-
PDF
- Split View
-
Views
-
Cite
Cite
Ukihide Tateishi, Koichiro Kimura, Junichi Tsuchiya, Daisuke Kano, Tadashi Watabe, Norio Nonomura, Katsuhiko Saito, Kota Yokoyama, Ken Yamagiwa, Takuya Adachi, Yuji Kojima, Soichiro Yoshida, Yasuhisa Fujii, Phase I/IIa trial of 18F-prostate specific membrane antigen (PSMA) 1007 PET/CT in healthy volunteers and prostate cancer patients, Japanese Journal of Clinical Oncology, Volume 54, Issue 3, March 2024, Pages 282–291, https://doi.org/10.1093/jjco/hyad166
Close - Share Icon Share
Abstract
18F-PSMA 1007 is a promising PET tracer for prostate cancer. We aimed to examine the safety, biodistribution, radiation dosimetry, and clinical effectiveness in Japanese healthy volunteers and patients with prostate cancer.
Part A evaluated the pharmacokinetics and exposure doses in three healthy volunteers. Part B evaluated the diagnostic accuracy in patients with untreated preoperative prostate cancer (Cohort 1, n = 7) and patients with biochemical recurrence (Cohort 2, n = 3). All subjects received a single dose of 3.7 MBq/kg 18F-PSMA 1007. Results: 18F-PSMA 1007 was found to be safe and well tolerated in all subjects. No serous AEs or drug-related AEs were identified during the present study. The average blood radioactivity concentration reached a maximum of 47.87 ± 1.05 (percentage of injected dose [%ID]/ml) at 5 min and then decreased to 1.60 ± 0.78 in 6 h. The systemic radioactivity reached a maximum of 211.05 ± 6.77 (%ID|$\times$|103) at 5 min and decreased to 7.18 ± 3.91 in 6 h. The sensitivity and positive predictive value were 100% and 100% based on both pathologic and imaging confirmation as gold standard. In Cohort 1, 15 primary foci (11.9%) were >5 mm in the largest diameter and identified in 39 of 126 segments (30.1%). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for 60 min uptake time acquisition were 80.0, 96.5, 91.4, 91.2 and 91.3%, respectively.
Our study revealed that 18F-PSMA 1007 was safe, well tolerated and showed high accuracy in the diagnosis of prostate cancer.
Introduction
Prostatic specific membrane antigen (PSMA) is a well-known target for the treatment and imaging of primary or metastatic prostate cancer (1,2). Detection of the abnormal uptake of PSMA by PSMA PET/CT provides valuable staging information and dictates the need for radionucleotide therapy using alpha- or beta- emitters. The magnitude of PSMA accumulation influences decisions on the appropriateness of surgery, hormone therapy, anti-cancer drugs and radiation therapy. Accordingly, various kinds of PSMA ligands have been developed for assessment of prostate cancer (3,4).
68Ga PSMA-11 has been used for PET imaging and was approved by the FDA as the first PET probe targeting PSMA (5–8). Moreover, 18F-labelled PSMA ligands have advantages including abundant availability and higher synthetic yield compared to 68Ga PSMA-11. 18F-PSMA 1007 can be applied not only to diagnosis but also radionucleotide therapy, because it has a similar structure to PSMA-617 which can chelate metals (9).
The diagnostic accuracy of 18F-PSMA 1007 PET/CT for prostate cancer indicates that it is a significant advance in this field (10,11). Giesel et al. (12) prospectively analyzed data from patients with nodal staging of prostate cancer and biochemical recurrence (BCR) of prostate cancer after radical prostatectomy to determine the diagnostic performance of 18F-PSMA 1007 PET/CT as validated by histopathology. To address the uncertainty of BCR detection in the Japanese population, Watabe et al.(13) examined the accuracy of selected elements of retrospective evaluation by 18F-PSMA 1007 PET/CT. This study identified abnormal accumulations of 18F-PSMA 1007 suggesting recurrence or metastasis in 93% (n = 26/28) of patients with BCR. More importantly, by focusing on sites with abnormal uptake, local recurrence was detected in 58% (n = 15/26). Of these sites, sites with focal uptake were detected adjacent to the bladder on 18F-PSMA 1007 PET/CT in 53% (n = 8/15) patients. That is, rapid excretion via the urinary tract resulting in intense uptake in the bladder leads to an underestimate of the incidence of BCR, but agents with slower urinary excretion rate such as 18F-PSMA 1007 are better tools for diagnosis of BCR in the prostatic bed.
18F-PSMA 1007 has been approved in France (2020) and the EU (2023) and evaluated in a phase III clinical trial in the US. However, its safety and effectiveness have never been evaluated in the Japanese population. The purpose of the present study was to assess the safety, biodistribution, radiation dosimetry and clinical effectiveness of 18F-PSMA 1007 in Japanese healthy volunteers and patients with prostate cancer.
