes seleccionaron positivamente las presas con preferencia por corriente rápida y sustrato de bloques, indicando su preferencia de hábitat por los rápidos. Por otro lado, seleccionaron positivamente presas con tamaño potencial grande—pero no el mayor—, que vivían en la columna de agua o en la hojarasca, y no dentro de los sedimentos. También eligieron presas ágiles, presas nadadoras o adheridas al sustrato, presas con alta flexibilidad corporal y presas que viven expuestas y agrupadas. En general, nuestros resultados ofrecen una imagen de las preferencias tróficas del desmán y apuntan a que los rasgos biológicos de las presas describen las preferencias tróficas de los consumidores mejor que su identidad taxonómica.
The conservation of endangered species is often limited by a lack of detailed biological knowledge, including factors that determine distribution, habitat, or diet requirements (Morrison et al. 2006). Diet studies traditionally rely on direct observation or visual identification of prey remains in predator feces and guts. However, these methods are currently being replaced by molecular tools such as DNA metabarcoding (Taberlet et al. 1999; Sheppard and Harwood 2005; Bohmann et al. 2014). Whatever the technique, most studies assess diet from taxa composition. However, taxonomic identity may be irrelevant for consumers, who likely select prey depending on other characteristics including abundance, detectability, or nutritional quality (Symondson 2002; Almenar et al. 2013). Therefore, alternative trait-based approaches can better describe the relationships between species and their environments (Luck et al. 2012). For instance, trait-based studies revealed prey selection patterns where taxonomic studies depicted opportunistic diets (Spitz et al. 2014), or unveiled a broad functional trophic spectrum in species previously considered strict prey specialists (Arrizabalaga-Escudero et al. 2019). Trait studies do not lack limitations, such as incomplete data sets, context dependency, or intertrait correlations (e.g., Poff et al. 2006; Cesar and Frid 2012; Kremer et al. 2017). Nevertheless, some traits are likely more relevant for the predator than taxonomic identity (Schmitz 2017) and thus could reveal factors that drive predator foraging preferences.
The Pyrenean Desman (Galemys pyrenaicus, Eulipotyphla, Talpidae) is a semiaquatic insectivorous mammal that lives in cold, clean mountain streams—endemic to the northern Iberian Peninsula and the Pyrenees. However, its distribution area has shrunk severely during the last decades, and the IUCN currently lists it as Endangered (Quaglietta 2021). The desman strongly prefers fast-flowing riffles over slower runs or pools (Esnaola et al. 2018b), even when there are no abundance differences in benthic invertebrates—the principal prey for the desman—between habitats (Esnaola et al. 2021).
In this paper, we reanalyzed the results of a desman diet study (Esnaola et al. 2021), here comparing DNA metabarcoding of feces to prey availability in two contrasting streams. We examined desman preferences from a trait-based approach, which could offer essential information for effective conservation measures. Our study aimed at: (1) describing the biological traits of prey consumed by the Pyrenean Desman; (2) assessing trait-based diet selection; and (3) assessing the level of consistency in diet preferences, despite differences in available prey taxa, between streams of contrasting conservation status.
The desman is uniquely adapted to detect and capture underwater prey. First, it uses fast-flowing riffles to counteract buoyancy and stay attached to the riverbed with the least possible effort (Richard 1986). Second, it detects prey by haptic perception, through the vibrissae and Eimer’s organs on its trunk (Argaud 1944; Bauchot et al. 1973), and possibly also through smell, and exhaling and inhaling air bubbles, as do other aquatic insectivores (Catania 2006; Ivlev et al. 2013). These detection systems require close contact with prey, which probably elicits escape or protection responses. Therefore, we first hypothesized that desmans eat mostly prey living on the riverbed of fast-flowing riffles, with limited capacity to flee. Additionally, according to optimal foraging theory (Werner and Hall 1974), we hypothesized that the desman should prefer larger, more palatable, clustered prey that would be energetically more profitable. Furthermore, we also hypothesized that trait preferences would remain constant across streams, despite differences in diet availability when assessed by taxonomic composition (Esnaola et al. 2021).
Materials and Methods
Study area.
The data analyzed here were collected in 2016, in two streams in the northern Iberian Peninsula (the Basque Country). Elama is a second-order headwater stream draining an uninhabited basin of 1,415 ha over granite and schist in Artikutza valley. It has been managed as a strict Nature Reserve since 1919, having no extractive activity and is mostly covered by beech and oak forests (Castro 2009). The physical habitat is dominated by riffles and runs (45% of the total bottom surface each), whereas pools cover only 10% (Esnaola et al. 2018b). Leitzaran is a fourth-order stream draining a basin of 12,402 ha over limestone, slate, and sandstone. Contrasting with Elama, in the headwaters of Leitzaran there are two towns totaling 3,150 inhabitants. Further downstream the stream runs through a long, uninhabited valley of approximately 25 km, where forestry and hydropower diversion schemes are the only human activities (Izagirre et al. 2013). In Leitzaran, runs are dominant (60%), followed by riffles (30%), and pools (10%; Esnaola et al. 2018b). According to the EU Water Framework Directive (Council of the European Communities 2000), both streams have good ecological status and belong to the EU Natura 2000 network of protected areas (Council of the European Communities 1992). However, the absence of human activities results in better conservation status in Elama.
