-
PDF
- Split View
-
Views
-
Cite
Cite
Qiwei Claire Xue, Lisa Larrimore Ouellette, Innovation policy and the market for vaccines, Journal of Law and the Biosciences, Volume 7, Issue 1, January-June 2020, lsaa026, https://doi.org/10.1093/jlb/lsaa026
Close - Share Icon Share
Abstract
Vaccines play a crucial role in improving global public health, with the ability to stem the spread of infectious diseases and the potential to eradicate them completely. Compared with pharmaceuticals that treat disease, however, preventative vaccines have received less attention from both biomedical researchers and innovation scholars. This neglect has substantial human and financial costs, as vividly illustrated by the COVID-19 pandemic. In this article, we argue that the large number of ``missing'' vaccines is likely due to more than lack of scientific opportunities. Two key aspects of vaccines help account for their anemic development pipeline: (1) they are preventatives rather than treatments; and (2) they are generally durable goods with long-term effects rather than products purchased repeatedly. We explain how both aspects make vaccines less profitable than repeat-purchase treatments, even given comparable IP protection. We conclude by arguing that innovation policy should address these market distortions by experimenting with larger government-set rewards for vaccine production and use. Most modestly, policymakers should increase direct funding—including no grants and public-private partnerships—and insurance-based market subsidies for vaccine development. We also make the case for a large cash prize for any new vaccine made available at low or zero cost.
I. Introduction
Developing new interventions to improve global health is of critical humanitarian and economic importance. This problem has been starkly illustrated by the COVID-19 pandemic, which has already wiped trillions of dollars of market value from U.S. stock indices and could cause over one million deaths in the United States alone.1 And COVID-19 is only one of many threats to global health. Infectious diseases—including HIV, tuberculosis, hepatitis, and malaria—are responsible for a staggering one-quarter of global deaths, and over two-thirds of deaths in children under age five.2 In 2014, infectious diseases killed nearly 150,000 people in the United States,3 and global health has been recognized as an issue of U.S. national security4 and a driver of global economic growth.5 Research on new medical technologies to combat these diseases has substantial social benefits. But in a competitive market, firms often capture only a small part of this value, leading to underinvestment.6 To address this concern, policymakers have created innovation institutions such as intellectual property (IP) law and direct public spending,7 which lead to hundreds of billions of dollars spent on global biomedical research each year.8
A growing body of recent legal scholarship has examined the interactions and relative efficacy of these biomedical innovation policies.9 Most of this literature has focused on a particular class of interventions: therapeutic drugs, including small-molecule drugs and more complex biologic therapeutics. These prescription drugs—and ways to reduce their prices—are also the focus of current healthcare policy debates.10 But where are the policy discussions around medical innovations that prevent consumers from contracting infections and needing pharmaceuticals in the first place? One problem is that some health interventions cannot be protected through IP law, making it difficult for firms to capture a return on their research investments.11 For example, it is hard to patent a new lifestyle intervention or an innovation in healthcare quality. But this literature on comparative institutional analysis has largely ignored a field of patentable innovation beyond therapeutic drugs: preventative vaccines.12
This omission is important because it is far from clear that the current policy menu provides optimal incentives for developing vaccines relative to other health interventions. In the most recent example of the difficulty interesting private firms in vaccine development, even after the threat of COVID-19 became apparent, a top official at the U.S. National Institutes of Health (NIH) said it was ‘very difficult and very frustrating’ that no major pharmaceutical company was willing to manufacture a vaccine that was under NIH development—even though the research process had already been funded by the NIH and a large public–private coalition.13 And an earlier project to develop a vaccine for the coronavirus SARS, which might have provided cross-protection against COVID-19, was killed off in 2016 for lack of funding.14 As summarized by The Economist, ‘profits in vaccine making are low,’ and vaccine manufacturers ‘are generally wary of developing vaccines for pandemics, not least because developing vaccines for diseases that then vanish is even less profitable.’15
The limited interest in preventative vaccine development is pervasive. Consider Lyme disease. This infection is the most prevalent tick-borne disease in the United States and is spreading worldwide.16 Treating Lyme disease has been estimated to cost the U.S. healthcare system around $1 billion each year.17 A Lyme disease vaccine was approved in 1998 but withdrawn in 2002 after safety concerns led to poor market performance.18 Or consider hepatitis C. An estimated 3.4 million Americans and 181 million people worldwide are estimated to be living with this virus, which is a leading cause of death due to its association with liver cirrhosis and cancer.19 Drugs have been developed to cure hepatitis C, with price tags over $50,000.20 No vaccine is available.21 Or consider HIV, which currently infects about one million Americans and 38 million people worldwide.22 Antiretroviral therapy has substantially improved the life expectancy of these patients since 1996, albeit at a high price—the cost of treating just those Americans diagnosed with HIV in 2009 has been estimated to be over $16 billion.23 The daily-use drug Truvada was approved for HIV prevention in 2012 (8 years after its approval as an HIV treatment), with a list price around $2000 for a 30-day supply.24 But despite the potential benefit for public health, there is no vaccine to prevent HIV transmission.25 From 2014 to 2018, the U.S. Food and Drug Administration (FDA) approved only nine vaccines, compared with 213 therapeutic drugs.26
In this article, we argue that the large number of ‘missing’ preventative vaccines for infectious diseases is likely due to more than lack of scientific opportunities. Moreover, this is a pressing social problem. In addition to directly fighting these costly diseases and reducing suffering for immunized patients who would otherwise be infected, vaccines can create substantial social benefits for persons other than vaccinated patients. In particular, vaccines create positive externalities for those who benefit from herd immunity, those who avoid the costs of others’ illness (such as employers), and future generations who may benefit from disease eradication.27 The measles outbreaks in the United States due to declining vaccination rates illustrate the costs of losing herd immunity, including to babies who have contracted measles from older unvaccinated children.28 Enormous social costs from COVID-19 might have been avoided if earlier coronavirus vaccine research had not been shut down for lack of funding.29 In short, vaccines and vaccine research will be undersupplied due to a double externality problem: producers do not capture all of the knowledge spillovers from their research efforts, and patients do not capture the social benefits like herd immunity from their choice to vaccinate. Thus, all else equal, we think society should prefer that innovators increase their research efforts on preventative vaccines, and that vaccine use be more heavily subsidized.
In some cases, lower private-sector interest in vaccines than therapeutics may be due to differences in scientific opportunities, development cost, and regulatory regimes, and we emphasize that we are not ruling out any of these concerns.30 But this is not the full story. Even when there are no differences across these dimensions, vaccines are not on a level playing field. Rather, as we explain in this article, preventative vaccines typically have two key aspects that make them fundamentally different from therapeutic drugs: (1) they are preventatives rather than treatments; and (2) they are generally durable goods with long-term effects rather than products purchased repeatedly.31 Both aspects exacerbate the externality problems by making vaccines less profitable than repeat-purchase therapeutic drugs, even when the products have comparable periods of IP protection. And the expectation of lower profits corresponds to lower ex ante incentives to develop vaccines in the first place.
One set of problems arises from irrational preferences by purchasers, including both patients and healthcare payers.32 If the risk of contracting a particular disease is 1 per cent and the value of treating the disease is $100,000, then for rational, risk-neutral purchasers,33 an innovator should receive the same revenues by either (1) charging $100,000 to treat the 1 per cent of patients who contract the disease, either as a lump sum for a one-time cure or split into multiple payments for a repeat-purchase treatment, or (2) charging $1000 per person to inoculate everyone in the population (with appropriate time discounting). If purchasers are risk averse, then the vaccine should be preferred. But studies from behavioral economics suggest that purchasers may underestimate the likelihood of getting sick, undervalue statistical lives, and overestimate the risks of vaccine side effects, such that they will prefer the treatment over the preventative.34 For example, these effects may help explain relatively low uptake of annual flu vaccines,35 or why the Gardasil vaccine for HPV has been adopted more slowly and less widely than expected.36 And if purchasers undervalue costs that are in the future or divided into separate charges—problems known in the behavioral economics literature as present bias and partitioned pricing—they will prefer repeat-purchase interventions to durable vaccines.37
The degree to which these problems of consumer irrationality distort investment away from vaccines depends on factors including the salience of the particular disease and the structure of healthcare payment systems. For example, given the high salience of COVID-19 in 2020, there would likely be substantial demand for a COVID-19 vaccine if it were available—although there might be disproportionately more demand for a COVID-19 treatment, particularly if measured by willingness-to-pay. And insurers might have incentives to correct for these behavioral biases and to account for the externality of herd immunity—although in a country where most people routinely switch health care plans, insurers may have little incentive to pay for preventative care.38
But even for rational, risk-neutral purchasers, both key aspects of vaccines still prevent monopolists from extracting the same profits as they can for repeat-purchase therapeutics. This result is counterintuitive: if each purchaser is indifferent between making a payment now based on their disease risk to prevent a disease and making multiple payments with the same net present value to treat the disease in the future, would not manufacturers be indifferent between selling a repeat-purchase treatment and a durable vaccine? The answer turns out to be no. First, disease risk is often heterogeneous: patients may realize they have a high or low risk of a particular disease due to family history or lifestyle choices. As explained by economists Michael Kremer and Christopher Snyder, if a monopolist cannot price discriminate by selling at a higher price to the high-risk patients, vaccine profits will be lower than for a corresponding treatment.39 And this problem is likely exacerbated by the greater ease of price discrimination for treatments than for preventatives.40 Second, as the economics literature has recognized in other contexts, monopolists typically find non-durable goods to be more profitable than durable goods.41
Combined, these behavioral and non-behavioral effects suggest that absent significant government intervention in healthcare markets—such as mandatory or free vaccination42—the prospect of monopoly profits will under-incentivize the development of vaccines relative to treatments. In particular, traditional market-based IP incentives may be specifically insufficient for promoting vaccine development, despite the outsized social benefits of vaccines. And IP-based allocation is also ill-suited to the vaccine context, as illustrated most recently by the many calls to make any COVID-19 vaccine affordable and available to all.43 Instead, we draw on innovation policy scholarship that has increasingly recognized the value of non-IP innovation incentives and allocation mechanisms for counteracting distortions stemming from market-based rewards.44 These show promise both for correcting against monopolists’ biases toward repeat-purchase treatments and for expanding access to preventative medicine.45 For example, governments should supplement market-based rewards by increasing the use of public funding on policies such as grants, public–private partnerships such as the Coalition for Epidemic Preparedness Innovation (CEPI), and insurance-based market subsidies for vaccine development.46 In addition, we argue that the United States—ideally in collaboration with other governments—should offer a large cash prize for any new vaccine made available at low or zero cost, with the prize size based on the number of patients vaccinated.47
In short, vaccine markets are vulnerable to a singular set of distortions and foregone surpluses that has been underappreciated in the legal literature on biomedical innovation, with substantial implications for public health, including pandemic preparedness. Part II of this article describes the current regulatory environment and market for vaccines, including important differences from the markets for other pharmaceutical products. Part III then argues that two key features of vaccines—that they are generally (1) preventative and (2) durable—likely lead to both undersupply and underconsumption in the current market. Finally, Part IV explores how vaccine innovation institutions might be improved in light of these insights.
II. The Global Market for Vaccines
The vaccine market has many parallels to the market for therapeutic drugs, with similar legal institutions incentivizing and regulating access to new products. Because of these similarities, these markets are often lumped together in scholarly analyses, with a greater focus on the trillion-dollar global pharmaceutical market (including both small-molecule drugs and complex biologics)48 than on the comparatively tiny vaccine market, with estimated global revenues around $50 billion U.S. dollars.49 In this part, we highlight the key regulatory differences between the markets for vaccines and therapeutics—ie, the differences that will affect the choice of research direction even when scientific opportunities are equivalent.50 Section II.A describes the stages of vaccine development. Section II.B focuses on the legal frameworks for regulating vaccine safety and efficacy, both through ex ante approval and ex post liability. Section II.C provides a taxonomy of innovation incentives for vaccine development. Finally, Section II.D presents evidence of the comparatively anemic vaccine development pipeline.
A. Vaccine Development Stages
Like for therapeutic drugs, the development process for preventative vaccines is long and risky, typically taking over a decade.51 The process begins with exploratory research, such as basic science research to look for antigens that may be helpful in preventing a particular disease.52 Once a promising vaccine candidate is identified, development enters the preclinical stage, during which in vitro tests and in vivo animal studies are used to better understand the vaccine’s cellular effects and predict its safety and efficacy in humans.53
If the preclinical results are sufficiently promising, the vaccine can move on to clinical testing in humans, which requires the permission of relevant government regulators.54 In the United States, the developer submits to the FDA an investigational new drug (IND) application, which includes details about the vaccine, how it is manufactured, why it is believed to be effective (including the preclinical results), and the proposed clinical testing protocol.55 Clinical testing typically involves three phases: Phase I enrolls around 20–80 subjects to evaluate safety and tolerability and to get preliminary immune response data; Phase II enrolls several 100 subjects to provide a preliminary demonstration of efficacy; and Phase III enrolls 100s to 1000s of subjects to establish safety and efficacy for approval.56 If all goes as planned, the total time for clinical development and licensure is typically over 10 years.57 (Efforts to bring a COVID-19 vaccine to market in 12–18 months reflect an extraordinary and ethically controversial change to usual protocols, including skipping animal trials.58).
In broad strokes, these stages are similar to those for therapeutic drugs, whether small-molecule or biologic. But they differ in important details. Most notably, because vaccines focus on preventing rather than curing or treating a disease, the study population is generally healthy individuals rather than those who have contracted the disease.59 This difference has two important implications for study design. First, investigators cannot look for elimination of disease symptoms of evidence of efficacy. They can look for differences in disease rates, although this requires a large study population. More typically, they look for a particular immune response, known as a surrogate endpoint, such as an antibody level that indicates protection from the disease.60 Second, administering vaccines to healthy patients means that there is a low tolerance for adverse side effects, and the need for sufficient statistical power to detect rare side effects typically increases the size of Phase III trials.61
Another important difference between vaccines and at least small-molecule therapeutic drugs is that the decision to build manufacturing capacity must be made much earlier in the development process.62 Vaccine manufacturing plants cost around $50 to $300 million, and regulatory burdens mean that the commitment to build a plant for a given vaccine is typically made about 4–6 years before expected licensure.63 (The lengthy process of developing manufacturing capacity is why Bill Gates has committed to funding factories for seven potential COVID-19 vaccines before any have demonstrated efficacy, even though most of them are likely to be abandoned.64) As discussed further below, all else equal, these differences may raise the expected cost of developing a vaccine relative to a small-molecule drug.
B. Regulatory Framework
Government regulators are involved throughout the development process described in Section II.A. Manufacturers must seek ex ante approval to market a new vaccine by providing evidence that it is consistently safe and effective. And after a vaccine is on the market, manufacturers may have to conduct additional post-marketing studies and report adverse events, and they can face ex post liability for resulting failures. In this section, we briefly review this regulatory framework in the United States and other jurisdictions.
1. United States
Oversight of the efficacy and safety of vaccines in the United States falls to the FDA’s Center for Biologics Evaluation and Research (CBER).65 Vaccines are licensed as ‘biological products’ under Section 351 of the Public Health Service Act.66 Like other medicines, a vaccine begins as an IND application and then proceeds through the three phases of clinical trials described above.67 Vaccine sponsors then submit a Biologics License Application (BLA)—the FDA does not have a vaccine-specific application.68 Currently, CBER has approved over 80 vaccines for use in the United States covering more than 25 diseases.69
Approved vaccines are available for purchase by U.S. consumers, but the push for adoption of a vaccine typically comes from recommendations by the Centers for Disease Control and Prevention (CDC). Specifically, the CDC’s Advisory Committee on Immunization Practices (ACIP) examines existing literature on the disease and the vaccine, consults with organizations such as the American Academy of Pediatrics, and then votes on a categorization based on a rubric, which the CDC refers to as a ‘Grading of Recommendations, Assessment, Development and Evaluation’ approach.70 Recommendations are made by age and by indication.71 Importantly, the ACIP schedules cover diseases, not individual vaccine products. Thus, the ACIP draws no distinction between vaccines for the same disease, such as Recombivax HB (Merck) and Engerix-B (GlaxoSmithKline), both vaccines for hepatitis B. The ACIP also does not have a preference between a combination vaccine, such as Kinrix (which immunizes against diphtheria, tetanus, pertussis, and polio), and individual shots—this choice is left to the discretion of the doctor and the patient.
The ACIP’s recommendations help shape the U.S. vaccine market. Every U.S. state has childhood vaccination mandates with only limited exemptions, enforced by vaccination prerequisites for attending school or day care, many of which draw on the ACIP schedules.72 The Affordable Care Act (ACA) went further in requiring that insurers cover these recommended vaccinations with no cost-sharing,73 although it is not year clear how this policy change will affect vaccine uptake.