Patients and methods
Study design
This study is an open-label, single arm, uncontrolled, single assignment, phase I/IIa study to investigate the safety of 18F-PSMA 1007 produced by the MPS200 synthesizer (MPS200PSMA-F). Part A evaluated the pharmacokinetics and exposure doses in healthy volunteers. Part B evaluated the ability of 18F-PSMA 1007 PET/CT to detect lesions in patients with untreated preoperative prostate cancer (Cohort 1) and patients with BCR after radiation therapy (Cohort 2). All subjects were administered a single dose of 3.7 MBg/kg 18F-PSMA 1007 within 10 s and their catheters were flushed with 3–5 ml saline. All patients in Cohort 1 received prostatectomy within 2 months after PET/CT study. The study was performed in accordance with the ethical principles established in the Declaration of Helsinki and Good Clinical Practice guidelines and was registered at www.clinicaltrials.jp (jRCT2032210711). A written informed consent was obtained from each subject, and all study procedures were approved by the institutional review boards of Tokyo Medical and Dental University Hospital (TMD20-MRAD-101).
Inclusion and exclusion criteria
Cohort 1 included Japanese patients with untreated preoperative prostate cancer who were scheduled receive prostatectomy within three months; had serum PSA level > 10 ng/ml; had Gleason score > or = 7; and had clinical stage T2b or more. Cohort 2 included Japanese patients with BCR after radiation therapy and 2.0 ng/ml or more increase in serum PSA level from nadir. Patients had sufficient organ functions meeting the following criteria: WBCs ≥2000/μl; neutrophils ≥1500/μl; hemoglobin >9.0 g/dl; serum creatinine ≤2 × upper limit of normal (ULN); total bilirubin ≤1.5 × ULN; aspartate aminotransferase (AST) ≤ 3 × ULN; alanine aminotransferase (ALT) ≤ 3 × ULN; ECOG Performance Status 0 to 2; age ≥ 20 years and < 80 years at the time of providing the written informed consent for participation in the study. Patients with a diagnosis of any malignant tumor other than prostate cancer or who had a past history of any malignant tumor other than prostate cancer within 5 years, who had insufficient renal function with estimated glomerular filtration rate (eGFR) <60 ml/min, or who were currently receiving treatment for prostate cancer, were excluded from the present study.
Radiosynthesis of 18F-PSMA 1007
The precursor of 18F-PSMA 1007 was purchased from ABX Advanced Biochemical Compounds GmbH (Dresden, Germany). For other materials, we used products listed in the Japanese Pharmacopeia or reagents of comparable quality. 18F- PSMA 1007 was produced by one-step synthesis by nucleophilic aromatic substitution (SNAr) using [18F]TBAF and unprotected precursor (P/N 99432, patented by ABX). The radiolabeling process was in accordance with ABX method similar to the method by Cardinale et al. (14). The manufacturing process for 18F-PSMA 1007 with SPE method on the MPS200 synthesizer (MPS200PSMA-F) was introduced by Sumitomo Heavy Industries Ltd., Tokyo, Japan.
Safety monitoring
Vital signs (i.e. blood pressure, heart rate, body temperature, respiratory rate and oxygen saturation) and 12-lead ECG parameters were evaluated before and 360 min after administration of 18F-PSMA 1007. Investigators conducted a final phone call (day 8) after administration of 18F-PSMA 1007. Adverse events (AEs) were coded by MedDRA/J Version 22.0, and the number and percentage of subjects who had developed AEs by severity and causal relationship to 18F-PSMA 1007 were recorded.
Image acquisition
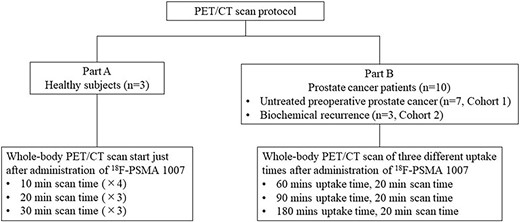
PET/CT scan protocol is summarized in Fig. 1. In part A, sequential whole-body emission scans from head to knee were performed by the following pattern: 10 minutes (× 4 times) + 20 minutes (× 3 times) + 30 minutes (× 3 times), respectively. Whole-body scans at three different uptake times including 60, 90 and 180 mins were performed in part B. Two machines (Celesteion, time-of-flight [TOF] PET/CT and Cartesion Prime, semi-conductor [SC] PET/CT, Canon Medical Systems, Otawara, Tochigi, Japan) were used in our study. PET images of TOF PET/CT were reconstructed using parameters featuring three dimensional-ordered subset expectation maximization (3D-OSEM) into a 336 × 336 matrix; voxel size 8.47 μl (2.04 × 2.04 × 2.04 mm) with 3-iterations, 10-subsets, a 6.0-mm 3D-Gaussian filter, scatter, time-of-flight (TOF) and point-spread function (PSF). PET images of SC PET/CT were reconstructed using parameters featuring 3D-OSEM into a 336 × 336 matrix; voxel size 9.44 μl (2.11 × 2.11 × 2.11 mm) with 2-iterations, 12-subsets, a 3.0-mm 3D-Gaussian filter and active corrections by scatter, TOF and PSF. Reconstructed images were evaluated by quantitative methods. Image analysis was performed using PMOD Ver. 4.4 (PMOD Technologies, LLC, Fällanden, Switzerland).