Sampling was conducted on the same stretches and periods as with the radiotracking work published previously (Esnaola et al. 2018b) on a 4-km section of Elama (from 43°12ʹ40″N, 1°48ʹ36″W to 43°11ʹ14″N, 1°48ʹ4″W; mean altitude, 330 m; mean width 7.08 m) and on a 10-km section of Leitzaran (from 43°8ʹ57″N, 1°57ʹ26″W to 43°6ʹ55″N, 1°56ʹ9″W; mean altitude, 290 m; mean width, 12.42 m). Section lengths differed because of the greater difficulty in trapping desmans in Leitzaran.
Prey availability.
As explained in Esnaola et al. (2021), aquatic macroinvertebrates were sampled with a Surber net (30 cm × 30 cm; 1-mm mesh) across three habitat types (Overton et al. 1997): (1) “riffles” with fast, turbulent water, uneven surface level, and white water; (2) “runs” with close to laminar flow and even depth; and (3) “pools” with the slow flow on riverbed depressions.
Ten samples were collected per habitat and stream in randomly selected locations, resulting in 60 samples. These were stored in plastic jars and preserved with 70% ethanol until identification. Macroinvertebrates were sieved with a 2-mm mesh, sorted, counted, and identified under a dissecting microscope (Tachet et al. 2002). Taxonomic resolution reached the genus level for most taxa, except for some Diptera, Coleoptera, Oligochaeta, Hirudinidae, and Nematoda, which were identified at the family or order levels. A total of 59 invertebrate taxa were identified—40 in Elama and 38 in Leitzaran (Esnaola et al. 2021). We computed the density of each taxon per stream by weighing density (individuals m2) at each habitat (riffle, run, or pool) by the proportion of streambed covered by a habitat at a stream. Then, we assigned biological traits to each taxon (see below). Trait availability at each stream was expressed in weighted percentage of occurrence (wPOO, the percentage of occurrence for each food item in the total data set, weighted by the total number of prey found in a given sample; Andriollo et al. 2019; Deagle et al. 2019).
Desman diet.
Esnaola et al. (2021) assessed desman diet by metabarcoding feces collected in Elama and Leitzaran streams in September and October 2016, using artificial shelters designed for this species (González-Esteban et al. 2018). A total of 188 droppings (94 per stream) were collected and stored in tubes containing 98% ethanol and frozen at −80°C. DNA was extracted, then PCR of the COI barcode region was amplified and sequenced employing high-throughput sequencing as described by Esnaola et al. (2018a) (Supplementary Data SD1). Metabarcoding yielded 75 invertebrate taxa in the desman diet (Esnaola et al. 2021). We assigned biological traits to the consumed taxa (next section). Results were expressed in wPOO (the percentage of occurrence for each food item in the total data set, weighed, in this case, by the total number of prey found in a given dropping). Although measuring the diet in wPOO can overestimate prey consumed often in small quantities and underestimate others consumed rarely but in large quantities (Deagle et al. 2019), the high number of samples collected reduced this bias (Mata et al. 2019).
Trait assignation.
We assigned biological traits to available and consumed prey taxa based on De Crespin and Usseglio-Polatera (2002) and Sánchez-Hernández et al. (2014). We examined nine traits potentially affecting desman selection (Table 1), each trait having 2–5 categories with affinity scores (from 0 = low affinity, to 5 = high affinity) expressed using fuzzy coding (Supplementary Data SD2). The fuzzy coding procedure positively describes the affinity of taxa for categories of a given variable (i.e., trait) in such a way that, instead of linking a taxon with a single (most preferred) trait category, it gives a range of affinities for different categories (Chevenet et al. 1994). For instance, the rheophilic mayfly Epeorus is assigned an affinity of 3 for fast, 1 for moderate, and 0 for slow current velocity. These traits were selected to assess the influence of invertebrate spatial proximity, accessibility, conspicuousness, and handling efficiency in desman prey selection. Two of these (current velocity and substrate) indicate the type of habitat within the stream (riffle, pool, or run). Current velocity describes a preference for slow, moderate, or fast water. Substrate preference describes an association with different types of substrata (blocks, gravel, etc.). The remainder of the traits yield information on other biologically important prey characteristics. Vertical location describes whether a taxon lives inside the substrate, on the substrate, or in the free-flowing water. Flow exposure describes whether invertebrates live exposed to or protected from the flow. Type of mobility/attachment to the substrate describes whether they are attached to the substratum, crawl on it, or swim. Agility describes the capacity to quickly return to the bottom after being entrained in the drift. Aggregation tendency depicts the tendency to live in groups. Potential size describes a maximal body size. Finally, body flexibility depicts the ability to be twisted and is linked, among others, to the absence of a protecting case or shell.