After a vaccine is marketed, the FDA has a number of regulatory tools to assess the vaccine’s continued safety, which can be enforced through warning letters, product recalls, and consent decrees.74 The agency may inspect manufacturing facilities, and manufacturers must perform specific tests of each lot of vaccine and submit results to CBER before releasing the lot.75 Approval might be contingent on completing more clinical trials or pediatric studies by a certain deadline.76 In addition, manufacturers and healthcare providers must report adverse events that occur after a vaccination, and the FDA reviews this data to determine whether revisions such as labeling changes are warranted.77
Vaccine manufacturers are partially shielded from liability for injuries caused by their products through the National Vaccine Injury Compensation Program (VICP).78 This no-fault system covers vaccines designated by the CDC for ‘routine administration to children’ and is funded by an excise tax on covered vaccines.79 Patients injured by these vaccines may only file a civil suit if VICP compensation is denied.80 In fiscal year 2019, the program provided 653 compensated awards with total outlays of $225 million.81 Although this system was intended to streamline adjudication and make compensation awards more consistent, it has been criticized as failing to meet these goals.82 Claims for injury compensation outside the VICP are largely foreclosed.83
2. Other Jurisdictions
The process for approval of vaccines in other developed countries is generally similar to in the United States, with a national regulatory authority tasked with ensuring the quality of marketed pharmaceuticals and biological products. In the European Union, the European Medicines Agency (EMA) has coordinated the evaluation of vaccines and other medicines for safety and efficacy since 1995, and the EMA and FDA try to provide coordinated scientific advice.84
National governments in Europe, Canada, and other developed countries—particularly those with single-payer healthcare systems—play a larger role in procurement and pricing of both vaccines and other medicines than in the United States.85 However, few of these countries mandate vaccination as the United States does, making it difficult to draw conclusions about the impact of differing levels of government involvement on uptake.86 Generally, in contrast to pharmaceuticals, vaccines are less likely to be distributed globally, suggesting high country-specific fixed costs.87 European countries and Canada typically have more licensed producers per vaccine than the United States, perhaps indicating lower regulatory barriers.88
Developing countries often obtain their vaccines through the United Nations, which relies on the WHO for scientific advice about quality, safety, and efficacy.89 The United Nations Children’s Fund procures vaccines on behalf of 80–100 low- and middle-income countries, with significant financial support from Gavi, the Vaccine Alliance (an international public–private partnership).90 The Pan American Health Organization procures vaccines for 41 Latin American and Caribbean countries, primarily using their national budgets.91 Together, these programs account for about 40 per cent of global volume but less than 10 per cent of global market value.92
Once vaccines are on the market, many other countries—19 as of 2018—have national vaccine compensation programs along the lines of the VICP, although there is large variation in what vaccines are compensated, the size of financial awards, and procedural details.93
C. Innovation Incentives
Because of the regulatory barriers and other costs of bringing vaccines to market, governments use a wide variety of policies to spur vaccine development, including market-based incentives and more tailored government spending.94 This section provides a brief overview of these innovation policies, with a focus on how incentives for vaccine research and development (R&D) differ from those for new therapeutic drugs. Our focus is on monetary incentives and the problem of firms dropping projects from their development pipelines due to insufficient expected profits,95 although we note that non-monetary motivations may also affect incentives, particularly for individual researchers who want the reputational reward from developing interventions with large public benefits.96
1. Intellectual Property and Market Incentives
Innovation scholars have conventionally focused on IP as the key policy tool to promote development of new knowledge goods.97 Under IP laws and other market-based incentives, policymakers set relatively neutral ground rules and then allow market forces to determine the size of the financial reward for a given innovation.98 In theory, these policies aggregate private information from market actors about the relative value of different knowledge goods. In the vaccine context, market incentives include patents, trade secrets, regulatory exclusivity, and R&D tax incentives.
Many aspects of vaccines can be protected with patents, including the composition, manufacturing process, and delivery devices.99 Unlike for small-molecule drugs, manufacturers of biologics—including vaccines and therapeutic biologics—are not required to publicly declare what patents they believe cover these products, making empirical comparisons challenging. Access-to-medicines advocates have pointed to patents as a key barrier to competition in vaccine markets,100 suggesting that patents provide some effective market power for manufacturers.
But even after patent expiration, competitors are only able to enter the market if they can obtain approval for a similar product from the relevant regulatory authority. In the United States, the 2010 Biologics Price Competition and Innovation Act (BPCIA) paved the way for an abbreviated FDA approval process under which generic biologics—known as biosimilars—could rely on an earlier entrant’s clinical data after 12 years.101 But few biosimilars have been approved and launched in the U.S. market, and none for vaccines.102
Patents and regulatory exclusivity are not the only market-based incentives for vaccines. Trade secrets can be used simultaneously with patents to protect different aspects of vaccine development,103 and they play a more critical role for biologics manufacturing than for small molecules.104 Vaccine producers can also take advantage of R&D tax incentives, including both general provisions available for most R&D expenditures105 and the specific tax credit for vaccines designated as orphan drugs.106
Overall, the effective monopoly period can be longer for vaccines than for small-molecule drugs.107 For example, the measles, mumps, and rubella (MMR) vaccine has no competition in the United States after 40 years,108 perhaps due to the high degree of concentration in the U.S. vaccine market.109 In theory, this robust exclusivity period can aggregate dispersed information about the social value of new vaccines.110 With market-based rewards, governments do not have to decide whether providing R&D funding to Pfizer or Gilead will lead to better vaccines; they can let the market decide. But market-based innovation institutions have predictable failures.111 For example, public health scholars have argued that ‘[m]any infectious disease markets are small and therefore do not offer sufficient profit potential even under monopoly conditions.’112 We have seen little analysis, however, of how these market incentives differ for vaccines relative to traditional therapeutics for a given disease.
2. Ex Ante Government Spending: Grants and National Labs
Policymakers can try to correct for the IP system’s biases through government-set innovation incentives, including direct ex ante funding for R&D.113 For example, as Amy Kapczynski has explored in detail, the international system for developing vaccines for pandemic and seasonal flu viruses is largely coordinated by the Global Influenza Surveillance and Response System (GISRS) run by the World Health Organization (WHO), which is supported by about $56 million annually from national governments and largely operates without formal IP.114 Recently, the WHO has encouraged governments to leverage the GISRS to improve COVID-19 surveillance.115
Most public funding for vaccine development, however, operates as a complement to, not substitute for, IP systems. The world’s largest research funder, the NIH, spent over $2 billion on vaccine-related research in fiscal year 2018,116 including through intramural research at its Vaccine Research Center117 and through extramural research programs, generally at universities.118 Additional vaccine R&D is conducted by a host of other federal agencies.119 Non-governmental organizations like the Bill and Melinda Gates Foundation increasingly play a similar role in funding vaccine development that seems to have insufficient incentives from the private sector.120
The United States allows the recipients of public research funding to patent any resulting inventions under the Bayh–Dole and Stevenson–Wydler Acts, and many countries have adopted similar regimes.121 Out of the 28 vaccines approved by the FDA from 1998 through 2009,122 one study found that nine involved public-sector patents,123 although three of these vaccines have been discontinued.124
3. Ex Post Government Spending: Prizes and Subsidies
In addition to incentivizing vaccine R&D through ex ante spending, governments can reward successful vaccines ex post, either through a pure prize system that provides a lump sum for a product that meets certain criteria (eg $3 billion for an effective malaria vaccine) or a market-based prize that provides a payment based on the number of patients served (eg $15 for every patient inoculated with an effective malaria vaccine).125
Pure prize systems for vaccines remain largely theoretical,126 but market-based prizes are used to spur vaccine development and distribution.127 An advance market commitment (AMC) funded by national governments and the Bill and Melinda Gates Foundation is being used to incentivize distribution of pneumococcal vaccines internationally.128 And more mundanely, demand-side government subsidies such as public healthcare and insurance are a form of market-based prize, particularly when reimbursement is based on a government assessment of the value of a health technology.129 By increasing the number of patients covered or the amount of reimbursement for a given health technology, governments can tailor innovation incentives for that technology.130
Indeed, the first empirical demonstration that government policies to expand healthcare utilization can spur R&D was in the vaccine context. Economist Amy Finkelstein identified three discrete U.S. policies that affected the return to vaccine development for particular disease classes: the 1991 CDC recommendation that infants receive hepatitis B vaccines, the 1993 Medicare decision to cover influenza vaccinations, and the 1986 introduction of the VICP, which indemnified manufacturers from lawsuits related to polio, diphtheria-tetanus, MMR, and pertussis vaccines.131 She found that these policies were associated with a statistically significant 2.5-fold increase in the number of new vaccine clinical trials for the affected diseases.132 One would expect a similar effect from other policies focused on increasing vaccination rates, such as school vaccination requirements133 and the Vaccines for Children program.134
D. The Anemic Vaccine Development Pipeline
Whether the incentives described in the prior Section are leading to a growing or flattening development pipeline depends on one’s metrics. One study found that ‘the proportion of new vaccine candidates entering all stages of clinical trials increased by 3–5 percentage points from 1990 to 2012.’135 But a 2019 McKinsey report noted signs of slowing innovation in the vaccine market, including ‘the share of growth from new vaccines launched down from almost 50 per cent in 2011 to less than 15 per cent in 2017—the lowest level in 20 years’ and ‘higher attrition rates for vaccine-development programs relative to other biologics.’136
Even if the vaccine development pipeline is growing, it remains a trickle relative to the pipeline for new pharmaceuticals.137 As noted above, from 2014 to 2018, the FDA approved only nine vaccines, compared with 213 therapeutic drugs.138 One recent review refers to the number of new vaccine approvals as ‘perennially low.’139 And there are many infectious diseases that lack any approved vaccine, including not just diseases like tuberculosis and malaria that primarily affect low-income populations, but also diseases like HIV and norovirus that regularly infect many high-income patients.140 Low prices, including congressional price caps for older vaccines, have contributed to vaccine shortages in the United States,141 and uncertainty about future prices increases the risk of new vaccine development.142
The limited vaccine development pipeline seems concerning on its face, although we emphasize that based on current evidence, we cannot conclude that overall vaccine R&D investments are too low from a social welfare perspective. For example, low investments may simply reflect a lack of scientific opportunities.143 But even if a vaccine project is equally technically feasible to a potential therapeutic drug, we think there are economic reasons that a private firm would eschew the vaccine. If companies’ decisions about which products to invest in are based on these non-scientific factors, that seems concerning as a health policy matter. The remainder of this article focuses on these underexplored aspects of vaccine development.
III. The Economics of Underinvestment in Vaccine R&D
As noted above, the comparatively anemic vaccine development pipeline may have multiple root causes, including the underlying science and the legal distinctions discussed in Part II, both of which vary across vaccines. But even if a potential vaccine and a drug candidate have the same likelihood of success and the same expected period of monopoly protection, vaccines generally differ from therapeutic drugs along two dimensions, as illustrated in Figure 1: (1) they are preventatives rather than treatments; and (2) they are durable goods with long-term effects rather than repeat-purchase products.
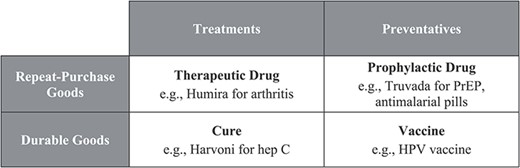
These dimensions are really continuous spectra rather than discrete boxes: some vaccines may have therapeutic as well as preventative benefits,144 and although most vaccine sequences do not exceed three doses, a few require more regular doses.145 But we think focusing on the polar ends of each spectrum helps illuminate the relevance of these dimensions, including the extent to which incentives are inadvertently tilted toward certain technologies.146 Also worth noting is that both cures (durable treatments) and prophylactic drugs (repeat-purchase preventatives) do confer some of the benefits of vaccines and suffer from some of the same distortions.
In this part, we explain why these properties render vaccines less profitable than treatments, whether biological or small-molecule, all else equal. These two attributes combined suggest that on both the supply and demand sides of the vaccine market, private incentives do not coincide with social welfare maximization.
Suppose a pharmaceutical company is considering developing a product targeted toward a particular virus. The company’s research team informs management that there are two products that seem scientifically promising: a new drug that would treat people infected with the virus when taken daily, and a vaccine that would prevent people from contracting the virus in the first place. The firm holds all the relevant IP for each product over an equal period,147 and both have the same likelihood of success. Developing both the vaccine and the drug would create the risk of having one product cannibalize the market for the other, so management decides to pursue only one of these options.148 How does it choose, and how might that private choice differ from the one a social planner would make?
A profit-maximizing firm should consider the risk-adjusted development cost and the expected return if the project is successful. As described in Section III.A, vaccines may be somewhat more expensive to develop than therapeutic drugs, although there is little reliable data on actual development costs. This difference could mean that vaccines are simply costlier from a social welfare perspective. As discussed in Section III.B, however, preventatives usually have greater positive externalities, such that society will often prefer the firm to focus on the vaccine, all else equal.
Even if the costs of developing the drug and vaccine were equivalent, the profit side of the equation also skews incentives toward the drug. Section III.C considers how expected returns will be affected by effects described in the behavioral economics literature on consumer preferences, while Section III.D considers economic effects that apply even absent consumer irrationality.
A. Development Cost
When compared with therapeutic drugs—particularly small-molecule drugs—biologic vaccines may be more expensive to design and test.149 The difference is not just that vaccines are all complex biologics: as discussed above, because preventatives are generally tested on healthy individuals rather than sick patients, a larger study population may be needed to observe a statistically significant effect, and there is a lower tolerance for adverse side effects.150 However, the ability to rely on surrogate endpoints such as the presence of antibodies helps reduce clinical trial costs.151 It is not obvious which of these are most important.
Unfortunately, data on the relative expense of vaccine versus small-molecule development are spotty. Estimates for the cost of vaccine development typically range in the hundreds of millions for clinical trials—well above most lower bounds on small-molecule clinical trial costs, but also within most upper bounds.152 Estimates that include failed attempts and opportunity costs range in the low billions, again comparable to estimates for small-molecule drug development.153 One review suggests this literature ‘implies the average capitalized costs are likely not statistically different from one another.’154
Nevertheless, the possibility that preventatives are costlier to produce is worth keeping in mind for the remainder of this article. If preventatives are simply more expensive to develop than treatments and thus costlier from a social welfare perspective, then society should prefer that firms focus on treatments, all else equal. But as discussed in the following section, preventatives have net social benefits that, based on current evidence, seem likely to outweigh their difference in costs.
B. Net Social Benefits and Externalities
Although vaccines may cost more to develop than therapeutics, they also have the potential to provide far greater benefits to society. These benefits for individuals other than the vaccinated patient—known as positive externalities or spillovers—could justify the extra costs of development from a social welfare point of view. However, to the extent that private actors are not able to capture them, these benefits may also contribute to substantial distortions in vaccine innovation markets.
As we explain in this section, the health economics literature has documented large positive consumption externalities of vaccines due to their preventative nature.155 Furthermore, as durables, vaccines can be on aggregate less costly to distribute and administer, since they need to be given only once (or a handful of times) per person. The WHO notes that vaccines are ‘accessible to even the most hard-to-reach and vulnerable populations.’156 Together, these factors contribute to the understanding in public health that vaccines are ‘among the most cost-effective health interventions.’157
1. Treatments Versus Preventatives: Herd Immunity and Eradication
As preventatives, vaccines provide benefits to society beyond those they immediately provide to individual consumers. Indeed, conventional wisdom in public health, or even common sense (‘An ounce of prevention is worth a pound of cure’ comes to mind), points toward prevention being preferable to treatment. The CDC maintains, ‘It is always better to prevent a disease than to treat it after it occurs.’158 Scholars similarly note that ‘the benefits of vaccination extend beyond prevention of specific diseases in individuals.’159 Although a full cost-benefit analysis of a preventative versus a treatment is a disease-specific exercise and outside the scope of this paper,160 the array of positive externalities associated with preventatives suggests that even if vaccines are costlier than treatments, they likely provide societal benefits far beyond these extra costs.