Biodistribution
In Cohort 1, descriptive statistics were calculated for PK parameters and radioactivity distribution rates in the whole body, organs, and tissues. Radioactivity in venous blood samples was assayed at 2, 5, 10, 15, 30, 45, 60, 80, 120, 180, 240 and 360 min post injection. Blood radioactivity was measured in a 4-ml blood sample. The radioactivity distribution in plasma was calculated for two categories: radioactivity per unit volume (percentage of injected dose [%ID]/ml) and radioactivity per systemic blood volume (%ID). During the dosimetry evaluation, the urinary radioactivity excretion rate was measured by collecting urine samples at the following intervals: 0–2, 2–4 and 4–6 h. The urinary excretion rate was calculated as the cumulative urinary excretion rate (%ID). Parameters of blood radioactivity including area under the curve (AUC), Cmax and Tmax were calculated at the following intervals: 0–5 min, 5 min–6 h, and 0 min–6 h. The radioactivity distribution rate (%ID) for each target organ (brain, stomach wall, heart, kidney, liver, red bone marrow, salivary gland, thyroid, bladder and whole body) was calculated from the PET image data obtained at each imaging time point. Besides, the residence time was determined based on the body distribution data. The absorbed radiation dose (mGy/MBq) was calculated for each item/target organ by the medical internal radiation dose (MIRD) method and the ED per unit activity administered (mSv/MBq) was calculated for each subject.
Internal radiation dosimetry
We calculated the absorbed doses, effective doses, and effective dose equivalents in major organs. Radioactivity was calculated by volume-of-interest analysis. Based on the amount of radioactivity accumulated in each organ, the absorbed dose for each organ was estimated using the MIRD adult model from the International Commission on Radiological Protection-endorsed IDAC 1.0 package.
Definitions and statistical analysis
We calculated the diagnostic accuracy by qualitative evaluation of PSMA expression on a 4-point scale: 0, below the blood pool level; 1, equal to or above blood pool level and lower than the liver level; 2, equal to or above the liver level and lower than the parotid gland level; 3, equal to or above the parotid gland level. Positive PET/CT finding was defined as score > or = 2. We also examined the relationship of the PSMA-RADS Ver.1.0 scoring system to that of the 4-point scale, where a PSMA-RADS score of 4 or 5 was defined as a positive finding (15). We calculated diagnostic accuracy using pathologic confirmation (n = 7) and imaging confirmation (n = 3) other than 18F-PSMA PET/CT obtained until the 92nd day after 18F-PSMA PET/CT as the gold standard. Three axial planes (basal, midgland and apex) of the prostate were selected and divided into 18 segments (Fig. 2) on the macroscopic level for correlation with PET/CT images. Primary tumor foci >5 mm in the largest diameter were used for assessment of diagnostic accuracy in patients in Cohort A. The maximum standardized uptake value (SUVmax) was compared to initial PSA level using the Pearson correlation test.
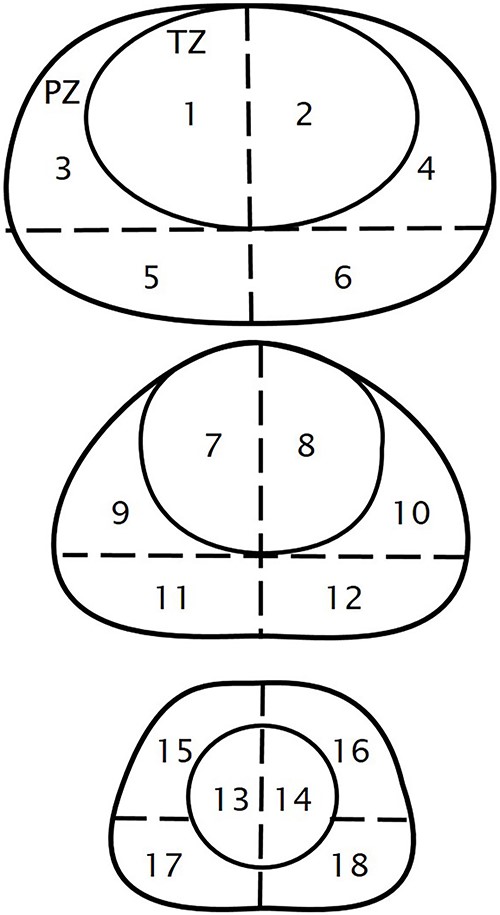
Prostatic segments three axial planes (basal, midgland, and apex) of the prostate are selected and divided into 18 segments: TZ, transitional zone; PZ, peripheral zone.