Traits and their categories used to characterize desman prey. Trait categories, first described by De Crespin and Usseglio-Polatera (2002), have been renamed and reclassified here. Original categories according to Sánchez-Hernández (2014).
| Trait . | Abbreviation . | Categories . | Sánchez-Hernández’s original categories . |
|---|---|---|---|
| Current velocity | CUV1 | Slow | Still/slow (0–25 cm/s) |
| CUV2 | Moderate | Moderate (25–75 cm/s) | |
| CUV3 | Fast | Fast (>75 cm/s) | |
| Substrate | SUS1 | Blocks | Blocks (>256 mm) |
| SUS2 | Gravel-cobble | Fine gravel/gravel-cobble (2–256 mm) | |
| SUS3 | Fine sediment | Silt (0.001–0.2 mm) + sand-silt (0.2–2 mm) + mud | |
| SUS4 | Macrophytes-roots | Bryophytes + other macrophytes + roots | |
| SUS5 | Litter | Litter, organic detritus | |
| Vertical location | DEP1 | Hyporheic | Hyporheic “burrower” + hyporheic “interstitial” |
| DEP2 | Epibenthic | Epibenthic erosional + epibenthic depositional | |
| DEP3 | Water column | Water column | |
| Flow exposure | FLE1 | Protected | Protected |
| FLE2 | Exposed | Exposed | |
| Mobility/attachment to substrate | MAS1 | Swimmer | Swimmer |
| MAS2 | Crawler | Crawler/walker + crawler/slider | |
| MAS3 | Attached | Permanently attached + temporarily attached | |
| Agility | AGI1 | None | None |
| AGI2 | Weak | Weak | |
| AGI3 | High | High | |
| Aggregation tendency | AGT1 | High | High |
| AGT2 | Weak | Weak | |
| Potential size | POS1 | ≤2 mm | ≤2 mm |
| POS2 | 2–8 mm | >2–4 mm + >4–8 mm | |
| POS3 | 8–32 mm | >8–16 mm + >16–32 mm | |
| POS4 | >32 mm | >32 mm | |
| Body flexibility (including cases/tubes) | BOF1 | None | None (<10°) |
| BOF2 | Weak | Weak (10–45°) | |
| BOF3 | High | High (>45°) |
| Trait . | Abbreviation . | Categories . | Sánchez-Hernández’s original categories . |
|---|---|---|---|
| Current velocity | CUV1 | Slow | Still/slow (0–25 cm/s) |
| CUV2 | Moderate | Moderate (25–75 cm/s) | |
| CUV3 | Fast | Fast (>75 cm/s) | |
| Substrate | SUS1 | Blocks | Blocks (>256 mm) |
| SUS2 | Gravel-cobble | Fine gravel/gravel-cobble (2–256 mm) | |
| SUS3 | Fine sediment | Silt (0.001–0.2 mm) + sand-silt (0.2–2 mm) + mud | |
| SUS4 | Macrophytes-roots | Bryophytes + other macrophytes + roots | |
| SUS5 | Litter | Litter, organic detritus | |
| Vertical location | DEP1 | Hyporheic | Hyporheic “burrower” + hyporheic “interstitial” |
| DEP2 | Epibenthic | Epibenthic erosional + epibenthic depositional | |
| DEP3 | Water column | Water column | |
| Flow exposure | FLE1 | Protected | Protected |
| FLE2 | Exposed | Exposed | |
| Mobility/attachment to substrate | MAS1 | Swimmer | Swimmer |
| MAS2 | Crawler | Crawler/walker + crawler/slider | |
| MAS3 | Attached | Permanently attached + temporarily attached | |
| Agility | AGI1 | None | None |
| AGI2 | Weak | Weak | |
| AGI3 | High | High | |
| Aggregation tendency | AGT1 | High | High |
| AGT2 | Weak | Weak | |
| Potential size | POS1 | ≤2 mm | ≤2 mm |
| POS2 | 2–8 mm | >2–4 mm + >4–8 mm | |
| POS3 | 8–32 mm | >8–16 mm + >16–32 mm | |
| POS4 | >32 mm | >32 mm | |
| Body flexibility (including cases/tubes) | BOF1 | None | None (<10°) |
| BOF2 | Weak | Weak (10–45°) | |
| BOF3 | High | High (>45°) |
Traits and their categories used to characterize desman prey. Trait categories, first described by De Crespin and Usseglio-Polatera (2002), have been renamed and reclassified here. Original categories according to Sánchez-Hernández (2014).