Perhaps the most basic externality associated with vaccination is herd immunity: each individual’s immunity reduces disease risk for the rest of the population by preventing the spread of infection.161 This is a classic example of a positive consumption externality. Consumption of a vaccine benefits not only the vaccinated patient but also the people around them, leading to an aggregate social benefit that is greater than the sum of all individual benefits. The inability of individuals to fully account for this excess social benefit in their decision-making leads to socially suboptimal outcomes: some people will choose not to be vaccinated, believing that their private costs outweigh their private benefits, even though the social benefits of their being vaccinated exceed the social costs. The devastating effect these individual decisions can have is strikingly illustrated by the rising infection rates associated with the anti-vaccination movement.162 For monopolist vaccine producers, this behavior drives a wedge between the social benefit granted by the product and the profits that the firm can extract. Crucially, this externality is a benefit associated with vaccines that is not conferred to nearly the same degree by current treatments: typically, treating patients after they have become infected will not stem infection in the same way as immunizing patients before they become infected will, particularly because patients are often infectious before manifesting symptoms.163 For example, asymptomatic, pre-symptomatic, and mildly symptomatic COVID-19 patients—known as ‘silent spreaders’—may play an important role in fueling the pandemic.164
By preventing people from getting sick both directly and through herd immunity, vaccines help maintain an overall healthier population. The health of a population is strongly associated with economic growth. Though causality likely runs in both directions in this relationship, there does exist evidence that health can promote growth.165 Even conservative estimates of effect size show it is substantial. Among other channels, the healthier a person, the more productive they are likely to be, missing fewer days of work and performing better when they do work.166 The global market collapse in response to COVID-19 provides a stark illustration of how significant these costs can be.167 Health is also associated with greater educational attainment and human capital formation, which contribute greatly to both a country’s and an individual’s future productivity.168 These impacts on growth could manifest as a reduction in between-country income inequality—many of the countries that stand to gain the most from disease elimination also have the lowest per capita GDPs.
The ultimate result of widespread immunity may be the local elimination or global eradication of a given disease—an externality above and beyond even herd immunity. Elimination typically refers to the achievement of a zero rate of infection in a well-defined geographic area, whereas eradication refers to the same outcome worldwide.169 Only two infectious diseases have ever been eradicated—smallpox and the livestock disease rinderpest—and vaccines played a key role in both cases.170 This does not mean that diseases cannot be eradicated without vaccination.171 However, it makes sense that eradication efforts focus on prevention. Vaccines are central, for example, to the global campaign to eradicate polio because the disease is contagious before it is symptomatic: by the time the patient is aware that treatment is needed, they have already spread the disease.172 This challenge is circumvented if the patient is unable to contract the disease in the first place. Immunity gaps thus have been a major obstacle for the eradication of polio.173 Similarly, preventative approaches have been critical for controlling infectious diseases for which vaccines do not exist, such as hepatitis C174 and dracunculiasis (infection by the parasitic guinea worm).175 In short, prevention is a key step in achieving the positive externality of eradication.
The benefits of elimination or eradication are incalculably far-reaching: mortality and disease morbidity are strong measures of national welfare. Declining mortality around the world has greatly promoted growth in welfare, even just by naively measuring the additional consumption that a longer life grants. 176 Perhaps most significantly, disease elimination and eradication benefit not only current but also future generations. Therefore, financing disease eradication with a deficit is welfare-improving in that it forces future generations to contribute toward an effort that benefits them.177 Eradication can be seen as an investment: by spending a little more on eradication now, society can avoid the cost of all vaccinations in the future.178 This sort of intergenerational externality justifies even greater amounts of societal spending on vaccines than the simple static externality of herd immunity would suggest. At the same time, these intergenerational effects are even more difficult for consumers—who likely discount the future at a much higher rate than society as a whole—to internalize.
While quantifying externalities is notoriously difficult, attempts have been made to estimate the value of vaccines. One estimate found that common vaccines save millions or tens of millions of disability-adjusted life years (DALYs) annually.179 The same work provides an estimate that smallpox eradication has saved $300 million dollars per year globally in direct costs;180 other estimates for the same figure that include indirect costs such as missed work run as high as $1.35 billion.181 Even discounting the future, this is hundreds of millions of dollars of benefit that a treatment could not have provided.
2. Repeat-Purchase Versus Durable Goods: Distribution and Adherence
Durable pharmaceuticals also seem to be associated with positive social benefits. They may be more difficult to invent or produce, but they are also often less costly to distribute on aggregate over time. Unlike repeat-purchase goods, they need to be distributed to each consumer only once. However, since more than 90 per cent of pharmaceutical sales in the United States are routed through a distributor,182 this lower social cost of distribution does not necessarily translate into higher profits for the innovating firm that paid the up-front research cost. This is true particularly if distributors have some market power of their own, which American distributors likely do: just three distributors, known as ‘The Big Three,’ account for more than 85 per cent of drug distribution revenue.183 While the magnitudes of differences in both R&D and distribution costs are unclear, lower distribution costs would certainly serve to offset in part the higher development costs.184
In addition, durable medical products including vaccines solve issues of non-compliance with treatment regimes. Lack of adherence is recognized as an important issue in public health: the WHO estimated in 2003 that in developed countries, adherence rates average only about 50 per cent, with numbers even lower in countries and among populations with less access to health resources.185 More recent disease-specific studies have shown little improvement on average.186 Granted, adherence is an issue even for vaccines.187 However, both dose frequency and regimen complexity contribute to non-compliance, and relative to repeat-purchase preventatives, vaccines can greatly mitigate these issues.188
HIV provides an illustrative example because a repeat-purchase preventative exists in the form of tenofovir (Viread). Adherence is central to efficacy but is unfortunately difficult to observe, and studies have found a wide range of adherence rates.189 Nevertheless, the presence of rates below 30 per cent in some of these studies is disturbing. Rates are similarly low for patients already suffering from HIV/AIDS. The WHO estimates that only one-third of patients overall comply with their treatment regimens, and the number remains low even among patients who comprehend the consequences of non-adherence.190 A vaccine that could be administered once or a small number of times and combined with other vaccinations would substantially alleviate this burden.
In sum, durable preventatives such as vaccines have significant positive externalities that will not be accounted for by our hypothetical pharmaceutical firm that is considering developing either a new therapeutic drug or a new vaccine for a particular virus. In contrast, the firm will be closely focused on its expected profit if the new product is successful—an issue we turn to for the remainder of this Part.
C. Irrational Purchaser Preferences
A firm’s expected profit for a new vaccine or drug will be determined by the demand for that product. For rational purchasers with uniform demands, the profit would be the same for a vaccine or a drug, all else equal (including development costs). For example, recall our example from the Introduction: if treating a disease with a 1-per cent risk has a value of $100,000, then the firm should receive the same revenues by charging either (1) $100,000 to treat the 1 per cent of patients who contract the disease or (2) $1000 per person for a vaccine. But as behavioral economists have documented, real-world purchasers deviate from this rational-actor model in many ways. In this part, we examine the effects of irrational purchasers for vaccine and drug markets.
Two preliminary caveats: First, firms themselves can suffer from behavioral biases, which might mitigate some of these consumer irrationalities.191 However, current evidence in the behavioral economics literature suggests that firms, particularly larger firms such as pharmaceutical producers, are less irrational and more able to safeguard against irrationality than consumers.192
Second, the relevant irrationalities likely differ depending on who is actually making purchasing choices. The U.S. market for drugs and vaccines is heavily regulated and beset with agency-cost problems, and allocative choices are often made by medical professionals or institutional payers rather than by individual consumers. Professionals are of course not immune to irrational behavior, and it is not obvious how entities such as insurers will affect this market. On the one hand, insurers may have a strong incentive to help patients overcome the problems described in this Section to reduce overall healthcare costs.193 On the other hand, patients may switch insurers often enough that a given insurer is less likely to internalize the costs of a patient’s failure to vaccinate.194 As Rachel Sachs explains, the fragmentation of the U.S. healthcare system decreases the incentive of insurers to provide preventive care.195 Unfortunately, testing the effect of other insurance regimes, such as single-payer health insurance, on purchases and uptake is difficult because few countries with single-payer healthcare also require childhood vaccination.196 In any case, we think keeping the relevant decisionmaker in mind is important for evaluating a given distortion.
1. Treatments Versus Preventatives: Errors in Risk Assessment
Along our first dimension—treatments versus preventatives—we think misguided risk assessments due to both errors in probabilistic reasoning and misinformation due to anti-vaccination campaigns will generally skew incentives toward treatments.
Errors in probabilistic reasoning can point in different directions,197 but on average, individuals have an optimistic bias when assessing personal risk, in that they underestimate the likelihood of potential harm to themselves.198 For example, in medical contexts such as heart disease, lung cancer, diabetes, and HIV, individuals have been shown to underestimate their likelihood of getting sick.199 If the hypothetical patient above thinks his risk of contracting a disease is one-tenth of a per cent rather than 1 per cent, then he will be willing to pay only $100 for the vaccine rather than $1000.
This over-optimism might also cause consumers to underestimate the risks of receiving a vaccine, but this effect is countered by an effect known as omission bias. As summarized by one review, ‘many studies have shown that individuals are more averse to the risks associated with an action—getting an ‘unsafe’ vaccine—than to the risks associated with inaction—taking a chance of contracting a [vaccine-preventable disease].’200 And this overweighting of the risks of adverse effects of vaccines becomes more problematic when coupled with misinformation about the true likelihood of those risks due to the anti-vaccination movement.201 The controversy over Merck’s Gardasil vaccine for HPV—sparked in part by the fear that it would increase unsafe sex by adolescent girls—provides one recent example.202
Together, these effects should make consumers prefer a treatment to a preventative, all else equal. We emphasize that this is only a hypothesis—other cognitive biases such as general risk aversion may point in the opposite direction. But our hypothesis finds at least some support in survey work in which consumers are asked about their willingness to pay for different medical interventions.
In one study, consumers were willing to pay about three times more to treat a food-borne virus than to prevent it, even when it was made clear that both interventions led to the same reduction in the likelihood of dying from the virus.203 In another study, participants preferred treatments to preventatives for lung cancer, with the preference seeming to be driven by the perceived urgency of the intervention.204 In short, once individuals have contracted a disease—making the disease particularly salient and eliminating the need for probabilistic reasoning—they are willing to pay a lot to get better. But they are not willing to pay that high treatment price times the actual epidemiological risk of the disease in order to prevent the disease in the first place.
Consumers do recognize the social benefits of preventions over treatments when they focus on abstract members of society rather than on themselves or another identifiable person who needs medical care. For example, when asked to compare the value of different policies for 100,000 people—such as a policy that ‘reduces pesticides in foods that cause colon and bladder cancer’ resulting in ‘10 fewer deaths over 5 years’ versus another that ‘treats [people] who have leukemia’ resulting in ‘5 fewer deaths over 30 years’—consumers expressed a willingness to pay twice as much for avoiding deaths via prevention policies rather than treatment policies.205
These results might make one more optimistic about allowing healthcare resources to be allocated by government decisionmakers who can make more abstract judgments. Of course, whether bureaucrats undervalue preventions will depend on the degree to which they in fact place themselves in this social planner position. There are at least two reasons to suspect that treatments would still receive disproportionate attention and resources over preventatives. First is an epistemic problem: when conducting cost-benefit analysis in a wide variety of contexts, decisionmakers tend to undervalue statistical lives (eg people who have a risk of dying from a disease if they are not vaccinated) relative to identifiable lives (eg people who are currently sick with a disease).206 And second is a public choice problem: the people who are currently sick with a disease and need treatment are a more concentrated interest than the people who would benefit from a vaccine.207
2. Repeat-Purchase Versus Durable Goods: Present Bias and Partitioned Pricing
Behavioral effects from consumers may also skew incentives along our second dimension, allowing producers to extract higher profits for repeat-purchase than for durable goods.208 In this section, we consider two well-documented behavioral consumer tendencies. First, the behavioral economics literature suggests that purchasers are likely to overvalue present benefits and undervalue future costs, including both financial costs and costs to health and wellbeing, a problem known as present-biased preferences. Second, purchasers are likely to underestimate bills that are divided into separate charges, a problem known as partitioned pricing. Here, we consider these effects in turn.
The struggle many people have with focusing too much on the present has long been recognized across a variety of disciplines.209 More recently, behavioral economists have provided formal models for understanding this trend.210 The most common model is known as ‘hyperbolic discounting’ (or ‘quasi-hyperbolic discounting’), meaning that people discount future payments (or costs) at a higher rate when the delay occurs sooner in time.211 Hyperbolic discounting leads to time-inconsistent preferences, such as preferring to receive $100 now over $110 in 1 year, but not preferring $100 in 1 year over $110 in 2 years.212 More generally, empirical work has consistently found that consumers value money now substantially more than economics would suggest, as if the annualized rate of return were around 30 per cent,213 or even higher for empirical work involving non-monetary rewards.214 This pattern is consistent with hyperbolic discounting or other models of time-inconsistent choice.
This puzzle of intertemporal choice—consumers making decisions based on an unusually high rate of return—has important implications for our hypothetical firm that is considering whether to develop a repeat-purchase product or a durable product for a given disease. At an annual interest rate of 5 per cent, consumers should slightly prefer paying $150,000 now over paying $1000 per month for 20 years.215 But if consumers act as if the rate of return is 30 per cent, then they would choose the $1000 monthly payments over paying even $40,000 now, making the repeat-purchase product far more profitable than the durable good that would last for 20 years.216
Consumers may prefer repeat-purchase products over durable goods not only due to undervaluing the future financial costs of repeat purchases, but also due to undervaluing or underestimating non-monetary costs, including the costs in time and wellbeing of actually getting repeated treatments or of having a condition worsen.217 This is part of why some patients have difficulty with adherence to a treatment regimen, as discussed above.218
Given the robust empirical result on consumers’ present focus across a variety of contexts, there is good reason to think that firms will be able to extract greater profit from a repeat-purchase product than from a durable good. Furthermore, empirical evidence exists that firms are aware of these consumer biases in at least some areas.219 This should lead to some distortion away from producing durables and toward producing non-durables.
It is worth noting that there is hope for consumers—another empirical regularity of this literature on intertemporal choice is that people make better decisions when they are making the decision for the future rather than for the present.220 If these decisions for the future are binding, people can lock themselves into good behaviors. Hence, multiple studies have found that reminders led to statistically significantly higher rates of preventative medicine screening.221 This implies that there may be ways to alleviate this distortion, although such interventions are unlikely to occur in a vacuum or to come from pharmaceutical producers.
The problem of present-biased preferences affects repeat-purchase goods because some of the purchases occur in the future. A second and distinct problem arises because repeat-purchase goods divide the price into separate charges. Dividing total cost into separate costs has long been a popular marketing strategy for firms. Consistent empirical evidence has shown that consumers perceive these partitioned prices to be lower.222 In some cases, this perception actually increases overall demand for the good: one experiment found that buyers do not fully account for auction fees even when they are explicit, causing them to pay a higher total cost in an auction with fees.223 Another study has shown that even experienced consumers fail to consider ancillary shipping and handling costs at all.224
Indeed, partitioned pricing schemes have become increasingly prevalent over time, particularly with the rise of the internet, showing seller awareness of the phenomenon.225 While the psychological impact of partitioned pricing is surely not the only factor driving firms to produce repeat-purchase goods, it is undoubtedly a beneficial feature of these goods from the producer’s perspective. This phenomenon coupled with discounting future costs would cause a consumer to perceive repeated smaller payments as being much preferable to a single larger payment.
D. Other Difficulties Extracting Social Value
The degree to which manufacturers will underinvest in vaccine R&D due to the problems of consumer irrationality discussed in Section III.C depends on a number of empirical factors, including consumer perceptions of the disease at issue, the structure of healthcare payment systems, and firms’ beliefs about how irrational consumers are. But even at the other extreme, if firms make investment decisions under the assumption that consumers are perfectly rational and risk-neutral, both of the vaccine attributes we have highlighted—that they are preventatives and that they are durable goods—tend in general to reduce the surplus captured by monopolists. Thus, we argue that wherever consumers fall on the spectrum of irrationality—and wherever firms think they fall—vaccines tend to be less profitable than repeat-purchase treatments. We explain these counterintuitive results in this Section. We emphasize that empirically disentangling, distinguishing, or parametrizing the effects discussed below would be monumentally challenging, particularly given the presence of the effects discussed above. Nevertheless, we believe they play some role in making investment in durable vaccines less attractive than in repeat-purchase therapeutics.
1. Treatments Versus Preventatives: Heterogeneous Disease Risk
Michael Kremer and Christopher Snyder were the first in the economics literature to model the difference between innovation incentives for preventatives and those for treatments.226 They showed that under plausible assumptions about disease risk, incentives for firms to invest in the development of a preventative versus a treatment may differ vastly and may not align with the social welfare-maximizing outcome. In particular, preventatives may be less profitable than treatments when disease risk is heterogeneous in the population, even if a preventative is cheaper to produce or provides more social benefit.227 This is easiest to demonstrate in a simplified example, such as the one below with a discrete, two-point distribution of disease risk. We emphasize, however, that this contrived example illustrates the economic issue that Kremer and Snyder identified, but does not describe the real-world vaccine market. The original paper shows that the same distortion exists under far richer assumptions about disease risk, development cost, revenue, and market structure, and that the result is robust across a variety of possible realistic variations.