All grades of adverse events were estimated according to common terminology criteria for adverse events (CTCAE ver. 4.0). In patient characteristics, duration data were analyzed by the Wilcoxon Rank Sum test, and other continuous variables were analyzed using t-tests; all categorical data were analyzed with the Fisher exact test.
IBM SPSS Statistics 29 (IBM Inc.), SAS Release 9.4 (SAS Institute Inc.), WinNonlin Version 8.0 (Certara, LP) and OLINDA/EXM Ver1.1 (Vanderbilt University) were used for statistical processing, pharmacokinetic parameter calculation and radiation dose analysis.
Results
The enrollment of subjects
There were 13 enrolled subjects: 3, 7 and 3 in the Part A, Part B-Cohort 1 and Part B-Cohort 2, respectively. Patient demographics are presented in Table 1. All seven patients of the Part B-Cohort 1 received total prostatectomy within three months. All three patients of the Part B-Cohort 2 completed radical radiation therapy (two patients for IMRT, one for heavy ion radiotherapy) at least 3 months before enrollment. No significant difference was found in mean age between Cohort 1 and Cohort 2 patients (P = 0.5). There was no statistically significant difference in height, body weight, BMI, ECOG performance status, medical history, complications, allergy history, smoking history and drinking history. There was a statistically significant difference in mean serum PSA level between Cohort 1 and Cohort 2 (10.05 vs. 3.74, P = 0.031), but not in median time from initial diagnosis to enrollment. In Cohort 2, one patient received hormonal therapy with leuprorelin and bicalutamide, and others did not receive any drug treatment before the radical radiation therapy. No patients received chemotherapy or immunotherapy prior to study. There was no significant difference in prior therapies within Cohort 2. Because of a single pelvic nodal lesion detected by 18F-PSMA 1007 PET/CT, lymph node dissection was performed after this study in one patient in Part B-Cohort 2. All subjects proceeded to the follow-up phase and are still alive.
| No.a . | Age (year) . | Height (m) . | Body weight (kg) . | BMI (kg/m2) . | Serum creatinine (mg/dl) . | 18F-PSMA-1007 (MBq) . | Gleason scoreb . | Initial PSA (ng/ml)b . | TNM classification . | Metastasis or recurrence . | SUVmax (60 m)c . | SUVmax (90 m)c . | SUVmax (180 m)c . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 1.72 | 69.9 | 23.6 | 1.03 | 259 | 4 + 3 | 11.5 | pT3a, pN0, L0, V0, Pn1 | — | 87.59 | 102.29 | 81.99 | none |
| 2 | 59 | 1.81 | 80.7 | 24.6 | 1.02 | 299 | 4 + 3 | 15.9 | pT2c, pN0, L0, V0, Pn0 | — | 49.2 | 57.05 | 63.85 | none |
| 3 | 75 | 1.80 | 73.4 | 22.7 | 0.96 | 272 | 3 + 4 | 7.66 | pT2c, pN0, L0, V0, Pn0 | — | 32.54 | 38.67 | 44.25 | none |
| 4 | 73 | 1.70 | 70.7 | 24.5 | 1.12 | 262 | 5 + 5 | 5.86 | pT2c, pN0, L0, V0, Pn1 | — | 54.16 | 74.08 | 101.27 | none |