| Trait . | Abbreviation . | Categories . | Sánchez-Hernández’s original categories . |
|---|---|---|---|
| Current velocity | CUV1 | Slow | Still/slow (0–25 cm/s) |
| CUV2 | Moderate | Moderate (25–75 cm/s) | |
| CUV3 | Fast | Fast (>75 cm/s) | |
| Substrate | SUS1 | Blocks | Blocks (>256 mm) |
| SUS2 | Gravel-cobble | Fine gravel/gravel-cobble (2–256 mm) | |
| SUS3 | Fine sediment | Silt (0.001–0.2 mm) + sand-silt (0.2–2 mm) + mud | |
| SUS4 | Macrophytes-roots | Bryophytes + other macrophytes + roots | |
| SUS5 | Litter | Litter, organic detritus | |
| Vertical location | DEP1 | Hyporheic | Hyporheic “burrower” + hyporheic “interstitial” |
| DEP2 | Epibenthic | Epibenthic erosional + epibenthic depositional | |
| DEP3 | Water column | Water column | |
| Flow exposure | FLE1 | Protected | Protected |
| FLE2 | Exposed | Exposed | |
| Mobility/attachment to substrate | MAS1 | Swimmer | Swimmer |
| MAS2 | Crawler | Crawler/walker + crawler/slider | |
| MAS3 | Attached | Permanently attached + temporarily attached | |
| Agility | AGI1 | None | None |
| AGI2 | Weak | Weak | |
| AGI3 | High | High | |
| Aggregation tendency | AGT1 | High | High |
| AGT2 | Weak | Weak | |
| Potential size | POS1 | ≤2 mm | ≤2 mm |
| POS2 | 2–8 mm | >2–4 mm + >4–8 mm | |
| POS3 | 8–32 mm | >8–16 mm + >16–32 mm | |
| POS4 | >32 mm | >32 mm | |
| Body flexibility (including cases/tubes) | BOF1 | None | None (<10°) |
| BOF2 | Weak | Weak (10–45°) | |
| BOF3 | High | High (>45°) |
| Trait . | Abbreviation . | Categories . | Sánchez-Hernández’s original categories . |
|---|---|---|---|
| Current velocity | CUV1 | Slow | Still/slow (0–25 cm/s) |
| CUV2 | Moderate | Moderate (25–75 cm/s) | |
| CUV3 | Fast | Fast (>75 cm/s) | |
| Substrate | SUS1 | Blocks | Blocks (>256 mm) |
| SUS2 | Gravel-cobble | Fine gravel/gravel-cobble (2–256 mm) | |
| SUS3 | Fine sediment | Silt (0.001–0.2 mm) + sand-silt (0.2–2 mm) + mud | |
| SUS4 | Macrophytes-roots | Bryophytes + other macrophytes + roots | |
| SUS5 | Litter | Litter, organic detritus | |
| Vertical location | DEP1 | Hyporheic | Hyporheic “burrower” + hyporheic “interstitial” |
| DEP2 | Epibenthic | Epibenthic erosional + epibenthic depositional | |
| DEP3 | Water column | Water column | |
| Flow exposure | FLE1 | Protected | Protected |
| FLE2 | Exposed | Exposed | |
| Mobility/attachment to substrate | MAS1 | Swimmer | Swimmer |
| MAS2 | Crawler | Crawler/walker + crawler/slider | |
| MAS3 | Attached | Permanently attached + temporarily attached | |
| Agility | AGI1 | None | None |
| AGI2 | Weak | Weak | |
| AGI3 | High | High | |
| Aggregation tendency | AGT1 | High | High |
| AGT2 | Weak | Weak | |
| Potential size | POS1 | ≤2 mm | ≤2 mm |
| POS2 | 2–8 mm | >2–4 mm + >4–8 mm | |
| POS3 | 8–32 mm | >8–16 mm + >16–32 mm | |
| POS4 | >32 mm | >32 mm | |
| Body flexibility (including cases/tubes) | BOF1 | None | None (<10°) |
| BOF2 | Weak | Weak (10–45°) | |
| BOF3 | High | High (>45°) |
We transformed affinities into relative affinities by dividing each value by the sum of affinities for each trait of each taxon. For taxonomic levels with no trait data (e.g., Chironomidae), the average value of all the lower taxonomic levels was computed (Sánchez-Hernández 2014; Supplementary Data SD2). Taxa with no data (annelids, Lepidoptera, and some terrestrial Coleoptera, all rarely consumed; Esnaola et al. 2021) were excluded from the analyses (Supplementary Data SD2).
Prey selection.
With wPOO in each stream, we built “taxa for stream × traits” arrays for availability (dimension = 140 × 28) and diet (dimension = 98 × 28). These values were summed and transformed into relative proportions for each stream (building “stream × traits” arrays for availability and diet; dimension = 2 × 28). Differences in trait category values between streams for prey availability and diet were analyzed with Pearson’s Chi-squared tests (function “chisq.test” of the R package; R Core Team 2014).