If the disease will be equally severe for every patient, one might imagine that there exists a common price, p, that each ex post identical patient will be willing to pay for a treatment. The company, then, can expect its revenue to be 35p.
By contrast, with risk-neutral or most forms of risk-averse consumers, the group with the lower disease risk will be willing to pay less for a preventative than the group with the higher disease risk. In the case of risk-neutral agents, the 20 consumers with a higher disease risk should be willing to pay 0.75p, while the other 80 will be willing to pay only 0.25p. If, as is typically the case, the firm cannot effectively price discriminate based on disease risk, it will either have to settle for revenues of 20 × 0.75p = 15p from only high-risk consumers or revenues of 100 × 0.25p = 25p from all consumers.229 Even the more lucrative of these options, selling to all consumers at a monopoly price of 0.25p, still generates less revenue than the firm would receive from the treatment the treatment: 25p < 35p. Indeed, risk averse patients would need to be willing to pay more than 0.35p—at least 40 per cent more than the risk-neutral price—before revenues from a preventative could surpass those of the treatment.
If costs of development are equal, the treatment is clearly more profitable. Indeed, even if the treatment costs more to develop—and thus would be less efficient from a societal welfare perspective—it might still generate greater profits than the preventative: consider the case where the treatment costs 10p to develop while the preventative only costs 5p. In this case, even absent the positive externalities descried in Section III.B, society ought to prefer the preventative, as it is half as costly. However, the treatment generates profits of 25p while the preventative only generates profits of 20p, meaning that the firm will prefer the treatment. Therefore, when risk is distributed heterogeneously in a population and firms cannot easily observe any individual’s risk, firms will be biased toward producing treatments, which provide higher expected returns, over preventatives. This occurs independent of the value of either pharmaceutical to society or to patients—the firm bases its decision not on the social value, but on how much profit it can extract. Thus, even if the social value of the preventative is substantially higher, as suggested by our analysis in Section III.B, the monopolist will not change its analysis.
Populations are heterogeneous in their disease risk for most diseases, but Kremer and Snyder find certain power-law distributions of disease risk render preventatives particularly unprofitable relative to treatments.230 Risk distribution is typically determined by the epidemiology of a disease—sexually transmitted infections and geographically restricted pathogens, for example, tend to have the problematic power-law risk distribution. Two diseases that fall into these categories for which therapies exist but vaccines do not are HIV and malaria.231 This epidemiology-driven heterogeneity across diseases allows Kremer and Snyder to test their theory empirically. Using a linear probability model, the authors find that among significant but non-ubiquitous diseases, those with biasing characteristics are statistically significantly less likely to have had a vaccine developed than they are a treatment.232
The example constructed above is a highly simplified version of the authors’ model. Relaxing many of its assumptions, such as the notion that consumers directly buy therapeutics from the producing firm or that the consumers are identical but for their disease risk, does not qualitatively change the results. In short, even absent consumer irrationality, producers generally will not be able to earn as much profit from a preventative as from a treatment, which will lower incentives to invest in preventatives in the first place.
2. Repeat-Purchase Versus Durable Goods: Pricing Commitment Problems
A similar problem occurs when considering the other key dimension along which vaccines differ from many therapeutic drugs: that they are typically long-lasting durable goods rather than repeat-purchase products. The law and economics literature has long distinguished between durable and non-durable goods:233 a durable good, such as a washing machine, retains its functionality after many uses, whereas a non-durable good, such as a bag of popcorn, can be consumed only once before it needs to be replaced. A corollary of this definition is that durable goods need only be purchased rarely—in some cases, only once—while non-durable goods must be purchased repeatedly. We argue that in this sense, vaccines economically act like durable goods, in contrast to repeat-purchase preventatives.234
The impact of durability on monopoly profits has been formalized in the economics literature perhaps most famously by an argument known as the Coase conjecture, published by Ronald Coase in 1972.235 Coase argued that a monopolist who would be in business for multiple periods selling a durable good would be happy to sell at the monopoly price at first, but would not be able to commit to a high price in the future, knowing that its primary hope for additional revenue will come from consumers who have not yet bought the good and who are therefore not willing to pay as much.236 Rational, patient consumers would anticipate the firm’s commitment problem and would be willing to wait for the firm to drop the price before buying, forcing the firm to charge a lower price from the outset if it wants to sell at all.
For illustration, consider a book publisher. Books are durable and may be read many times; most consumers buy only one copy of a given book; and publishers are generally monopolists over a given work.237 A monopolist publisher has the ability to choose both the price at which a new book will be sold and the quantity it will print. However, the publisher is still constrained by consumers’ willingness-to-pay (ie, the market demand curve). Although the publisher may choose an arbitrarily high price, if nobody is willing to pay $10 million for the newest Harry Potter book, the publisher will make zero revenue. A profit-maximizing publisher, therefore, chooses a pair of price and quantity that is consistent with consumers’ willingness-to-pay (ie, lies on the demand curve). Generally, the mathematics of profit maximization dictates that the optimal quantity occurs where marginal revenue is equal to marginal cost, and the optimal price is the highest price at which this quantity will be bought. Under most conditions, the monopoly price substantially exceeds the marginal cost of production: the monopoly price of a book might be $20 while the marginal cost of printing a copy of the book may be only $5. Thus, the firm is foregoing some potential sales—for example, from consumers willing to pay $15—because selling to fewer consumers but charging a higher price is more profitable. If the firm were producing a non-durable good, it could continually sell at $20 to the same, albeit limited, set of consumers. However, for an infinitely durable good, the firm will no longer make any sales after this first wave of consumers has purchased the good. If it wants to continue selling the same product, its only option is to lower the price—which it can afford to do because the monopoly price is so far above its costs.238 Therefore, a rational, profit-maximizing firm will lower its price, for example to $15, to squeeze out some more sales. The monopolist knows, however, that some fraction of consumers may anticipate this future price drop and wait the monopolist out, diminishing its revenue at the initial high price. Importantly, not every consumer needs to anticipate the price drop for the monopolist’s optimal price to change—just enough that selling a larger quantity at a lower price becomes more profitable than selling a smaller quantity at a higher price. Moreover, it need not be the case the consumers would actually have waited the monopolist out—only that it thought they would.
Extensions of this basic argument demonstrate that monopolists will also choose a lower-than-optimal level of durability when producing a more durable good has an associated cost. In particular, rather than charging a lower price for a durable good, a monopolist may choose to produce a less durable good, thereby expanding the size of its market in the future. While this behavior benefits the monopolist, it generally decreases social welfare.239 This behavior often manifests as planned obsolescence or contrived durability: planned obsolescence consists of introducing new versions of a product artificially often, rendering past versions deprecated, as often occurs in textbook publishing or software development, while contrived durability entails purposefully manufacturing a less durable product.240 The lifespan of the effect of a pharmaceutical, however, is generally determined by laws of nature rather than by simple manufacturing choices. Consequently, the equivalent behavior in the pharmaceutical industry consists of making a different product with a shorter lifespan, rather than shortening the lifespan of a given product.
Firms may not fear consumer patience for every vaccine. For example, vaccines that are likely to become mandatory childhood vaccines may suffer less from this particular distortion. However, even uncertainty about the likelihood of an ACIP recommendation, as might exist for an HIV vaccine, for example, could cause this effect to kick in. Moreover, durable-good concerns may be particularly salient for firms considering development of a vaccine for a current pandemic, such as COVID-19. In addition to social pressures to keep the price of such a vaccine low,241 the knowledge that each consumer will purchase a vaccine only once, and thus, that prices will be depressed and the market will dry up with time, makes development of a COVID-19 vaccine less attractive than development of an effective treatment.
In sum, just as monopolists find treatments more profitable than preventatives, they also find non-durable goods more profitable than durable goods. These expected profits have clear implications for the hypothetical pharmaceutical company with which we began this part. Recall that the firm is choosing whether to develop a new drug or a vaccine for a particular disease, where the two products have equal likelihood of success and equal periods of monopoly protection. Although society will often prefer the firm to focus on the vaccine due to the positive externalities, a profit-maximizing firm will instead focus on the expected profits from each product. And as we have discussed, these profits seem likely to be systematically skewed toward the drug rather than the vaccine. The resulting misalignment between social welfare and private R&D incentives has substantial implications for public health.
IV. Improving Vaccine Innovation Institutions
So far, we have presented a number of reasons to think that all else equal, preventative vaccines will be less profitable than therapeutic drugs, and that the expectation of lower profits creates lower market incentives to develop vaccines in the first place. What does this mean for vaccine innovation policy? In this final part, we highlight five lessons for policymakers interested in designing more effective biomedical innovation institutions, both for pandemic infections like COVID-19 and for other diseases.
First, as described in Part II, current policies surrounding vaccination in the United States do not demonstrate complete naïveté to the inefficiencies that would occur in an unregulated vaccine market. Programs such as immunization requirements for children,242 vaccine subsidies for certain groups,243 the VICP liability shield for manufacturers,244 and the ACA requirement of vaccine coverage with no cost-sharing245 serve to push consumption above its low equilibrium level. These policies each help recapture social surplus that would otherwise be foregone, although none guarantees achievement of the socially optimal outcome. For example, while vaccine mandates are probably welfare improving given the magnitude of the externalities (particularly the intergenerational externality),246 in some cases they may lead to costly ‘over-vaccination’—more money being spent on vaccination than is needed to achieve herd immunity. Without a more detailed quantitative analysis, we cannot place current policy relative to the optimum.
Second, the lack of rigorous evidence on the effects of current vaccine incentives is part of a broader problem of empirical certainty in innovation law. No one knows the ‘right’ amount of innovation, either in general or on vaccines in particular. As recently summarized by health economist Daris Lakdawalla, ‘whether innovation is too high or too low is a first-order—perhaps the first-order—policy question in the economics of the pharmaceutical industry.’247 It is also unclear how much innovation is actually spurred by innovation policies such as IP. Scholars have been unable to show that stronger patent rights lead to more research investments,248 much less that any such innovation benefit outweighs the costs of the patent system.249 Policymakers are thus operating under conditions of high empirical uncertainty.
Third, innovation policies must be made despite this uncertainty, and there are good theoretical reasons and suggestive empirical evidence to think that incentives are skewed toward therapeutic drugs rather than preventative vaccines, as presented in Part III. Given the positive externalities of vaccines, this distortion seems highly problematic. In other words, IP fails to capture society’s preference for durable preventatives over repeat-purchase treatments. Indeed, any purely market-set incentive is likely to fall short on this front: a monopolist cannot extract surplus from a durable vaccine to the degree it can from a repeat-purchase pharmaceutical treatment.
Fourth, and most importantly, policymakers can correct for these market failures with government-set rewards such as grants, prizes, and subsidies.250 An oft-noted disadvantage of government-set rewards is their inability to incorporate researchers’ private information about the value of innovation.251 However, in the case of vaccines, the market is almost sure to undervalue the invention—a strong argument for exogenously determined incentives to correct for these market failures. Most straightforwardly, government and non-profit research funders could increase direct spending on vaccine research through grants and national laboratories, or could increase tax incentives for vaccine research as through the Orphan Drug Act.252 Policymakers should also increase funding for public–private partnerships like CEPI, which was created after the Ebola outbreak.253 The NIH’s recent announcement that it will create a public–private partnership focused on COVID-19 seems like a step in the right direction.254
It also seems worth experimenting with more radical policy changes, such as offering a large monetary prize to the first firm to introduce a vaccine for a given disease. Health economists use metrics such as quality-adjusted life years (QALYs) to estimate the social burden of different diseases,255 and the prize amount could be tied to the net present value of the future lost QALYs.256 Such a prize could be modeled on the AMC that has been successfully implemented for pneumococcal disease.257 For example, one of us has proposed a $500 per patient vaccine for COVID-19, which would make such a vaccine one of the most profitable in history.258
Because vaccines’ positive externalities are conferred upon consumption and not simply upon invention or production, society needs to also incentivize use of these drugs. Crucially, these incentives need to be structured in a way that does not undermine innovation incentives—forcing firms to sell at low prices and giving them no other compensation would not be effective. After all, firms are unable even to capture all of consumers’ private benefits—much less the societal benefits—making them disinclined to invest in vaccine development. One of us has discussed the importance of decoupling innovation incentives and allocation mechanisms in this way, both in general259 and in the context of COVID-19 vaccines.260 In that spirit, a market with the following features could correct for the inefficiencies discussed above: innovators should be compensated in accordance with the social surplus their inventions generate, incentivizing innovation for which costs are high but benefits are even higher; meanwhile, consumers should have subsidized or low-cost access, allowing them to internalize some of the extra benefits to society.261 For example, government insurance programs such as Medicare and Medicaid can provide high reimbursement to producers and low-cost access to consumers.262 The federal government has already committed to paying for COVID-19 treatment for the uninsured at Medicare rates,263 and we think there is a strong argument that the government should also commit to covering the cost for any vaccine that proves to be effective.
The idea that governments should provide stronger incentives for new vaccines is not controversial among health policy scholars; however, we think this article goes beyond prior work in developing a framework for understanding the economic factors that lead to underinvestment in vaccines relative to traditional therapeutic pharmaceuticals. For example, Ana Santos Rutschman argues that public R&D funding for vaccines for outbreak diseases is particularly low.264 A number of other scholars have similarly pointed to private-sector gaps in vaccine financing and the need for greater investment from the government and non-profit sectors.265 The challenge, we think, is less in figuring out the direction policy should move and more in developing the political will to make it happen. And the difficulty of improving economic incentives through political markets should not be underestimated.266 As one of us has explained in the context of incentives for pain and addiction treatment, ‘non-market solutions are not a panacea: failures of the political market can be just as devastating, and comparisons of innovation institutions must consider the imperfections of each policy choice.’267
Our fifth and final suggestion for policymakers interested in improving vaccine innovation institutions is that they approach this task with intellectual humility, including a willingness to design policies in ways that will help answer some of the open questions in this area. Although we think the best existing theory and evidence suggests that incentives for developing new vaccines are insufficient relative to the incentives for therapeutic drugs, we have canvassed a number of questions that lack satisfactory answers. Most obviously, it would help to have more transparency about the actual costs and expected revenues from developing and distributing vaccines and pharmaceuticals. And innovation scholars still have a very imperfect understanding of how research investments respond to different policy changes.268
To address this empirical uncertainty, policymakers should look for opportunities to test different interventions in ways that improve the evidence base for innovation policy.269 For example, consider our above proposal to offer a standing prize to the first firm to successfully market a vaccine for a given disease.270 Rather than simply introducing this prize for all diseases that currently lack a vaccine, policymakers could offer a prize for a random set of diseases. Then one could see whether there are more research expenditures, clinical trials, and marketed vaccines for the diseases that had the offer of a prize reward.271 Without the use of randomization, it would be difficult to conclude that any increase in vaccine development effort was in fact caused by the prize rather than by other factors, such as increased scientific opportunities or changes in the costs of development. We think many academic teams would welcome the opportunity to collaborate with vaccine innovation institutions—including government agencies and non-profit funders—to design and evaluate pilots of the most promising policy interventions.
V. Conclusion
The COVID-19 pandemic is tragically demonstrating the human cost of underinvestment in vaccine research and infrastructure. As of this writing, many firms are pursuing COVID-19 vaccine candidates with unprecedented speed.272 But before the death toll began rising, there was little private-sector investment in vaccines for coronaviruses and similar problems—vaccines that might have been helpful in the early stages of the COVID-19 pandemic had been killed off during development as insufficiently profitable,273 and biomedical firms that invested in Ebola vaccines took major financial hits.274
In this article, we have argued that one cause for this underinvestment is that producers have difficulty capturing the social surplus generated by vaccines because of their preventative and durable nature, and that the problem is exacerbated by the large positive externalities associated with vaccine consumption. It remains unclear at this time whether the firm that first produces an effective COVID-19 vaccine will be rewarded with any significant profit. Instead, we think a pluralistic innovation policy environment that contains both a way of correcting the failures of market incentives and an allocation mechanism that incentivizes additional consumption would generate more social surplus overall. Prizes, market subsidies, grants, and tax incentives could all be used to accomplish these goals. The key point is simply that a COVID-19 vaccine—like vaccines for other infectious diseases—should be both profitable and broadly accessible, and that there is a vast innovation policy toolkit for accomplishing both.