| 5 | 74 | 1.62 | 55.8 | 21.3 | 0.68 | 206 | 4 + 3 | 3.95 | pT2c, pN0, L0, V1, Pn0 | — | 36.11 | 45.71 | 51.42 | none |
| 6 | 69 | 1.73 | 60.8 | 20.3 | 0.73 | 225 | 4 + 3 | 18.33 | pT2c, pN0, L0, V0, Pn0 | — | 25 | 33.1 | 41.37 | none |
| 7 | 65 | 1.74 | 76.4 | 25.2 | 1.17 | 283 | 4 + 3 | 7.18 | pT2c, pN0, L0, V1, Pn1 | — | 8.64 | 10.67 | 12.15 | none |
| 8 | 71 | 1.69 | 77.6 | 27.2 | 1.15 | 287 | 3 + 3 | 5.54 | — | Prostate and bone | 19.16 | 22.64 | 31.92 | none |
| 9 | 76 | 1.60 | 63.7 | 24.9 | 0.90 | 236 | NA | 3.12 | — | Lymph node | 74.35 | 81.65 | 100.54 | none |
| 10 | 72 | 1.67 | 76.3 | 27.4 | 1.03 | 282 | 3 + 4 | 2.55 | — | Prostate | 8.64 | 10.67 | 12.15 | none |
| No.a . | Age (year) . | Height (m) . | Body weight (kg) . | BMI (kg/m2) . | Serum creatinine (mg/dl) . | 18F-PSMA-1007 (MBq) . | Gleason scoreb . | Initial PSA (ng/ml)b . | TNM classification . | Metastasis or recurrence . | SUVmax (60 m)c . | SUVmax (90 m)c . | SUVmax (180 m)c . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 1.72 | 69.9 | 23.6 | 1.03 | 259 | 4 + 3 | 11.5 | pT3a, pN0, L0, V0, Pn1 | — | 87.59 | 102.29 | 81.99 | none |
| 2 | 59 | 1.81 | 80.7 | 24.6 | 1.02 | 299 | 4 + 3 | 15.9 | pT2c, pN0, L0, V0, Pn0 | — | 49.2 | 57.05 | 63.85 | none |
| 3 | 75 | 1.80 | 73.4 | 22.7 | 0.96 | 272 | 3 + 4 | 7.66 | pT2c, pN0, L0, V0, Pn0 | — | 32.54 | 38.67 | 44.25 | none |
| 4 | 73 | 1.70 | 70.7 | 24.5 | 1.12 | 262 | 5 + 5 | 5.86 | pT2c, pN0, L0, V0, Pn1 | — | 54.16 | 74.08 | 101.27 | none |
| 5 | 74 | 1.62 | 55.8 | 21.3 | 0.68 | 206 | 4 + 3 | 3.95 | pT2c, pN0, L0, V1, Pn0 | — | 36.11 | 45.71 | 51.42 | none |
| 6 | 69 | 1.73 | 60.8 | 20.3 | 0.73 | 225 | 4 + 3 | 18.33 | pT2c, pN0, L0, V0, Pn0 | — | 25 | 33.1 | 41.37 | none |
| 7 | 65 | 1.74 | 76.4 | 25.2 | 1.17 | 283 | 4 + 3 | 7.18 | pT2c, pN0, L0, V1, Pn1 | — | 8.64 | 10.67 | 12.15 | none |
| 8 | 71 | 1.69 | 77.6 | 27.2 | 1.15 | 287 | 3 + 3 | 5.54 | — | Prostate and bone | 19.16 | 22.64 | 31.92 | none |
| 9 | 76 | 1.60 | 63.7 | 24.9 | 0.90 | 236 | NA | 3.12 | — | Lymph node | 74.35 | 81.65 | 100.54 | none |
| 10 | 72 | 1.67 | 76.3 | 27.4 | 1.03 | 282 | 3 + 4 | 2.55 | — | Prostate | 8.64 | 10.67 | 12.15 | none |
Note.- SUV, standardized uptake value; NA, not available.
aNo. 1 to 7 were patients with treatment-naïve prostate cancer, and No. 8 to 10 were patients with biochemical recurrence after radical radiation therapy.
bThe highest Gleason scores of the radical prostatectomy specimens from patients No. 1 to 7 and target biopsy specimens from patients No. 8 and 10 are shown.
cThe number in parenthesis is the uptake time or acquisition time. Values represent the highest focal uptake in the prostate lesions from patient No. 1 to 8 and 10 and the abnormal uptake in the pelvic lymph node from patient No. 9.