A multivariate analysis was performed looking for similarities between diet and availability data, and between streams and among habitats, using a “method-category × traits” array (where method-categories were Elama diet, Leitzaran diet, Elama riffle availability, Elama run availability, Elama pool availability, Leitzaran riffle availability, Leitzaran run availability, and Leitzaran pool availability; dimension = 8 × 28). In this case, availability data (in wPOO) were weighed by the relative habitat surface of each habitat in the studied section of each stream. As a multivariate analysis, a fuzzy principal component analysis (FPCA; using the “prep.fuzzy.var” and “dudi.fpca” functions from the ade4 package in R; Dray and Dufour 2007) was performed, taking into account all categories of all traits together. FPCA is a robust modification of PCA (Cundari et al. 2002) for fuzzy coded data. A redundancy analysis (RDA, with a Hellinger distance matrix and the functions “rda” and “anova.cca” from the vegan package in R; Oksanen et al. 2019) was used to assess the significance of stream, habitat, and diet versus availability in explaining the variation of trait categories. We summarize the relative importance of each trait, explaining the overall differences between the two streams, the three habitats, and between diet and availability through inertia values.
Desman prey selection was estimated from diet-to-availability ratios. A log10 (diet/availability) ratio was calculated for each trait and sample category, dividing the diet value of each stream by each of the availability categories. Availability data were also weighed by available habitat surfaces. To deal with zeros in availability when computing the ratios, we added a constant to every value (half of the minimum value in the database) before calculating the ratio. Finally, average ratio and confidence intervals were calculated for each stream. These ratios served to test whether desmans select (positively or negatively) specific prey trait categories. Pearson’s Chi-squared test (function “chisq.test” of the stats package for R; R Core Team 2014) was performed to analyze whether the selection of trait categories was stream dependent. The significance level of all tests was set at P < 0.05. All analyses were performed using R 3.4.3 (R Core Team 2014).
Results
Prey availability.
Prey availability was very similar in both streams when expressed in biological traits. For current velocity trait categories, wPOO was highest for slow (39.6%), followed by moderate (36.6%) and fast (23.8%). Most invertebrates were associated with gravel-cobble (32.6%), followed by macrophytes-roots (29.4%), blocks (15.9%), fine sediment (15.3%), and litter (6.7%). According to vertical location, the wPOO was highest (72.9% on average) for epibenthic, followed by hyporheic (18.9%), and water column invertebrates (8.1%; Fig. 1). The protected category in the flow exposure trait averaged 59.4%. Regarding mobility/attachment, crawlers averaged 78.7%, followed by swimmer (11.1%), and attached (10.2%) categories. Most invertebrates (62.1% on average) had weak agility, followed by high (28.9%), and none (8.9%) categories. Invertebrates with high aggregation tendency averaged 58.3%. In terms of potential size, the 8–32 mm category averaged 69.4%, being followed by 2–8 mm (24.3%), >32 mm (6.0%), and ≤2 mm (0.3%). Finally, concerning body flexibility, invertebrates with high flexibility had the highest wPOO (36.4%), followed by weak (34.7%), and none (28.9%). Differences in prey availability between streams were not significant for any trait (P > 0.05).
![Availability (in weighted percentage of occurrence [wPOO]) of the main biological traits in the Elama (best conservation status) and Leitzaran (affected by hydropower) streams.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jmammal/104/6/10.1093_jmammal_gyad061/1/m_gyad061_fig1.jpeg?Expires=1747995155&Signature=IQ8BPlkRQ~uM~eIpjafPQXwFGdg2-pk4pPN9N~rrIfr-DuFkAGUEI2b9D0mfQD~S17kABIZfk2KP6g6XX8JGlA4P2vmTUfvd2q~wnWFwryvDBvfXTyRaSiczz47JJ-RxGlp1xvXs~mZMYzwL1r8SB74gpPAoSwCwP~9oCgvF-9OzuLIpQzh9ES-X3n6EHMu8y1moCc5pLalpFktdQxp-8sN93sx9nEN~2aqrrunraGFiU~gAd0-elW1y7sfpxNCB-geT--XLSKACAxEdBfRPFfNKQx8orsEyYtCfcVRjddL5aPyIYincWohu2WfPZrBGIlQGCFSWr-g03gVeOFaF8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Availability (in weighted percentage of occurrence [wPOO]) of the main biological traits in the Elama (best conservation status) and Leitzaran (affected by hydropower) streams.
Desman diet.
The relative contribution of the various trait categories to the desman diet was almost identical in both streams (Fig. 2), as differences between streams were not significant for any of the traits (P > 0.05). Differences were negligible among traits related to current velocity (moderate 35.2%, slow 34.4%, fast 30.4%). Regarding the substrate categories, desmans mainly consumed prey associated with gravel-cobble (31.4%) and macrophytes-roots (29.8%), followed by blocks (19.6%), fine sediment (11.0%), and litter (8.3%). Regarding vertical location preference, desmans consumed species predominantly associated with epibenthic habitats (73.5% on average), followed by hyporheic (15.0%), and water column (11.5%). The flow exposure trait showed minor differences (53.7% for exposed versus 46.3% for protected); whereas, regarding mobility/attachment to the substrate, desmans more frequently consumed prey with a crawler lifestyle (70.1%) than belonging to the rest of the categories. Prey with no agility were the least frequent by a large margin (4.1% versus 52.5% for weak and 43.4% for high agility, respectively). Prey items with high aggregation tendencies were more frequent in the diet (64.8%) than those with a weak tendency (35.2%). In terms of potential size, desmans mainly consumed prey with medium-sized affinity (8–32 mm = 64.8%, 2–8 mm = 33.2%), very rarely consumed prey with potential size affinity > 32 mm (2.0%), and rarely prey with potential size affinity ≤ 2 mm. Finally, the body flexibility trait showed the highest relative contribution for high flexibility (48.7%), followed by weak (35.0%), and none (16.3%) categories.