Footnotes
*For helpful comments, we thank Wendy Epstein, Jacob Goldin, Daniel Hemel, Brad Larsen, Mark Lemley, Amy Madl, Jonathan Masur, Ana Santos Rutschman, Rachel Sachs, Jake Sherkow, Heidi Williams, Samantha Zyontz, participants at the 2019 IP Scholars Conference and the 2020 Works-in-Progress Intellectual Property Colloquium, and two anonymous reviewers.
See Sheri Fink, Worst-Case Estimates for U.S. Coronavirus Deaths, N.Y. Times (Mar. 13, 2020), https://www.nytimes.com/2020/03/13/us/coronavirus-deaths-estimate.html; After the Market’s Worst Day in Decades, Where Do We Go from Here?, N.Y. Times (Mar. 13, 2020), https://www.nytimes.com/2020/03/13/business/dealbook/coronavirus-markets-stocks.html (describing the loss of $4.7 trillion on a single day) (accessed May 8, 2020).
Nat’l Inst. of Med., What You Need to Know About Infectious Disease 11 (2001).
See Victoria Hansen et al., Infectious Disease Mortality Trends in the United States, 1980–2014, 316 JAMA 2149, 2149 (2016).
See Nat’l Acads. of Sci., Eng’g & Med., Global Health and the Future Role of the United States 43 (2017).
See, eg Philippe Aghion, Peter Howitt & Fabrice Murtin, The Relationship Between Health and Growth: When Lucas Meets Nelson-Phelps, 2 Rev. Econ. & Institutions 1 (2011); Robert J. Barro, Health and Economic Growth, 14 Annals Econ. & Fin. 305 (2013); David E. Bloom & David Canning, The Health and Wealth of Nations, 287 Science 1207 (2000); David N. Well, Accounting for the Effect of Health on Economic Growth, 122 Q.J. Econ. 1265 (2007).
See generally Nicholas Bloom, John Van Reenen & Heidi Williams, A Toolkit of Policies to Promote Innovation, J. Econ. Persp., Summer 2019, at 163, 168 (summarizing evidence).
See id.; Daniel J. Hemel & Lisa Larrimore Ouellette, Innovation Policy Pluralism, 128 Yale L.J. 544 (2019) (describing these institutions).
See One Year on, Global Observatory on Health R&D Identifies Striking Gaps and Inequalities, World Health Org. (Feb. 2018), https://www.who.int/features/2018/health-research-and-development/en (accessed May 8, 2020).
See, eg Rebecca S. Eisenberg & W. Nicholson Price, II, Promoting Healthcare Innovation on the Demand Side, 4 J.L. & Biosciences 3 (2017); Hemel & Ouellette, supra note 7; Rachel E. Sachs, Administering Health Innovation, 39 Cardozo L. Rev. 1991 (2018); Rachel E. Sachs, Prizing Insurance: Prescription Drug Insurance as Innovation Incentive, 30 Harv. J.L. & Tech. 153 (2016) [hereinafter Sachs, Prizing Insurance]; Jacob S. Sherkow, Cancer’s IP, 96 N.C. L. Rev. 297 (2018).
For a summary of recent policy proposals and the bipartisan support for lowering drug prices, see Mark A. Lemley, Lisa Larrimore Ouellette & Rachel E. Sachs, The Medicare Innovation Subsidy, 95 N.Y.U. L. Rev. 75 (2020).
See Amy Kapczynski & Talha Syed, The Continuum of Excludability and the Limits of Patents, 122 Yale L.J. 1900 (2013); Benjamin N. Roin, Unpatentable Drugs and the Standards of Patentability, 87 Tex. L. Rev. 503 (2009).
Preventative vaccines target the immune system to stimulate its response to disease. See U.S. Food & Drug Admin., Guidance for Industry: Content and Format of Chemistry, Manufacturing and Controls Information and Establishment Description Information for a Vaccine or Related Product 1 (1999), https://www.fda.gov/media/73614/download. While therapeutic vaccination has gained traction recently in the treatment of, for example, cancers, see Chunqing Guo et al., Therapeutic Cancer Vaccines: Past, Present, and Future, 119 Adv. Cancer Res. 421 (2013), this Article uses ‘vaccine’ in the traditional sense of immunizations intended to prevent—rather than treat—disease.
A number of law, economics, and medical articles have investigated aspects of the vaccine innovation ecosystem, but these works have generally not provided a framework for comparing the economic factors that lead to underinvestment in vaccines relative to traditional therapeutic pharmaceuticals. See, eg Nat’l Research Council, Vaccine Supply and Innovation (1985); Amy Finkelstein, Static and Dynamic Effects of Health Policy: Evidence from the Vaccine Industry, 119 Q.J. Econ. 527, 556–57 (2004); Amy Kapczynski, Order Without Intellectual Property Law: Open Science in Influenza, 102 Cornell L. Rev. 1539 (2017); Efthimios Parasidis, Recalibrating Vaccination Laws, 97 B.U. L. Rev. 2153 (2017); Ana Santos Rutschman, The Intellectual Property of Vaccines: Takeaways from Recent Infectious Disease Outbreaks, 118 Mich. L. Rev. Online 170 (2020); Ashley J. Stevens et al., The Role of Public-Sector Research in the Discovery of Drugs and Vaccines, 364 New Eng. J. Med. 535 (2011) (accessed May 8, 2020).
Nicholas Florko, Major Drug Makers Haven’t Stepped up to Manufacture NIH Coronavirus Vaccine, Top U.S. Health Official Says, STAT (Feb. 11, 2020), https://www.statnews.com/2020/02/11/major-drug-makers-havent-stepped-up-to-manufacture-coronavirus-vaccine-top-u-s-health-official-says (quoting Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases) (accessed May 8, 2020).
See Mike Hixenbaugh, Scientists Were Close to a Coronavirus Vaccine Years Ago. Then the Money Dried up., NBC News (Mar. 5, 2020), https://www.nbcnews.com/health/health-care/scientists-were-close-coronavirus-vaccine-years-ago-then-money-dried-n1150091 (accessed May 8, 2020).
Can the World Find a Good Covid-19 Vaccine Quickly Enough?, Economist (Apr. 16, 2020), https://www.economist.com/briefing/2020/04/16/can-the-world-find-a-good-covid-19-vaccine-quickly-enough.
See Brandee L. Stone et al., Brave New Worlds: The Expanding Universe of Lyme Disease, 17 Vector-Borne & Zoonotic Diseases 619 (2017).
See Emily R. Adrion et al., Health Care Costs, Utilization and Patterns of Care Following Lyme Disease, 10 PLoS One e0116767, at 12 (2015).
See Brittany Flaherty, Can a New Lyme Disease Vaccine Overcome a History of Distrust and Failure?, STAT (Aug. 22, 2019), https://www.statnews.com/2019/08/22/lyme-disease-vaccine-market (accessed May 8, 2020).
See Jane P. Messina et al., Global Distribution and Prevalence of Hepatitis C Virus Genotypes, 61 Hepatology 77, tbl. S1 (2015).
See Rebecca Robbins, The Lab Breakthrough that Paved the Way for Today’s Pricey Hepatitis C Cures, STAT (Sept. 13, 2016), https://www.statnews.com/2016/09/13/lab-breakthrough-hepatitis-c (accessed May 8, 2020).
See Jake Stone et al., The Potential Impact of a Hepatitis C Vaccine for People Who Inject Drugs: Is a Vaccine Needed in the Age of Direct-Acting Antivirals?, 11 PLoS One e0156213 (2016).
See HIV Statistics Overview, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/hiv/statistics/overview/index.html (accessed Nov. 21, 2019); Global Health Observatory (GHO) Data: HIV/AIDS, World Health Org., https://www.who.int/gho/hiv/en (accessed Feb. 11, 2020).
See HIV Cost-Effectiveness, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/hiv/programresources/guidance/costeffectiveness/index.html (accessed Oct. 31, 2019). First-line antiretroviral medicines have become more cost-effective over the past decade, making it possible to treat a large number of HIV patients in the developing world. See Luis Sagaon-Teyssier et al., Affordability of Adult HIV/AIDS Treatment in Developing Countries: Modelling Price Determinants for a Better Insight of the Market Functioning, 19 J. Int’l AIDS Soc’y 20619 (2016).
See Shefali Luthra & Anna Gorman, Rising Cost of PrEP to Prevent HIV Infection Pushes It out of Reach for Many, NPR (June 30, 2018), https://www.npr.org/sections/health-shots/2018/06/30/624045995/rising-cost-of-prep-a-pill-that-prevents-hiv-pushes-it-out-of-reach-for-many (accessed May 8, 2020).
See What Is a Preventative HIV Vaccine?, U.S. Dep’t Health & Human Servs., https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/19/96/what-is-a-preventative-hiv-vaccine (accessed June 27, 2019).
See Biological Approvals by Year, U.S. Food & Drug Admin., https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biological-approvals-year (accessed Feb. 19, 2020) (listing new vaccine approvals by year: one in 2018, two in 2017, one in 2016, three in 2015, two in 2014); Asher Mullard, 2018 FDA Drug Approvals, 18 Nature 85, 85 fig. 1 (2019) (showing 154 new small-molecule drugs and 59 new biologic drugs approved from 2014 through 2018); infra Section II.D.
See infra Section III.B.
See Pam Belluck & Adeel Hassan, Measles Outbreak Questions and Answers: Everything You Want to Know, N.Y. Times (Feb. 20, 2019), https://www.nytimes.com/2019/02/20/us/measles-outbreak.html (noting that for measles, ‘between 93 and 95% of people in a community need to be vaccinated to achieve herd immunity,’ and that ‘[d]uring the Disneyland outbreak in 2015, a 9-month-old child whose parents were planning to immunize contracted measles from an older child who had not been vaccinated’) (accessed May 8, 2020).
See supra note 14 and accompanying text.
For one discussion of scientific difficulties in vaccine development, see Ohid Yaqub & Paul Nightingale, Vaccine Innovation, Translational Research and the Management of Knowledge Accumulation, 75 Soc. Sci. & Med. 2143 (2012).
Each of these dimensions is a continuum rather than a dichotomy; some vaccines require boosters or have therapeutic as well as preventative benefits. But we think focusing on the polar ends illustrates the relevance of each dimension. See infra notes 144–146 and accompanying text.
For more on the role of insurance and healthcare payers, see infra notes 193–196 and accompanying text.
‘Risk-neutral’ means that purchasers are indifferent between levels of risk, such as the choice between facing a guaranteed $1000 cost or a 1% chance of a $100,000 cost. In contrast, a risk-averse purchaser would prefer paying more than $1000 to avoid the risk of a bigger loss.
See infra Section III.C.1.
See, eg Influenza Vaccination Coverage and Effectiveness, World Health Org., http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/influenza-vaccination-coverage-and-effectiveness (accessed May 2, 2020).
See generally Nat’l Vaccine Advisory Comm., Overcoming Barriers to Low HPV Vaccine Uptake in the United States: Recommendations from the National Vaccine Advisory Committee, 131 Pub. Health Rep. 17 (2016) (discussing low uptake of Gardasil).
See infra Section III.C.2.
Many Americans receive health care through their employer, and the median number of years that U.S. workers had been with their current employer was 4.2 years in 2018. See Press Release, U.S. Bureau of Labor Statistics, Employee Tenure Summary (Sept. 20, 2018), https://www.bls.gov/news.release/tenure.nr0.htm. Switching rates are also high on Affordable Care Act marketplaces. See Margot Sanger-Katz, High Rate of Shopping and Switching in Obamacare Plans Is a Good Sign, N.Y. Times (Feb. 26, 2015), https://www.nytimes.com/2015/02/27/upshot/high-rate-of-shopping-and-switching-in-obamacare-plans-is-a-good-sign.html. See also infra note 195 and accompanying text (accessed May 8, 2020).
Michael Kremer & Christopher M. Snyder, Preventatives Versus Treatments, 130 Q.J. Econ. 1167 (2015); see infra Section III.D.1.
Price discrimination refers to the ability to charge difference prices to difference consumers, rather than setting a uniform market price. When a monopolist patent holder can price discriminate, her profits are closer to the social value of her invention, and the deadweight loss of monopoly pricing is reduced. See generally Hemel & Ouellette, supra note 7, at 579–80.
See infra Section III.D.2.
For a discussion of existing programs in the United States and other jurisdictions, see infra Section II.B. Interestingly, single-payer healthcare is rarely combined with mandatory vaccination, making it difficult to draw conclusions about the impact of differing levels of government involvement on uptake. See infra note 86 and accompanying text.
See, eg Gavin Yamey, A Coronavirus Vaccine Should Be for Everyone, Not Just Those Who Can Afford It, STAT (Mar. 5, 2020), https://www.statnews.com/2020/03/05/coronavirus-vaccine-affordable-for-everyone; Ted Barrett, Manu Raju & Clare Foran, Vaccine Affordability a Last Sticking Point in Coronavirus Funding Package Talks in Congress, CNN (Mar. 3, 2020), https://www.cnn.com/2020/03/03/politics/coronavirus-funding-fight-congress/index.html (accessed May 8, 2020).
See infra Part IV.
See id.
See generally Ana Santos Rutschman, The Mosaic of Coronavirus Vaccine Development: Systemic Failures in Vaccine Innovation, J. Int’l Aff. (Mar. 21, 2020), https://jia.sipa.columbia.edu/online-articles/mosaic-coronavirus-vaccine-development-systemic-failures-vaccine-innovation (accessed May 8, 2020).
See Daniel Hemel & Lisa Larrimore Ouellette, Want a Coronavirus Vaccine, Fast? Here’s a Solution, Time (Mar. 4, 2020), https://time.com/5795013/coronavirus-vaccine-prize-challenge (accessed May 8, 2020).
See The Global Use of Medicine in 2019 and Outlook to 2023, IQVIA (Jan. 29, 2019), https://www.iqvia.com/institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023. As helpfully explained by Nicholson Price and Arti Rai, ‘[i]n terms of size and rough complexity, if an aspirin were a bicycle, a small biologic would be a Toyota Prius, and a large biologic would be an F-16 fighter jet.’ W. Nicholson Price II & Arti K. Rai, Manufacturing Barriers to Biologics Competition and Innovation, 101 Iowa L. Rev. 1023, 1023 (2016) (accessed May 8, 2020).
See Tara Azimi et al., Refueling the Innovation Engine in Vaccines, McKinsey & Co. (May 2019), https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/refueling-the-innovation-engine-in-vaccines (accessed May 8, 2020).
This Article focuses on removing distortions in incentives, all else equal, but we do not mean to imply that there are no differences in scientific opportunities. For some vaccines, technological hurdles may be a bigger deterrent to private investment than lack of incentives.
See R. Gordon Douglas & Vijay B. Samant, The Vaccine Industry, in Vaccines 33, 33 (Stanley A. Plotkin et al. eds., 6th ed. 2013); Stuart A. Thompson, How Long Will a Vaccine Really Take?, N.Y. Times (Apr. 30, 2020), https://www.nytimes.com/interactive/2020/04/30/opinion/coronavirus-covid-vaccine.html (accessed May 8, 2020).
Norman W. Baylor, The Regulatory Evaluation of Vaccines for Human Use, in 2 Vaccine Design: Methods and Protocols 773, 775 (Sunil Thomas ed., 2016).
Id. at 777; see also Brian Dean Abramson, Vaccine, Vaccination, and Immunization Law 2–7 to −11 (2018) (providing a detailed overview).
Baylor, supra note 52, at 778.
Id.
See id. at 778–79; Abramson, supra note 53, at 2–11 to −12; Douglas & Samant, supra note 51, at 34.
Douglas & Samant, supra note 51, at 34.
See Eric Boodman, Researchers Rush to Test Coronavirus Vaccine in People Without Knowing How Well It Works in Animals, STAT (Mar. 11, 2020), https://www.statnews.com/2020/03/11/researchers-rush-to-start-moderna-coronavirus-vaccine-trial-without-usual-animal-testing; Thompson, supra note 51.
Ernst R. Berndt, Rena N. Denoncourt & Anjli C. Warner, U.S. Markets for Vaccines: Characteristics, Case Studies, and Controversies 14 (2009).
Id. Where human efficacy studies are not ethical or feasible, the FDA may also permit approval of certain biological products based on animal studies, but ‘FDA rarely allows for approvals of vaccines under the Animal Rule.’ Abramson, supra note 53, at 2–10. There has been increasing use of surrogate endpoints for non-preventative medicines as well, see FDA Facilitates the Use of Surrogate Endpoints in Drug Development, U.S. Food & Drug Admin. (Nov. 5, 2018), https://www.fda.gov/drugs/fda-facilitates-use-surrogate-endpoints-drug-development-november-5-2018-issue, which may shorten clinical trials for these medicines, potentially exacerbating the bias toward developing treatments over preventatives (accessed May 8, 2020).