| No.a . | Age (year) . | Height (m) . | Body weight (kg) . | BMI (kg/m2) . | Serum creatinine (mg/dl) . | 18F-PSMA-1007 (MBq) . | Gleason scoreb . | Initial PSA (ng/ml)b . | TNM classification . | Metastasis or recurrence . | SUVmax (60 m)c . | SUVmax (90 m)c . | SUVmax (180 m)c . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 1.72 | 69.9 | 23.6 | 1.03 | 259 | 4 + 3 | 11.5 | pT3a, pN0, L0, V0, Pn1 | — | 87.59 | 102.29 | 81.99 | none |
| 2 | 59 | 1.81 | 80.7 | 24.6 | 1.02 | 299 | 4 + 3 | 15.9 | pT2c, pN0, L0, V0, Pn0 | — | 49.2 | 57.05 | 63.85 | none |
| 3 | 75 | 1.80 | 73.4 | 22.7 | 0.96 | 272 | 3 + 4 | 7.66 | pT2c, pN0, L0, V0, Pn0 | — | 32.54 | 38.67 | 44.25 | none |
| 4 | 73 | 1.70 | 70.7 | 24.5 | 1.12 | 262 | 5 + 5 | 5.86 | pT2c, pN0, L0, V0, Pn1 | — | 54.16 | 74.08 | 101.27 | none |
| 5 | 74 | 1.62 | 55.8 | 21.3 | 0.68 | 206 | 4 + 3 | 3.95 | pT2c, pN0, L0, V1, Pn0 | — | 36.11 | 45.71 | 51.42 | none |
| 6 | 69 | 1.73 | 60.8 | 20.3 | 0.73 | 225 | 4 + 3 | 18.33 | pT2c, pN0, L0, V0, Pn0 | — | 25 | 33.1 | 41.37 | none |
| 7 | 65 | 1.74 | 76.4 | 25.2 | 1.17 | 283 | 4 + 3 | 7.18 | pT2c, pN0, L0, V1, Pn1 | — | 8.64 | 10.67 | 12.15 | none |
| 8 | 71 | 1.69 | 77.6 | 27.2 | 1.15 | 287 | 3 + 3 | 5.54 | — | Prostate and bone | 19.16 | 22.64 | 31.92 | none |
| 9 | 76 | 1.60 | 63.7 | 24.9 | 0.90 | 236 | NA | 3.12 | — | Lymph node | 74.35 | 81.65 | 100.54 | none |
| 10 | 72 | 1.67 | 76.3 | 27.4 | 1.03 | 282 | 3 + 4 | 2.55 | — | Prostate | 8.64 | 10.67 | 12.15 | none |
| No.a . | Age (year) . | Height (m) . | Body weight (kg) . | BMI (kg/m2) . | Serum creatinine (mg/dl) . | 18F-PSMA-1007 (MBq) . | Gleason scoreb . | Initial PSA (ng/ml)b . | TNM classification . | Metastasis or recurrence . | SUVmax (60 m)c . | SUVmax (90 m)c . | SUVmax (180 m)c . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 1.72 | 69.9 | 23.6 | 1.03 | 259 | 4 + 3 | 11.5 | pT3a, pN0, L0, V0, Pn1 | — | 87.59 | 102.29 | 81.99 | none |
| 2 | 59 | 1.81 | 80.7 | 24.6 | 1.02 | 299 | 4 + 3 | 15.9 | pT2c, pN0, L0, V0, Pn0 | — | 49.2 | 57.05 | 63.85 | none |
| 3 | 75 | 1.80 | 73.4 | 22.7 | 0.96 | 272 | 3 + 4 | 7.66 | pT2c, pN0, L0, V0, Pn0 | — | 32.54 | 38.67 | 44.25 | none |
| 4 | 73 | 1.70 | 70.7 | 24.5 | 1.12 | 262 | 5 + 5 | 5.86 | pT2c, pN0, L0, V0, Pn1 | — | 54.16 | 74.08 | 101.27 | none |
| 5 | 74 | 1.62 | 55.8 | 21.3 | 0.68 | 206 | 4 + 3 | 3.95 | pT2c, pN0, L0, V1, Pn0 | — | 36.11 | 45.71 | 51.42 | none |
| 6 | 69 | 1.73 | 60.8 | 20.3 | 0.73 | 225 | 4 + 3 | 18.33 | pT2c, pN0, L0, V0, Pn0 | — | 25 | 33.1 | 41.37 | none |
| 7 | 65 | 1.74 | 76.4 | 25.2 | 1.17 | 283 | 4 + 3 | 7.18 | pT2c, pN0, L0, V1, Pn1 | — | 8.64 | 10.67 | 12.15 | none |
| 8 | 71 | 1.69 | 77.6 | 27.2 | 1.15 | 287 | 3 + 3 | 5.54 | — | Prostate and bone | 19.16 | 22.64 | 31.92 | none |
| 9 | 76 | 1.60 | 63.7 | 24.9 | 0.90 | 236 | NA | 3.12 | — | Lymph node | 74.35 | 81.65 | 100.54 | none |
| 10 | 72 | 1.67 | 76.3 | 27.4 | 1.03 | 282 | 3 + 4 | 2.55 | — | Prostate | 8.64 | 10.67 | 12.15 | none |
Note.- SUV, standardized uptake value; NA, not available.
aNo. 1 to 7 were patients with treatment-naïve prostate cancer, and No. 8 to 10 were patients with biochemical recurrence after radical radiation therapy.
bThe highest Gleason scores of the radical prostatectomy specimens from patients No. 1 to 7 and target biopsy specimens from patients No. 8 and 10 are shown.
cThe number in parenthesis is the uptake time or acquisition time. Values represent the highest focal uptake in the prostate lesions from patient No. 1 to 8 and 10 and the abnormal uptake in the pelvic lymph node from patient No. 9.