![Relative contribution (in weighted percentage of occurrence [wPOO]) of the main biological traits to the desman diet in Elama and Leitzaran streams.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jmammal/104/6/10.1093_jmammal_gyad061/1/m_gyad061_fig2.jpeg?Expires=1747995155&Signature=KXHyt9jKNIDg5TjZERoeP-T2xjawXON2ysQUhPoN8Mu0NuE5gxgtO~~~PqawsbLlMvu5MyAI2dNkXqrrgZqQZ8C0VJacndqzzwqR93h4Qi7GoHYmYZhXUmmBexr4qOOlXBgXMtgegI6LLbgTWrNx3ub5y7rmFV3i9KEvSCZWtULvdZZ9xEfKorAcYxuGVs1aOioWHbs6mWuDFMTaSbUTZT1DT6yTSqJcsnx582roEmP9205ytuQcuSJaKLy82QjIzqdlrTBeTnZPtFjpt~MScV0eU55LLL7HNybZ--ugJnBGHADacyCEPzzpLWC4CTveJSVeqB3odBT3AtXDZHlAwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Relative contribution (in weighted percentage of occurrence [wPOO]) of the main biological traits to the desman diet in Elama and Leitzaran streams.
Trait selection.
In the fuzzy PCA performed with all data (diet and availability; Fig. 3), body flexibility, agility, aggregation tendency, flow exposure, and potential size showed the highest inertia values (>0.003; Supplementary Data SD3), the first axis explained the 63.8% of the total variance, and the second 24.4%. Although overall availability in the two streams did not show large differences, the multivariate approach showed that diets were even more similar. The FPCA mostly showed differences between streams (Axis 1) and habitats (Axis 2). Most notably, regarding the desman diet, both streams had similar loadings in the first two FPCA axes, closer to the availability in riffles and furthest from the availability in pools. As for availability, habitat points were somewhat further apart for Elama than for Leitzaran, showing that differences among habitats were slightly larger in the best-preserved stream. An RDA showed differences to be statistically significant between streams (F1= 6.72, P = 0.003; 36.4% of the variance explained) and between diet and availability (F2 = 4.28, P = 0.042; 23.2%), but not among habitats (F1 = 2.23, P = 0.131; 24.1%; Supplementary Data SD4).
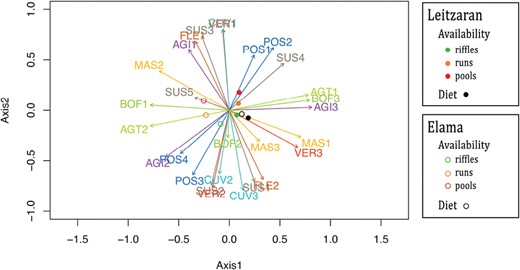
Fuzzy principal component analysis (FPCA) of the invertebrate traits in diet and availability in the stream. Arrows represent trait categories (see abbreviations in Table 1). Each trait is represented in a different color.
Diet-to-availability ratios showed that desmans positively selected prey associated with the areas of fast water (>75 cm/s), blocks, and litter, living in the water column, and exposed to water flow (Fig. 4). They positively selected either swimmers or prey with mechanisms to attach to the substrate, highly agile, with a strong tendency to form aggregations, 2–8 mm long, and with high body flexibility. On the other hand, desmans negatively selected prey associated with fine substrates and areas protected from the water flow (Fig. 4), crawlers, with a weak tendency to aggregate, smaller than 2 mm, longer than 32 mm, and without body flexibility. Despite subtle differences in preferences, the general selection pattern was the same for both streams (P > 0.05).
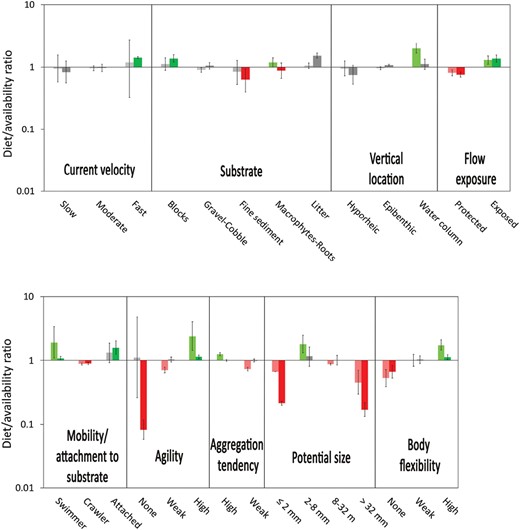
Selection of trait categories: positive, negative, and no selection. Colors are lighter for Elama and darker for Leitzaran streams.