Berndt, supra note 59, at 14–15 (noting ‘unusually large phase III trials,’ such as 140,000 children for a rotavirus vaccine).
Id. at 26.
See Douglas & Samant, supra note 51, at 34; see also Thompson, supra note 51 (‘Those factories follow strict guidelines governing biological facilities and usually take around 5 years to build, costing at least three times more than conventional pharmaceutical factories.’).
See Isobel Asher Hamilton, Bill Gates Is Helping Fund New Factories for 7 Potential Coronavirus Vaccines, Even Though It Will Waste Billions of Dollars, Bus. Insider (Apr. 3, 2020), https://www.businessinsider.com/bill-gates-factories-7-different-vaccines-to-fight-coronavirus-2020-4. For other ways the COVID-19 vaccine process might be accelerated, see Thompson, supra note 51 (accessed May 8, 2020).
See Vaccines, U.S. Food & Drug Admin., https://www.fda.gov/vaccines-blood-biologics/vaccines (accessed July 5, 2019); Norman W. Baylor & Valerie B. Marshall, Regulation and Testing of Vaccines, in Vaccines 1427, 1427 (Stanley A. Plotkin et al. eds., 6th ed. 2013).
42 U.S.C. § 262. Vaccines and other biologics are also regulated as ‘drugs’ under the Food, Drug and Cosmetic Act. For an overview, see Abramson, supra note 53, at 2–2 to −6.
See Abramson, supra note 53, at 2–11 to −13.
See id. at 2–16; see also 21 C.F.R. § 601.2 (requiring, among other submissions, ‘data derived from non-clinical laboratory and clinical studies which demonstrate that the manufactured product meets prescribed requirements of safety, purity, and potency’).
See Vaccines Licensed for Use in the United States, U.S. Food & Drug Admin., https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm093833.htm (accessed Feb. 7, 2020).
Evidence-Based Recommendations—GRADE, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/vaccines/acip/recs/GRADE/about-grade.html (accessed Oct. 23, 2018).
Immunization Schedules, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/vaccines/schedules/index.html (accessed Feb. 3, 2020).
See Abramson, supra note 53, at 6–11 to −12.
42 U.S.C. § 300gg-13(a) (‘A group health plan and a health insurance issuer offering group or individual health insurance coverage shall, at a minimum provide coverage for and shall not impose any cost sharing requirements for... (2) immunizations that have in effect a recommendation from the Advisory Committee on Immunization Practices.. ..’).
See Abramson, supra note 53, at 2–21 to −22.
See id. at 2–19, 2–21.
See id. at 2–19.
See id. at 8–2 to −3 (describing this Vaccine Adverse Event Reporting System).
42 U.S.C. §§ 300aa-1 to 300aa-34; see Abramson, supra note 53, at 9–3 to −34.
See Geoffrey Evans et al., Legal Issues, in Vaccines 1481, 1483 (Stanley A. Plotkin et al. eds., 6th ed. 2013); Covered Vaccines, Health Resources & Servs. Admin., https://www.hrsa.gov/vaccine-compensation/covered-vaccines/index.html (accessed Jan. 2020).
See Evans et al., supra note 79, at 1483.
Health Resources & Servs. Admin., National Vaccine Injury Compensation Program Monthly Statistics Report 9 (Feb 2020), https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/data-statistics-vicp.pdf.
See, eg Nora Freeman Engstrom, A Dose of Reality for Specialized Courts: Lessons from the VICP, 163 U. Pa. L. Rev. 1631 (2015); Peter H. Meyers, Fixing the Flaws in the Federal Vaccine Injury Compensation Program, 63 Admin. L. Rev. 785 (2011); Lainie Rutkow et al., Balancing Consumer and Industry Interests in Public Health: The National Vaccine Injury Compensation Program and Its Influence During the Last Two Decades, 111 Penn St. L. Rev. 681 (2007).
See Abramson, supra note 53, at 9–34 to −41; see also Bruesewitz v. Wyeth, 562 U.S. 223 (2011) (holding that the National Childhood Vaccine Injury Act of 1986, which established the VICP, preempts design-defect claims against vaccine manufacturers).
Falk Ehmann et al., Regulation of Vaccines in Europe, in Vaccines 1447, 1447–49 (Stanley A. Plotkin et al. eds., 6th ed. 2013).
See Patricia M. Danzon et al., Vaccine Supply: A Cross-National Perspective, 24 Health Aff. 706, 709 (2005).
The UK, Denmark, Finland, Norway, and Sweden all have true single-payer healthcare but do not require vaccines for school enrollment, though some European countries with multi-payer universal healthcare have such requirements. See Elena Bozzola et al., Mandatory Vaccinations in European Countries, Undocumented Information, False News and the Impact on Vaccination Uptake: The Position of the Italian Pediatric Society, 44 Italian J. Pediatrics 67 (2018). In Canada, only the provinces of Ontario and New Brunswick mandate a full set of vaccines, while Manitoba mandates a measles vaccine. See Erin Walkinshaw, Mandatory Vaccinations: The Canadian Picture, 183 CMAJ E1165, E1165 (2011); Is Immunization Mandatory in Canada?, Immunize Canada, https://immunize.ca/immunization-mandatory-canada (accessed Apr. 11, 2020).
Danzon et al., supra note 85, at 711–12.
Id. at 712.
See Lahouari Belgharbi et al., Regulation of Vaccines in Developing Countries, in Vaccines 1454 (Stanley A. Plotkin et al. eds., 6th ed. 2013); World Health Org., Global Vaccine Market Report (Oct. 2018), https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/MI4A_Global_Vaccine_Market_Report.pdf (accessed May 8, 2020).
Immunization, Vaccines and Biologicals: Procurement Mechanisms and Systems, World Health Org., https://www.who.int/immunization/programmes_systems/procurement/mechanisms_systems/pooled_procurement/en/index2.html (accessed Feb. 11, 2020). For an overview of the sources of vaccine assistance for low- and middle-income countries, see Annie Haakenstad et al., Vaccine Assistance to Low- and Middle-Income Countries Increased to $3.6 Billion in 2014, 35 Health Aff. 242 (2016). Even with this help, many children remain unvaccinated. See, eg Ramanan Laxminarayan & Nirmal Kumar Ganguly, India’s Vaccine Deficit: Why More than Half of Indian Children Are Not Fully Immunized, and What Can—and Should—Be Done, 30 Health Aff. 1096 (2011).
Immunization, Vaccines and Biologicals: Procurement Mechanisms and Systems, supra note 90.
See World Health Org., supra note 89, at 4, 8.
See Abramson, supra note 53, at 9–44; Mary S. Holland, Liability for Vaccine Injury: The United States, the European Union, and the Developing World, 67 Emory L.J. 415 (2018). For a proposal for international coordination on vaccine liability, see John D. Winter, Cassye Cole & Jonah Wacholder, Toward a Global Solution on Vaccine Liability and Compensation, 74 Food & Drug L.J. 1 (2019).
See generally Daniel J. Hemel & Lisa Larrimore Ouellette, Beyond the Patents–Prizes Debate, 92 Tex. L. Rev. 303 (2013) (providing a framework for comparing innovation incentives, including along this market-set versus government-set dimension); Lisa Larrimore Ouellette, Patentable Subject Matter and Nonpatent Innovation Incentives, 5 U.C. Irvine L. Rev. 1115, 1128–34 (2015) (using this framework to provide an overview of innovation incentives in the biomedical context).
See generally Roin, supra note 11, at 545–47 (describing this screening process for pharmaceuticals).
See generally Hemel & Ouellette, supra note 94, at 352–55. In some cases, for-profit pharmaceutical companies may pursue unprofitable projects for the reputational benefits or to develop distribution networks for future profits in emerging economies. See Donald G. McNeil Jr., Drug Companies Are Focusing on the Poor After Decades of Ignoring Them, N.Y. Times (June 24, 2019), https://www.nytimes.com/2019/06/24/health/drugs-poor-countries-africa.html (accessed May 8, 2020).
See, eg Dan L. Burk & Mark A. Lemley, Policy Levers in Patent Law, 89 Va. L. Rev. 1575, 1576 (2003) (‘Patent law is our primary policy tool to promote innovation... .’).
See Hemel & Ouellette, supra note 94, at 327–28.
Karen Durell, Vaccines and IP Rights: A Multifaceted Relationship, in 2 Vaccine Design 791, 796 (Sunil Thomas ed., 2016); see also Abramson, supra note 53, at 3–4 to −119 (providing an overview of patent law as applied to vaccines). Additionally, many jurisdictions—including the United States, some European countries, and Japan—allow patent terms to be extended to account for the time lost to regulatory approval. See Adam C. Krol et al., From the Bench to the Pharmacy: Protecting Innovation During Vaccine Development and Commercialization, in 2 Vaccine Design 813, 825 (Sunil Thomas ed., 2016).
See, eg Médecins Sans Frontières, A Fair Shot for Vaccine Affordability 2 (2017), https://msfaccess.org/sites/default/files/VAC_report_A%20Fair%20Shot%20for%20Vaccine%20Affordability_ENG_2017.pdf (concluding that patents ‘pose a threat to access to affordable versions of newer vaccines’); Catherine Saez, Access to Vaccines, Patents Growing Concern, Panellists Say (June 10, 2014), https://www.ip-watch.org/2014/06/10/access-to-vaccines-patents-growing-concerns-panellists-say (reporting that representatives from the WHO and Gavi said that ‘for some new vaccines, patents can constitute a barrier’ to access and that ‘patent thickets are becoming a problem for vaccines’) (accessed May 8, 2020).
Pub. L. No. 111–148, § 7002, 124 Stat. 804, 807 (2010). The BPCIA was based on the earlier Hatch–Waxman Act for small-molecule drugs. Drug Price Competition and Patent Term Restoration Act of 1984, Pub. L. No. 98–417, 98 Stat. 1585. In addition, drugs or biological products (including vaccines) for rare diseases can receive 7 years of market exclusivity under the Orphan Drug Act. 21 U.S.C. § 360 cc.
All biosimilar approvals have come not from CBER, but from its partner in regulating biologics, the Center for Drug Evaluation and Research (CDER), which regulates a different set of pharmaceutical products. See Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations, U.S. Food & Drug Admin., https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/purple-book-lists-licensed-biological-products-reference-product-exclusivity-and-biosimilarity-or (accessed July 2, 2019). Although there have been no biosimilar vaccine approvals, there have been cases in which more than one brand-name company has marketed a vaccines for the same disease, meaning that they each conducted their own clinical trials. For example, both Merck and GlaxoSmithKline market vaccines for hepatitis B under the brands Recombivax HB and Engerix-B. See supra note 69.
See Abramson, supra note 53, at 4–8 to −10; Durell, supra note 99, at 801–02.
See Price & Rai, supra note 48. But see Rebecca Weires, Recent Advances in Biologics Manufacturing Diminish the Importance of Trade Secrets: A Response to Price and Rai, Written Description (Mar. 4, 2019), https://writtendescription.blogspot.com/2019/03/recent-advances-in-biologics.html (arguing that scientific advances may decrease the importance of trade secrets in this context) (accessed May 8, 2020).
See Hemel & Ouellette, supra note 94, at 321–26 (explaining I.R.C. §§ 41, 174).
See id. at 379 (discussing I.R.C. § 45C). The orphan drug tax credit was cut from 50 to 25% by the Dec. 2017 tax reform. See Tax Cuts and Jobs Act, Pub. L. No. 115–97, § 13401(a). For an example of a vaccine receiving orphan drug status, see Christina Mattina, Vaccine for Glioblastoma Awarded Orphan Drug Status by FDA, Am. J. Managed Care (Aug. 10, 2017), https://www.ajmc.com/newsroom/vaccine-for-glioblastoma-awarded-orphan-drug-status-by-fda. On applying the orphan drug tax credit in the COVID-19 context, see Lisa Larrimore Ouellette, Does Gilead’s (Withdrawn) Orphan Designation Request for a Potential Coronavirus Treatment Deserve Your Outrage?, Written Description (Mar. 25, 2020), https://writtendescription.blogspot.com/2020/03/does-gileads-withdrawn-orphan.html (accessed May 8, 2020).
See Douglas & Samant, supra note 51, at 38–39 (noting that ‘unlike pharmaceuticals, old vaccines continue to be profitable for a variety of reasons,’ including the difficulty of generic approval and limitations on ‘access to know-how’).
Id. at 39. It is unclear to us why Merck, the MMR vaccine producer, has not raised the price significantly. But in any case, Merck may soon have a competitor. See Nicola P. Klein et al., Immunogenicity and Safety of a Measles-Mumps-Rubella Vaccine Administered as a First Dose to Children Aged 12 to 15 Months: A Phase III, Randomized, Noninferiority, Lot-to-Lot Consistency Study, 9 J. Pediatric Infectious Diseases Soc’y 194 (2020).
See generally Ana Santos Rutschman, The Vaccine Race in the 21st Century, 61 Ariz. L. Rev. 729, 741 fig. 1 (2019) (illustrating this consolidation).
See generally E. Glen Weyl & Jean Tirole, Market Power Screens Willingness-to-Pay, 127 Q.J. Econ. 1971 (2012) (explaining how above-marginal-cost pricing serves a screening function that distinguishes marginal technological improvements from more substantial ones).
See Hemel & Ouellette, supra note 7, at 555–56.
Jonathan J. Darrow, Michael S. Sinha & Aaron S. Kesselheim, When Markets Fail: Patents and Infectious Disease Products, 73 Food & Drug L.J. 361, 362 (2018). But see Mark Eccleston-Turner, The Economic Theory of Patent Protection and Pandemic Influenza Vaccines: Do Patents Really Incentivize Innovation in the Field?, 42 Am. J.L. & Med. 572, 572 (2016) (arguing that for pandemic influenza vaccines, ‘there is a very strong incentive to innovate because... manufacturers are selling a product for which demand exceeds supply to a captive market of nations and organizations’).
See Hemel & Ouellette, supra note 94, at 320–21.
Kapczynski, supra note 12, at 1566.
World Health Org., Operational Considerations for COVID-19 Surveillance Using GISRS (Mar. 2020), https://apps.who.int/iris/bitstream/handle/10665/331589/WHO-2019-nCoV-Leveraging_GISRS-2020.1-eng.pdf (accessed May 8, 2020).
See Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC), Nat’l Insts. of Health (Apr. 19, 2019), https://report.nih.gov/categorical_spending.aspx. This chart divides research into categories that are not mutually exclusive, and more specific categories indicate that in 2018 the NIH spent $37 million on HPV and cervical cancer vaccines, $57 million on malaria vaccines, $40 million on tuberculosis vaccines, and $562 on vaccines related to AIDS, suggesting that other vaccine spending was about $1.3 billion. Id (accessed May 8, 2020).
See Vaccine Research Center, Nat’l Inst. of Allergy & Infectious Diseases, https://www.niaid.nih.gov/about/vrc (accessed July 31, 2019).
See Douglas & Samant, supra note 51, at 35.
Other U.S. agencies that fund R&D related to vaccines include the Centers for Disease Control and Prevention (CDC), the Department of Defense (DOD) (including through the Walter Reed Army Institute of Research, the U.S. Army Medical Research Institute for Infectious Diseases, and the Naval Medical Research Center), the Biomedical Advanced Research and Development Authority (BARDA) within Health and Human Services, and the U.S. Agency for International Development (USAID). Id. at 35–36.
Id. at 36. On the Gates Foundation’s role in the COVID-19 response, see Bill Gates, The First Modern Pandemic, GatesNotes (Apr. 23, 2020), https://www.gatesnotes.com/Health/Pandemic-Innovation (accessed May 8, 2020).
For a review, see Lisa Larrimore Ouellette & Rebecca Weires, University Patenting: Is Private Law Serving Public Values? 2019 Mich. St. L. Rev. (forthcoming 2020), https://www.ssrn.com/abstract=3443692 (accessed May 8, 2020).
See Biological Approvals by Year, U.S. Food & Drug Admin. Archive, http://wayback.archive-it.org/7993/20170111000524/http://www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/BiologicalApprovalsbyYear/default.htm (accessed Jan. 11, 2017).
See Stevens et al., supra note 12, at supp. App. (listing 15 vaccines approved by the FDA between 1970 and 2009 with related public-sector patents, which we compared with the FDA lists of approvals by year to find nine in the 1998–2009 timeframe). As previously noted, unlike for small-molecule drugs, manufacturers of biologics are not required to publicly declare what patents they believe cover their products, so this list may be under-inclusive. The researchers looked for public announcements about successful academic licensing and, as a final check, sent their list to directors of university technology transfer offices. Id. at 538.