Safety and tolerability
18F-PSMA 1007 was found to be safe and well tolerated in all subjects. No serious AEs or drug-related AEs were identified during the present study. There were no errors in the process of 18F-PSMA 1007 synthesis by the W6CY346 synthesizer (MPS200PSMA-F). There were no abnormalities in vital signs and 12-lead ECG findings. None of the reported changes from baseline in hematology, coagulation, and clinical chemistry parameters were considered clinically significant.
Biodistribution and radiation dosimetry
Figure 3 shows the pharmacokinetics of 18F-PSMA 1007 in three healthy volunteers of the Phase 1 study. No significant difference was found in three healthy volunteers. The average blood radioactivity concentration (%ID/ml) reached a maximum of 47.87 ± 1.05 at 5 min after the administration of 18F-PSMA 1007, and then decreased rapidly to 16.40 ± 1.68 at 1 h, 7.67 ± 2.83 at 2 h, 4.33 ± 1.50 in 3 h and 1.60 ± 0.78 in 6 h (Fig. 3A). The systemic radioactivity (%ID|$\times$|103) reached a maximum of 211.05 ± 6.77 at 5 min after the administration of 18F-PSMA 1007, and decreased rapidly to 72.62 ± 11.39 at 1 h, 34.25 ± 14.45 at 2 h, 19.35 ± 7.76 in 3 h and 7.18 ± 3.91 in 6 h (Fig. 3B). The AUC, Cmax (%ID/ml) and Tmax (h) of the first 6 h were 52.78 ± 8.75, 47.87 ± 1.05 and 0.08, respectively. Cumulative urinary excretion of radioactivity after 18F-PSMA 1007 administration (%ID) was 0.80 ± 0.10 at 0–2 h, 1.37 ± 0.06 at 0–4 h and 1.93 ± 0.21 in 0–6 h (Fig. 3C). The systemic radioactivity distribution was 62.69 ± 5.01 at 5 min after 18F-PSMA 1007 administration, but then decreased to 40.45 ± 6.08 at 1 h, 26.13 ± 4.16 at 2 h, 17.21 ± 2.76 at 3 h, and 6.06 ± 1.05 at 6 h. The effective dose was 4.97 ± 0.53 mSv in all subjects. The effective dose per MBq in target organs and tissues after administration of 18F-PSMA 1007 is shown in Fig. 4. The five organs/tissues associated with the highest radioactivity distribution were kidneys, parotid glands, liver, adrenal gland and red marrow. The absorbed doses (mGy/MBq) in parotid glands, kidney, and liver were 0.15 ± 0.04, 0.10 ± 0.03 and 0.07 ± 0.02, respectively.
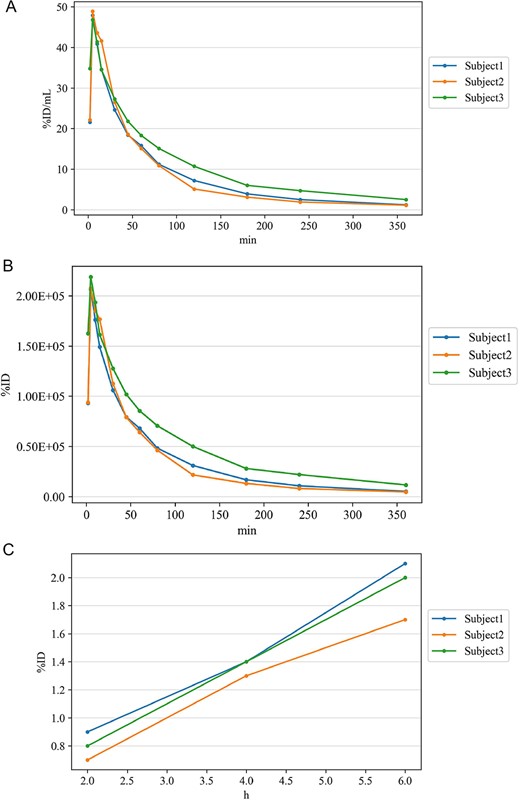
The pharmacokinetics of 18F-PSMA 1007 in three healthy volunteers (subject 1–3). (A). Average blood radioactivity concentration (%ID/mL). (B) Systemic radioactivity. (C) Cumulative urinary excretion of radioactivity.