Discussion
Our prey trait analysis was aimed at understanding the food and habitat preferences of a freshwater predator, the Pyrenean Desman. Our results help to explain its foraging decisions better than the hitherto used taxonomic identity of their prey and give important clues for managing this endangered species. Although prey selection does not depend on any single trait, consistency of the trait sets between two separate streams mirrors the environmental requirements of the desman.
Most studies have described desmans as generalist foragers consuming a variety of prey taxa (e.g., Biffi et al. 2017a, 2017b; Hawlitschek et al. 2018). Nevertheless, taxonomy-based descriptions of diet are hard to compare and interpret. For instance, in the Ulla stream, Santamarina (1992, 1993) found Trichoptera to be the prey most frequently consumed by the desman, whereas Santamarina and Guitian (1988) and Castién and Gosálbez (1995) found Ephemeroptera to be more frequent in the diet. It is hard to interpret whether these differences reflect temporal changes in food availability or other differences (e.g., methodological). Similarly, our previous taxonomic approach in the Elama and Leitzaran streams (Esnaola et al. 2021) also found some differences in consumed taxa, although these were hard to interpret.
Notably, our trait-based diet description depicts the Pyrenean Desman not as a generalist but as a predator with a high preference for riffles, showing a notably similar diet in both study areas. The trait-based diet descriptions and the multivariate analyses performed (FPCA) were consistent in both streams, thus indicating a clear pattern in the desman diet unaffected by the conservation status of the streams. The desman stands out as a habitat-specialist rheophilic mammal that mostly consumes riffle-dwelling prey. Evolutionarily, the desman is a mole separated from its terrestrial relatives about 37 Ma ago (Douady and Douzery 2003) that substituted an ancestral terrestrial excavator for an aquatic rheophilic lifestyle without appreciable change in its sensorial organs.
Our results also underscore the importance of the methodology used to characterize prey (taxonomy versus biological traits)—the biological traits approach allowed us to identify characteristics shared by prey from different families and the contrasting characteristics in genera within a family.
Trait-based prey selection.
Desmans positively selected prey with traits indicating a rheophilic lifestyle such as fast flow and block substrate that are especially abundant in riffles (Merritt et al. 2008). Thus, trait-based prey selection supports previous radiotelemetry results showing that riffles are highly preferred foraging habitats for desmans (Esnaola et al. 2018b)—this is probably because it is easier for desmans to use their strong claws to crawl along the bottom of fast-flowing sections.
Preference for prey exposed to the flow, and a negative selection for those in more protected habitats, may also be linked to the desman rheophilic lifestyle and suggests that desmans do not dig in the stream bottom nor overturn stones. Similarly, negative selection for prey with an affinity for fine substrates probably reflects that seeking buried prey is costly for desmans. Their preference for prey within the water column, and swimmers, reflects that desmans prefer to hunt prey such as Gammarids, which are the right size, appear on the surface of the bottom, and are relatively slow movers (Tachet et al. 2002).
Results for different traits are partially in line with predictions from optimal foraging theory. First, desmans positively selected prey with a high tendency to aggregate, consistent with the long periods spent by desmans foraging in specific riffles (hot spots) before moving elsewhere (Esnaola et al. 2018b). In the case of desman prey, aggregation tendency seems to be a consequence of individuals looking for similar conditions, such as simuliids gathering in high densities on the upper part of boulders, where they find optimal hydraulic conditions for filtering. In these situations, any desman finding such a patch would gain much energy by foraging intensively on it at a low cost. Accordingly, the preference for prey inhabiting litter seems to reflect the exceptionally high density of invertebrates, mainly large shredders, found in litter accumulations (Flores et al. 2017).
Regarding prey size, the selection against prey smaller than 2 mm follows the energetic criteria by Brose et al. (2006), who predicted that the consumer-resource body-mass ratio could not exceed six orders of magnitude. It is noteworthy that we sampled invertebrates with a 2-mm mesh net, which would capture a low proportion of invertebrates smaller than 2 mm. Still, their frequency was even lower in the diet than in our availability samples, thus showing a strong negative preference by the desman. On the other hand, desmans did not positively select the largest prey, expected to be energetically more profitable, rather selecting prey with a 2–8 mm potential size. The reason could be that some large prey (e.g., Perlidae) can flee quickly and could be harder to catch.