See Selected Discontinued U.S. Vaccines, Ctrs. for Disease Control & Prevention (May 2019), https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/discontinued-vac.pdf (listing Certiva, RotaShield, and LYMErix among discontinued vaccines) (accessed May 8, 2020).
See Hemel & Ouellette, supra note 94, at 317–19.
See, eg James Love & Tim Hubbard, Prizes for Innovation in New Medicines and Vaccines, 18 Annals Health L. 155 (2009).
See Heidi Williams, Innovation Inducement Prizes: Connecting Research to Policy, 31 J. Pol’y Analysis 752, 758–59 (2012).
See id. at 769; How the Pneumococcal AMC Works, Gavi: The Vaccine Alliance, https://www.gavi.org/investing-gavi/innovative-financing/pneumococcal-amc/how-it-works (accessed May 8, 2020).
See Benjamin N. Roin, Intellectual Property Versus Prizes: Reframing the Debate, 81 U. Chi. L. Rev. 999, 1011 (2014) (noting that ‘most developed countries already accomplish (or could accomplish) the same basic objectives of the prize system [for prescription drugs] through their national prescription-drug insurance programs’); Sachs, Prizing Insurance, supra note 9. In practice, demand-side subsidies differ from some prize proposals in that the reward is not limited to the first product to market, although subsidy or prize design could be adjusted to be more or less exclusive, and the optimal exclusivity will likely differ by medical product.
See Lemley et al., supra note 10.
Finkelstein, supra note 12, at 535.
Id. at 542.
As noted above, these exist in every U.S. state, see supra note 72 and accompanying text, but are considerably rarer outside of the U.S., see supra note 86 and accompanying text.
See Vaccines for Children Program (VFC): About VFC, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/vaccines/programs/vfc/about/index.html (accessed Feb. 18, 2016). The Vaccines for Children program has been effective at increasing update. See Brendan Walsh et al., Since the Start of the Vaccines for Children Program, Uptake Has Increased, and Most Disparities Have Decreased, 35 Health Aff. 356 (2016).
Thomas J. Hwang & Aaron S. Kesselheim, Vaccine Pipeline Has Grown During the Past Two Decades with More Early-Stage Trials from Small and Medium-Size Companies, 35 Health Aff. 219, 223 (2016).
Azimi et al., supra note 49.
For overviews of overall vaccine R&D funding, see Abramson, supra note 53, at ch. 5; Douglas & Samant, supra note 51, at 35.
See supra note 26 and accompanying text.
Darrow et al., supra note 112, at 362.
See Azimi et al., supra note 49.
See Lars Noah, Triage in the Nation’s Medicine Cabinet: The Puzzling Scarcity of Vaccines and Other Drugs, 54 S.C. L. Rev. 741 (2003); David B. Ridley, Xiaoshu Bei & Eli B. Liebman, No Shot: US Vaccine Prices and Shortages, 35 Health Aff. 235 (2016).
See Azimi et al., supra note 49.
See supra note 30 and accompanying text.
See supra note 12 (discussing therapeutic cancer vaccines).
See Darrow et al., supra note 112, at 369 (‘Although repeated vaccinations are possible, the CDC adult immunization schedule does not include recommendations for boosters for most vaccines, and a patient may receive a given vaccine sequence only once in his or her lifetime. Most vaccine sequences do not exceed three doses, and some immunizations consist of a single injection per vaccine or even less....’).
Cf. Hemel & Ouellette, supra note 7, at 555 (‘Some of the most oft-used dichotomies in legal analysis, such as rules versus standards and property rules versus liability rules, also admit of intermediate cases and blurred lines. Framing a problem in polar terms allows us to focus on the reasons why policy makers might prefer to tilt more in one direction than the other.. ..’).
This is a plausible assumption for many products, see supra Section II.C.1, but note that if either the drug or the vaccine has a longer expected IP life, that may skew incentives in its favor. See generally Eric Budish, Benjamin N. Roin & Heidi Williams, Do Firms Underinvest in Long-Term Research? Evidence from Cancer Clinical Trials, 105 Am. Econ. Rev. 2044 (2015) (demonstrating comparative underinvestment in R&D for cancer drugs with shorter effective patent term).
See generally Colleen Cunningham, Florian Ederer & Song Ma, Killer Acquisitions (Mar. 22, 2019) (unpublished manuscript), https://ssrn.com/abstract=3241707 (documenting how incumbent pharmaceutical firms acquire innovative targets solely to kill competing projects) (accessed May 8, 2020).
See generally Azimi et al., supra note 49 (suggesting that prospective vaccines have ‘increased opportunity cost as relative investment economics converge with other biologics,’ particularly given the number of blockbuster biologics, and ‘high technical complexity and commercial uncertainty compared with recent [vaccine] innovations’).
See supra notes 59–61.
See supra note 60 and accompanying text.
See Dimitrios Gouglas et al., Estimating the Cost of Vaccine Development Against Epidemic Infectious Diseases: A Cost Minimisation Study, 6 Lancet Global Health e1386 (2018); Donald W. Light et al., Estimated Research and Development Costs of Rotavirus Vaccines, 27 Vaccine 6627 (2009); Arianna Waye et al., Vaccine Development Costs: A Review, 12 Expert Rev. Vaccines 1495 (2013).
See id.
See Berndt et al., supra note 59, at 24–25.
See Frank A. Sloan, The Economics of Vaccines, in The Oxford Handbook of the Economics of the Biopharmaceutical Industry 524, 526–28 (Patricia M. Danzon & Sean Nicholson eds., 2012).
Immunization, World Health Org., https://www.who.int/topics/immunization/en (accessed Feb. 11, 2020). This does not mean that the distribution costs are insignificant. While the durable nature of vaccines makes them less costly to distribute than similar repeat-purchase goods, they do often need to be kept at certain temperatures through a ‘cold chain,’ which can be challenging in some countries. See World Health Org., Immunization in Practice ch. 2 (2015), https://www.who.int/immunization/documents/IIP2015_Module2.pdf (accessed May 8, 2020).
Tracy A. Lieu et al., Overcoming Economic Barriers to the Optimal Use of Vaccines, 24 Health Aff. 666, 666 (2005). A review of economic analyses for vaccines found that vaccines for a wide array of diseases were cost justified, some even without taking into account social benefits. Mark A. Miller & Alan R. Hinman, Economic Analyses of Vaccine Policies, in Vaccines 1413 (Stanley A. Plotkin et al. eds., 6th ed. 2013).
Why Are Childhood Vaccines So Important?, Ctrs. For Disease Control & Prevention, https://www.cdc.gov/vaccines/vac-gen/howvpd.htm (accessed May 16, 2018); see also World Health Org., The Impact of the Expanded Program on Immunization and the Polio Eradication Initiative on Health Systems in the Americas: Final Report of the ‘Taylor Commission’ 2 (1995) (emphasizing the importance of a ‘culture of prevention’ that the polio eradication initiative built).
F.E. Andre et al., Vaccination Greatly Reduces Disease, Disability, Death and Inequity Worldwide, 86 Bull. World Health Org. 140, 143 (2008).
See Miller & Hinman, supra note 157, for a review of some disease-specific studies.
See Paul Fine, Ken Eames & David L. Keymann, “Herd Immunity”: A Rough Guide, 52 Clinical Infectious Diseases 911 (2011).
See, eg Belluck & Hassan, supra note 28; Azhar Hussain et al., The Anti-Vaccination Movement: A Regression in Modern Medicine, 10 Cureus e2919 (2018). It is worth noting that the externality distortion is exacerbated by the fact that estimates even of private costs may be incorrect—for example, if people are worried about an improbable or impossible side effect.
Furthermore, the WTO has emphasized that for those diseases that would be treated with antibiotics, vaccines can slow the progress of antibiotic resistance by obviating their use. See Andre et al., supra note 159; Why Is Vaccination Important for Addressing Antibiotic Resistance, World Health Org. (Nov. 2016), https://www.who.int/features/qa/vaccination-antibiotic-resistance/en (accessed May 8, 2020).
See Pien Huang, What We Know About the Silent Spreaders of COVID-19, NPR (Apr. 13, 2020), https://www.npr.org/sections/goatsandsoda/2020/04/13/831883560/can-a-coronavirus-patient-who-isnt-showing-symptoms-infect-others (accessed May 8, 2020).
See supra note 5 and accompanying text.
See World Health Organization, WHO Guide to Identifying the Economic Consequences of Disease and Injury 31–32 (2003).
See supra note 1 and accompanying text.
Id. at 36. For an interesting case study, see Edward Miguel & Michael Kremer, Worms: Identifying Impacts on Education and Health in the Presence of Treatment Externalities, 72 Econometrica 159 (2004).
See Walter R. Dowdle, The Principles of Disease Elimination and Eradication, 76 (Supp. 2) Bull. World Health Org. 22 (1998).
See Frank Fenner, Global Eradication of Smallpox, 4 Revs. Infectious Diseases 916 (1982); David Brown, Rinderpest, or ‘Cattle Plague,’ Becomes Only Second Disease to Be Eradicated, Wash Post. (May 26, 2011), https://www.washingtonpost.com/national/rinderpest-or-cattle-plague-becomes-only-second-disease-to-be-eradicated/2011/05/26/AGMPENCH_story.html; Oyewale Tomori, From Smallpox Eradication to the Future of Global Health: Innovations, Application and Lessons for Future Eradication and Control Initiatives, 29 (Supp. 4) Vaccine D145, D146 (2011) (explaining how ‘preventative over curative approaches’ played an instrumental role in eradicating smallpox) (accessed May 8, 2020).
Elimination has been accomplished for some diseases for which a vaccine does not exist: for example, yaws, a debilitating bacterial infection of the skin and bones, has been eliminated in India. See Press Release, World Health Org., WHO Declares India Free of Yaws (May 11, 2016), https://www.who.int/neglected_diseases/news/India_free_of_yaws/en. However, yaws appears to be the exception rather than the rule (accessed May 8, 2020).
Brian Greenwood, The Contribution of Vaccination to Global Health: Past, Present and Future, 369 Philosophical Transactions Royal Soc’y B 20130433 (2014).
See Tomori, supra note 170.
See David L. Thomas, Global Control of Hepatitis C: Where Challenge Meets Opportunity, 19 Nature Med. 850, 853 (2013) (‘As with any infectious disease, the optimal method of HCV control is prevention.’).
See Dracunculiasis Eradication, World Health Org., https://www.who.int/dracunculiasis/eradication/en (accessed Feb. 11, 2020) (explaining that control and elimination have been achieved not by treating infections but by preventing them, since the parasite requires a host to reproduce).
See Charles I. Jones & Peter J. Klenow, Beyond GDP? Welfare Across Countries and Time, 106 Am. Econ. Rev. 2426 (2016).
See Pierre-Yves Geoffard & Tomas Philipson, Disease Eradication: Private Versus Public Vaccination, 87 Am. Econ. Rev. 222 (1997).
See Scott Barrett, Eradication Versus Control: The Economics of Global Infectious Disease Policies, 82 Bull. World Health Org. 683 (2004).
Jenifer Ehreth, The Global Value of Vaccination, 21 Vaccine 596, 599 (2003).
Id. at 598.
Frank Fenner et a., Smallpox and Its Eradication 1365 (1988).
Matan C. Dabora et al., Financing and Distribution of Pharmaceuticals in the United States, 318 JAMA 21 (2017).
Id. at 21 (‘The US distributor market is highly consolidated, with 3 companies accounting for more than 85% of market share: AmerisourceBergen, Cardinal Health, and McKesson.’). Distributors, by taking on some of the cost associated with providing a good, can capture a chunk of the surplus between consumer valuation and producer costs. Suppose consumers value a product in the range of $20–$100 and the product costs $10 to produce and $10 to distribute. In the absence of distributors, the monopolist would accept any price higher than $20 (but would try to push the price up toward $100), say $60. In a world with a competitive distributor market, the monopolist can earn the same profit by selling to distributors at a price of $50, leaving $10 of room for distributors to jump in. When the distributor market is truly competitive, no distributor would have the power to capture more than their $10 costs. However, in a world with three large distributors, the distributors may have enough market power to buy from producers at a lower price than they otherwise would, and to raise the price they charge to consumers. For example, rather than buying from producers at $50 and selling to consumers at $60, a distributor with market power might be able to buy at $40 and sell at $70. In such a world, a decrease in distribution costs might not cause a proportionate—or even any—change in the price paid by distributors to producers, and thus the surplus associated with the lower distribution cost might largely or entirely accrue to the distributor, rather than the manufacturer.
Some, though not all, of the purported benefits of more durable household goods, such as decreased environmental impact of manufacturing and distribution, are likely to apply to pharmaceuticals as well. For a summary of some of these benefits, see Carlos Montalvo et al., European Parliament, A Longer Lifetime for Products: Benefits for Consumers and Companies 10–11 (2016).
World Health Org., Adherence to Long-Term Therapies: Evidence for Action 7 (Eduardo Sabaté ed., 2003), https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (accessed May 8, 2020).
See, eg Marie T. Brown & Jennifer K. Bussell, Medication Adherence: WHO Cares?, 86 Mayo Clinic Proc. 304 (2011); Carmen Ortego et al., Adherence to Highly Active Antiretroviral Therapy (HAART): A Meta-Analysis, 15 AIDS & Behav. 1381 (2011).
See, eg Alan Gervaix et al., Pneumococcal Vaccination in Europe: Schedule Adherence, 36 Clinical Therapeutics 802; A. Schoeps et al., Socio-demographic determinants of timely adherence to BCG, Penta3, measles, and complete vaccination schedule in Burkina Faso, 32 Vaccine 96 (2013); Lea E. Widdice et al., Adherence to the HPV Vaccine Dosing Intervals and Factors Associated With Completion of 3 Doses, 127 Pediatrics 77 (2011).
World Health Org., supra note 185, at 30, 35.
See K. Rivet Amico & Michael J. Stirratt, Adherence to Preexposure Prophylaxis: Current, Emerging, and Anticipated Bases of Evidence, 59 Clinical Infectious Diseases S55 (2014).
World Health Org., supra note 185, at 95.
See generally Paul Heidhues & Botond Kőszegi, Behavioral Industrial Organization, in 1 Handbook of Behavioral Economics: Foundations and Applications 517, 574–87 (B. Douglas Bernheim et al. eds. 2018).
See id. at 574; see also Michael Kremer, Gautam Rao & Frank Schilbach, Behavioral Development Economics, in 2 Handbook of Behavioral Economics: Foundations and Applications 345, 415 (B. Douglas Bernheim et al. eds., 2019) (noting that ‘even behavioral industrial organization... has mostly assumed sophisticated, profit-maximizing firms responding to behavioral consumers,’ and explaining the ‘numerous justifications’ for the assumption that firms successfully maximizing profits).
See generally Eisenberg & Price, supra note 9 (describing how integrated health systems such as Kaiser have incentives to engage in some types of medical innovation).
See supra note 38 and accompanying text.
Rachel E. Sachs, Integrating Health Innovation Policy, 34 Harv. J.L. & Tech. (forthcoming 2020), https://ssrn.com/abstract=3564354. This is one of many reasons that greater integration or long-term insurance contracts might be preferable (accessed May 8, 2020).
See supra note 86 and accompanying text.
See generally Daniel J. Benjamin, Errors in Probabilistic Reasoning and Judgment Biases, in 2 Handbook of Behavioral Economics: Foundations and Applications 69 (B. Douglas Bernheim et al. eds., 2019) (reviewing the behavioral economics literature in this area).
See Neil D. Weinstein, Optimistic Biases About Personal Risk, 246 Science 1232 (1989).
See, eg Jean Hammond et al., Why Do Women Underestimate the Risk of Cardiac Disease? A Literature Review, 20 Austl. Critical Care 53 (2007); R.S. Gold & H.M. Aucote, ‘I’m Less at Risk than Most Guys’: Gay Men’s Unrealistic Optimism About Becoming Infected with HIV, 14 Int’l J. STD & AIDS 18 (2003); T. Rouyard et al., Perceptions of Risks for Diabetes-Related Complications in Type 2 Diabetes Populations: A Systematic Review, 34 Diabetic Med. 467 (2017); N.D. Weinstein et al., Smokers’ Unrealistic Optimism About Their Risk, 14 Tobacco Control 55 (2005). This bias also applies to assessing the benefits and risks of medical interventions. See Yaniv Hanoch et al., Reaping the Benefits and Avoiding the Risks: Unrealistic Optimism in the Health Domain, 39 Risk Analysis 792 (2019). Particularly salient diseases may have different average risk estimates; for example, women in the United States and Canada (but not the UK) tend to overestimate their risk of developing breast cancer. See Penelope Hopwood, Breast Cancer Risk Perception: What Do We Know and Understand?, 2 Breast Cancer Res. 387 (2000).
Eve Dubé et al., Vaccine Hesitancy: An Overview, 9 Hum. Vaccines & Immunotherapeutics 1763, 1769 (2013).
See Inst. of Med., Adverse Effects of Vaccines: Evidence and Causality (2012); Dubé et al., supra note 201.
See Nat’l Vaccine Advisory Comm., supra note 36; Dan M. Kahan, A Risky Science Communication Environment for Vaccines, 342 Science 53 (2013).
Phaedra S. Corso et al., Assessing Preferences for Prevention Versus Treatment Using Willingness to Pay, 22 Med. Decision Making S92, S97 (2002).
Ree M. Meertens et al., Prevention Praised, Cure Preferred: Results of Between-Subjects Experimental Studies Comparing (Monetary) Appreciation for Preventative and Curative Interventions, 13 BMC Med. Informatics & Decision Making 136 (2013).
Ryan Bosworth et al., Is an Ounce of Prevention Worth a Pound of Cure? Comparing Demand for Public Prevention and Treatment Policies, 30 Med. Decision Making E40, E43–E44, E47 (2010). Of course, these results depend on the population and the survey construction, and other studies have more ambiguous results. See, eg Magnus Johannesson & Per-Olov Johansson, A Note on Prevention Versus Cure, 41 Health Pol’y 181, 181 (1997) (finding, in a Swedish survey, that ‘1.2–1.4 lives saved in acute care is judged equivalent to saving one life through prevention’).
See generally Frank Ackerman & Lisa Heinzerling, Pricing the Priceless: Cost-Benefit Analysis of Environmental Protection, 150 U. Pa. L. Rev. 1553 (2002); George Lowenstein, Deborah A. Small & Jeff Strnad, Statistical, Identifiable, and Iconic Victims, in Behavioral Public Finance 32 (Edward J. McCaffery & Joel Slemrod eds., 2006).
See generally Deepak Hegde & Bhaven Sampat, Can Private Money Buy Public Science? Disease Group Lobbying and Federal Funding for Biomedical Research, 61 Mgmt. Sci. 2281 (2015).
Health law scholars have noted that vaccines require fewer doses than treatments, see Darrow et al., supra note 112, at 369, but they do not discuss why vaccine producers might not be able to charge higher prices to make up for the smaller number of doses. We think the behavioral effects discussed in this section and the distortions in the absence of irrationality discussed in Section III.D.2 are relevant.
See Keith Marzilli Ericson & David Laibson, Intertemporal Choice, in 2 Handbook of Behavioral Economics: Foundations and Applications 1, 2 (B. Douglas Bernheim et al. eds. 2019) (‘People seem to struggle when they make intertemporal tradeoffs, a phenomenon which has been extensively discussed by moral philosophers, political economists, psychologists, and policymakers. Writings about self-control and self-management are almost as old as written language itself.’); Richard H. Thaler, Misbehaving: The Making of Behavioral Economics 87–88 (2015).
See Ericson & Laibson, supra note 209, at 7–20 (reviewing these models).
See Kris N. Kirby, Bidding on the Future: Evidence Against Normative Discounting of Delayed Rewards, 126 J. Experimental Psychol. 54 (1997); David Laibson, Golden Eggs and Hyperbolic Discounting, 112 Q.J. Econ. 443 (1997); see also Ericson & Laibson, supra note 209, at 7–10 (reviewing this literature).
The most common model of time-consistent preferences, by contrast, is known as ‘exponential discounting.’ See Ericson & Laibson, supra note 209, at 2.
See Ericson & Laibson, supra note 209, at 22; see also Jonathan D. Cohen et al., Measuring Time Preferences, J. Econ. Lit. (forthcoming 2020), https://scholar.harvard.edu/files/laibson/files/cohen-ericson-laibson-white_forth-jel_2019.pdf (providing a deeper theoretical discussion of this literature) (accessed May 8, 2020)
See Ericson & Laibson, supra note 209, at 25–26.
The present value PV of a series of payments P over n periods at rate r per period is given by PV = P [1—(1 + r)–n]/r. For an online calculator, see Present Value of Annuity Calculator, FinancialMentor, https://financialmentor.com/calculator/present-value-of-annuity-calculator (accessed Feb. 11, 2020).
Id. A related but distinct problem is that households generally hold low levels of liquid assets, see Ericson & Laibson, supra note 209, at 36, making it difficult to make a large lump sum purchase in the absence of insurance.
See Marcella Alsan et al., A Commitment Contract to Achieve Virologic Suppression in Poorly Adherent Patients with HIV/AIDS, 31 AIDS 1765, 1766 (2017) (‘Individuals often intend to engage in healthy behaviors in the future, but when the moment to engage in such a behavior arrives, they frequently fail to follow through on their intentions, instead making choices that are expedient at the time.’).
See supra notes 185–190 and accompanying text.
See, eg Stefano DellaVigna & Ulrike Malmendier, Contract Design and Self-Control: Theory and Evidence, 119 Q.J. Econ. 353 (2004); Paul Heidhues & Botond Kőszegi, Exploiting Naïvete About Self-Control in the Credit Market, 100 Am. Econ. Rev. 2279 (2010).
See Ericson & Laibson, supra note 209, at 27–28.
See Katherine L. Milkman et al., Planning Prompts as a Means of Increasing Preventive Screening Rates, 56 Preventive Med. 92 (2013); Katherine L. Milkman et al., Using Implementation Intentions Prompts to Enhance Influenza Vaccination Rates, 108 Proc. Nat’l Acad. Sci. 10415 (2011).
See, eg Vicki G. Morwitz et al., Divide and Prosper: Consumers’ Reactions to Partitioned Prices, 35 J. Marketing Research 453 (1998); Eric A. Greenleaf et al., The Price Does Not Include Additional Taxes, Fees, and Surcharges: A Review of Research on Partitioned Pricing, 26 J. Consumer Psychol. 105 (2016); Kiljae Lee et al., Regulatory Focus as a Predictor of Attitudes Toward Partitioned and Combined Pricing, 24 J. Consumer Psychol. 355 (2014).
Morwitz et al., supra note 222, at 457. Consumer willingness to pay a higher price at every quantity is consistent with an outward shift of the demand curve.
John M. Clark & Sidne G. Ward, Consumer Behavior in Online Auctions: An Examination of Partitioned Prices on eBay, 16 J. Marketing Theory & Prac. 57 (2008).
Greenleaf et al., supra note 222, at 107.
Kremer & Snyder, supra note 39.
The economics literature has also noted more broadly that consumer heterogeneity in disease risk may lead to market failures in vaccine markets. See Frederick Chen & Flavio Toxvaerd, The Economics of Vaccination, 363 J. Theoretical Biology 105 (2014) (describing market failures associated with vaccines without any comparison to treatments).
See Who’s at Risk for Hepatitis B? Learning the Hep B Basics, Hepatitis B Found. (Sept. 13, 2017), https://www.hepb.org/blog/whos-at-risk-for-hepatitis-b-learning-the-hbv-basics (accessed May 8, 2020).
In this example, if the firm can discriminate based on disease risk, it can expect the same revenue from either a preventative or a treatment. This is not a particularly realistic outcome, however: disease risk is generally private information. Furthermore, one might imagine that it is even more difficult to conduct this type of discrimination with a more complex distribution of disease risk (and that the practice of charging higher-risk populations more for protection might be frowned upon).
Kremer & Snyder, supra note 39, at 1186.
While short-term protection against malaria exists, see David O. Freedman, Malaria Prevention in Short-Term Travelers, 359 New Eng. J. Med. 603 (2008), there is no reliable long-term prophylactic regimen, see Sumadhya Deepika Fernando, Chaturaka Rodrigo & Senaka Rajapakse, Chemoprophylaxis in Malaria: Drugs, Evidence of Efficacy and Costs, 4 Asian Pacific J. Tropical Med. 330 (2011). The WHO considers both vaccines valuable prospects and monitors research and development in both areas. See HIV Vaccine Development, World Health Org., https://www.who.int/immunization/research/development/hiv_vaccdev/en (accessed Feb. 11, 2020); Malaria Vaccines, World Health Org., https://www.who.int/immunization/research/development/malaria/en (accessed Feb. 11, 2020).
Kremer & Snyder, supra note 39, at 1217 tbl.v. The authors specifically focus on diseases classified by the CDC as ‘notifiable,’ for which the CDC monitors cases and outbreaks. Id. at 1215. The list includes diseases such as HIV, malaria, and rubella, but excludes the common cold and influenza (except when the latter causes pediatric mortality). The regressions include controls for disease characteristics such as the infectious agent (viral, bacterial, etc.) that may affect the overall difficulty of developing a vaccine versus a treatment and find a statistically significant effect even with these controls.
See generally Jean Tirole, The Theory of Industrial Organization 80 (1988) (explaining this distinction). Although the distinction between durables and non-durables is well accepted in general, the concept has rarely been applied to pharmaceuticals.
A parallel comparison may be made along the other column of our taxonomy between cures (durable therapeutics) and treatments (non-durable therapeutics).
R.H. Coase, Durability and Monopoly, 15 J.L. & Econ. 143 (1972).
Id. at 144.
Books can also be resold, a property that further complicates the market for books but not the market for vaccines, but which also is not necessary for the following analysis.
Many publishers circumvent this constraint by not selling the same product: for example, they price discriminate by releasing a paperback edition rather than lowering the price of the hardcover. Vaccine producers in general do not have an equivalent option.
See Jeremy Bulow, An Economic Theory of Planned Obsolescence, 101 Q.J. Econ. 729, 730 (1986) (‘Except under unusual cost conditions a monopolist not threatened by entry will produce goods with inefficiently short useful lives.’).
See Barak Y. Orbach, The Durapolist Puzzle: Monopoly Power in Durable-Goods Markets, Yale J. Reg. 67, 91–92 (2004). For further discussion of the implications of these behaviors for market power and their treatment by antitrust law, see id. at 92, 97.
See Daniel Hemel & Lisa Larrimore Ouellette, Pharmaceutical Profits and Public Health Are Not Incompatible, N.Y. Times (Apr. 8, 2020), https://www.nytimes.com/2020/04/08/opinion/coronavirus-drug-company-profits.html (accessed May 8, 2020).
See supra note 72 and accompanying text.
See supra notes 129–134 and accompanying text.
See supra notes 78–83 and accompanying text.
See supra note 73 and accompanying text.
See Geoffard & Philipson, supra note 177. Avoiding surplus loss and achieving herd immunity is a bigger challenge in other countries, such as India and parts of Europe where vaccination is not required. See Ramanan Laxminarayan & Mirmal Kumar Ganguly, India’s Vaccine Deficit: Why More Than Half of Indian Children Are Not Fully Immunized, and What Can—and Should—Be Done, 30 Health Aff. 1096 (2011); Measles Cases Hit Record High in the European Union, World Health Org. Eur. (Aug. 20, 2019), http://www.euro.who.int/en/media-centre/sections/press-releases/2018/measles-cases-hit-record-high-in-the-european-region (accessed May 8, 2020).
Darius N. Lakdawalla, Economics of the Pharmaceutical Industry, 56 J. Econ. Lit. 397, 444 (2018).
See Heidi L. Williams, How Do Patents Affect Research Investments?, 9 Ann. Rev. Econ. 441, 464 (2017) (‘[W]e still have essentially no credible empirical evidence on the seemingly simple question of whether stronger patent rights—either longer patent terms or broader patent rights—encourage research investments into developing new technologies.’).
See Lisa Larrimore Ouellette, Patent Experimentalism, 101 Va. L. Rev. 65, 75–87 (2015).
See Hemel & Ouellette, supra note 94. This shift will require overcoming political economy concerns that have led to preventative technologies receiving less government funding. See David Dranove, Is There Underinvestment in R&D About Prevention?, 17 J. Health Econ. 117 (1998).
See, eg Brian D. Wright, The Economics of Invention Incentives: Patents, Prizes, and Research Contracts, 73 Am. Econ. Rev. 691 (1983).
For a discussion of current spending, see supra notes 116–120 and accompanying text.
For an overview, see Rutschman, supra note 46.
See NIH to Launch Public-Private Partnership to Speed COVID-19 Vaccine and Treatment Options, Nat’l Insts. of Health (Apr. 17, 2020), https://www.nih.gov/news-events/news-releases/nih-launch-public-private-partnership-speed-covid-19-vaccine-treatment-options (accessed May 8, 2020).
For a non-technical overview, see Understanding Summary Measures Used to Estimate the Burden of Disease: All about HALYs, DALYs and QALYs, Nat’l Collaborating Ctr. for Infectious Diseases (2015), https://nccid.ca/publications/understanding-summary-measures-used-to-estimate-the-burden-of-disease (accessed May 8, 2020).
The optimal prize might actually be a fraction of the net present value of lost QALYs to avoid overinvestment due to a common pool problem. See generally Partha Dasgupta & Joseph Stiglitz, Uncertainty, Industrial Structure, and the Speed of R&D, 11 Bell J. Econ. 1, 18 (1980); Brian D. Wright, The Economics of Invention Incentives: Patents, Prizes, and Research Contracts, 73 Am. Econ. Rev. 691, 693–94 (1983).
See supra note 128 and accompanying text.
Hemel & Ouellette, supra note 47.
Hemel & Ouellette, supra note 7.
Hemel & Ouellette, supra note 241.
The issue of who pays and who should pay—whether some consumers should cross-subsidize others—is subtle in this case, as heterogeneity in disease risk itself gives rise to some of the issues discussed in this paper. Thus, by definition, the diseases for which surplus extraction is most difficult disproportionately affect certain populations. HIV and malaria, as discussed above, are two such diseases. This paper does not take a general stance on the normative issue of who ought to pay how much for the development of vaccines for such diseases. However, eradication of either of these diseases would likely benefit other groups than just high-risk populations, for example by facilitating economic development and exchange.
See Hemel & Ouellette, supra note 7, at 594–95; Sachs, Prizing Insurance, supra note 9.
See Mary Ellen McIntire, Government Plans to Pay Hospitals for COVID-19 Care for Uninsured, Roll Call (Apr. 3, 2020), https://www.rollcall.com/2020/04/03/government-plans-to-pay-hospitals-for-covid-19-care-for-uninsured (accessed May 8, 2020).
See Ana Santos Rutschman, IP Preparedness for Outbreak Diseases, 65 UCLA L. Rev. 1200, 1217 (2018); Ana Santos Rutschman, Vaccine Licensure in the Public Interest: Lessons from the Development of the U.S. Army Zika Vaccine, 127 Yale L.J. F. 651 (2018). Rutschman argues for limiting IP rights (such as through limits on exclusive licenses) to reduce transactional IP inefficiencies, although other policy interventions are needed to ameliorate the incentives problem. See Doug Lichtman, The Central Assumptions of Patent Law: A Response to Ana Santos Rutschman’s IP Preparedness for Outbreak Diseases, 65 UCLA L. Rev. 1268, 1268 (2018).
See, eg Darrow et al., supra note 112; Lieu et al., supra note 157.
See Mark V. Pauly, Improving Vaccine Supply and Development: Who Needs What?, 24 Health Aff. 680, 687–88 (2005) (describing the difficulty of designing appropriate incentives for public researchers in the vaccine context).
Daniel J. Hemel & Lisa Larrimore Ouellette, Innovation Institutions and the Opioid Crisis, J.L. & Biosciences (forthcoming 2020), https://ssrn.com/abstract=3534721.
See supra note 248 and accompanying text (accessed May 8, 2020).
Cf. Amy C. Madl & Lisa Larrimore Ouellette, Policy Experiments to Address Gender Inequality Among Innovators, 57 Hous. L. Rev. 813, 813 (2020) (‘[P]olicymakers should not overstate the existing evidence for potential interventions out of a desire for rapid progress. Nor should they use this lack of evidence as an excuse for inaction. Rather, we argue that institutions interested in this issue should look for opportunities to rigorously and transparently test the most promising interventions.’).
See supra notes 255–256 and accompanying text.
See Ouellette, supra note 249, at 92–95 (providing an overview of this kind of policy experiment).
See Tung Thanh Le, The COVID-19 Vaccine Development Landscape, 19 Nature Revs. Drug Discovery 305 (2020).
See supra note 14 and accompanying text.
See Matt Apuzzo & David D. Kirkpatrick, Covid-19 Changed How the World Does Science, Together, N.Y. Times (Apr. 1, 2020), https://www.nytimes.com/2020/04/01/world/europe/coronavirus-science-research-cooperation.html (accessed May 8, 2020).