Desmans positively selected prey with high body flexibility, mostly corresponding to invertebrates without cases or tubes, which are considered easier to manipulate and eat (Rychlik and Jancewicz 2002) and, thus, likely more profitable (Bertrand 1992). Regarding cased Trichopterans, researchers have reported contrasting results, from positive (Santamarina and Guitian 1988; Santamarina 1992, 1993) to negative preference (Bertrand 1992; Biffi et al. 2017b). Cases do not condition the selection for Trichopterans in dippers (Cinclus cinclus; Santamarina 1990; Ormerod and Tyler 1991; Taylor and O’Halloran 1997, 2001), which share the habitat and trophic preferences with desmans. These contrasts likely derive from the force with which the cases adhere to the substrate and body size because small prey with more fixed, thicker cases (e.g., Glossossomatidae and Goeridae) are less consumed than larger prey with less fixed cases (e.g., Limnephilidae, Odontoceridae, and Sericostomatidae; Vieira-Lanero 2000; Esnaola et al. 2021).
The preference for highly agile prey seems a counterintuitive criterion for desmans, likely linked to other traits such as the vertical location preference or exposure to water flow. At first glance, it seems that highly agile prey would be harder to catch for desmans and, therefore, negatively selected. Nevertheless, we cannot discard that they could be more easily detectable by desmans, which are almost blind (Trutat 1891) and nocturnal animals, and thus, will barely rely on vision to detect prey items. Instead, they have excellent chemoreceptors in their trunk-like nose (Richard 1985), as well as well-developed mechanoreceptors (Argaud 1944; Bauchot et al. 1973), which may be critical in detecting mobile prey such as swimmers. However, mechanoreception will be hampered by turbulence of fast-running waters, being only valuable to detect prey that are not too small and that move close enough. On the contrary, sudden movements of highly agile prey may make them more easily detectable by desmans in a short distance, explaining positive selection.
Desmans showed a similar selection pattern in both streams, although with minor differences, probably owing to the contrasting prey availability between streams and habitats. Similarity between selection patterns in both studied streams provided a basis for improved understanding of desman dietary needs and highlighted the trophic specialization of the species.
Esnaola et al. (2021) detected no differences in total prey availability among habitats that could explain the strong preference of desmans for riffles and suggested that such a choice would be related to factors other than availability. When examining prey traits, differences in availability between habitats were not significant (see the RDA of the multivariate analysis). Regardless, diet points were more similar to prey availability in riffles in Leitzaran (Fig. 3), the stream where riffles are less abundant and with lower conservation status. This greater similarity emphasizes preference for traits showing prey adaptations to living in riffles (i.e., current velocity and substrate preference traits). However, the fact that diet is more similar to availability in riffles than in runs and pools cannot fully account for the selection for riffles simply because of the food they harbor. Other ecomorphological, functional, or behavioral constraints to deal with buoyancy and physical habitat heterogeneity should also be considered (Richard 1986).
Although they prey upon a wide variety of taxa, the results of this study depict the desman as a highly specialized rheophilic species with morphofunctional and ecomorphological adaptations. Thus, the desman appears to be a habitat specialist adapted to forage in fast-flowing facies that depend on riffle availability. Almenar et al. (2013) observed in an insectivorous species that foraging patch choice fitted a hierarchical sequence, driven first by the species morphological specializations and ability to hunt in some spaces, then by the detectability of prey in specific areas, and finally by the relative abundance of prey.
Our results underscore the importance of riffles for the Pyrenean desman. Many human activities—such as water diversion, building weirs, and channelization—affect channel hydraulics and reduce the availability of riffle habitats. We suggest that these activities are detrimental for desmans and can partially explain the general decline of the species even in regions where the ecological status of streams has improved in the last decades. Trait analysis, thus, also helps in prescribing restoration actions (e.g., increasing environmental flows, taking down weirs) that might directly benefit the conservation status of the species.
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Metabarcoding of desman feces.
Supplementary Data SD2.—Affinity scores of each taxon for each trait category expressed through fuzzy coding (Chevenet et al. 1994; Sánchez-Hernández 2014). Taxa with no data were given the average value of all the lower taxonomic levels (gray) or were excluded from the analyses (“-”).
Supplementary Data SD3.—The inertia value of each trait in the fuzzy principal component analysis (FPCA).
Supplementary Data SD4.—ANOVA of the redundancy analysis (RDA) of the global analysis in trait-level.
Funding
This study was supported by the EU project LIFE IREKIBAI (LIFE14 NAT/ES/000186), as well as by the Provincial Council of Gipuzkoa, the City Council of San Sebastian, the Basque Government (156P/2016 and IT754-13), and the University of the Basque Country UPV/EHU. The Basque Government granted AE (grant number PRE_2015_1_0408). Authors declare no direct financial interest.
Acknowledgments
We thank Iñigo Mendiola and Aitor Lekuona (Provincial Council of Gipuzkoa), and Asunción Yarzabal and Iñaki Uranga (City Council of San Sebastián), for their assistance in making this work possible; Vanessa Mata, Hugo Rebelo, and Mafalda Galhardo (CIBIO-InBIO, Universidade do Porto), for helping with diet analyses; the Sequencing and Genotyping Unit–Genomic Facilities–SGIker (UPV/EHU/ERDF, EU) for the technical and human support provided; and Mark Brigham for correcting and helping to improve a draft.