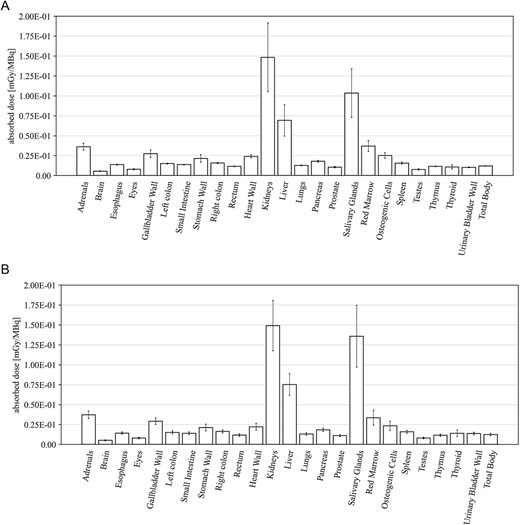
The effective dose per MBq in target organs and tissues. (A) Absorbed dose in subjects with Part A (mGy/MBq). (B) Absorbed dose in subjects with Part B (mGy/MBq).
Clinical effectiveness
Visual assessment revealed that 18F-PSMA 1007 PET/CT could detect primary and metastatic lesions in all patients. In the patient-by-patient analysis, the sensitivity and positive predictive value of 18F-PSMA 1007 PET/CT were 100% and 100%, when measured against both pathologic and imaging confirmation as the gold standard, respectively. Similar results were obtained in the assessment by PSMA-RAD Ver.1.0. Initial PSA level didn’t correlate with any SUVmax (r = 0.087, P = 0.853 for 60 min; r = 0.044, P = 0.925 for 90 min; r = −0.048, P = 0.918 for 180 min uptake time) of the primary tumor. In cohort A, 15 primary foci (11.9%) were identified in all 126 segments based on 18F-PSMA 1007 PET/CT. The largest diameter of primary foci ranged from 5 to 32 mm (median 18 mm; interquartile range [IQR], 13.8–2.3 mm) in the pathologic specimens. Primary foci were located in 39 segments (30.1%). In contrast, eight primary foci ranging from 1 to 8 mm (median 2 mm; interquartile range [IQR], 2.0–4.0 mm) in the pathologic specimens couldn’t be visualized on 18F-PSMA 1007 PET/CT (Fig. 5). In addition to these eight lesions from the analysis considering possible spatial resolution of PET/CT, there were missed tumors (false negative findings) in three segments of two patients (28.6%) (Fig. 6). These tumors were located in the peripheral zone within posterior elements of the prostatic lobe. However, these tumors were unrecognizable on both of diffusion-weighted and T2-weighted MR images. False positive findings in one segment in a patient (14.3%) were caused by non-specific accumulation within normal tissue. Corresponded diffusion-weighted and T2-weighted MR images showed normal findings. Primary tumors were correctly determined in 34 segments (27.0%) at both uptake times of 90 and 180 min on 18F-PSMA 1007 PET/CT, while at the 60 min uptake time, 18F-PSMA 1007 PET/CT failed to identify primary tumor in two segments. The specificity was similar between uptake times on 18F-PSMA 1007 PET/CT. Diagnostic accuracy values are summarized in Table 2. A segment-by-segment basis analysis found the highest diagnostic accuracy was 93.7% on 18F-PSMA 1007 PET/CT. Nodal metastasis of the left iliac node was pathologically proven in one patient (33.3%) of Cohort 2. This patient received surgical biopsy. The affected node was correctly identified on 18F-PSMA 1007 PET/CT. Verification of bone metastasis was accomplished with radiologic follow-up in one patient (33.3%) of Cohort B. Three bone metastases were correctly identified on 18F-PSMA 1007 PET/CT. A false positive result in one patient resulted from a benign rib tumor (an osteolytic but slightly sclerotic rim lesion) on the CT portion of PET/CT. In a retrospective review of routine CT scans, we found that this bone lesion hasn’t changed in 3 years (Fig. 7).
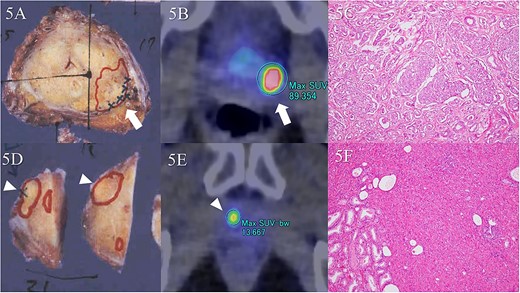
Comparison of pathologic specimens and 18F-PSMA 1007 PET/CT. There were two tumor foci in separate segments. Tumor lesion is in the left dorsal segment of the midgland on macroscopic observation (A, arrow). The largest diameter is 20 mm. A Gleason score of 4 + 3 demonstrates significant uptake (B, arrow). Microscopic observation reveals adenocarcinoma with Gleason score of 4 + 3 (C). In addition, the diameter of the tumor in the right ventral segment of the apex is a maximal 18 mm (D, arrowhead). Significant uptake is found in the corresponding area on 18F-PSMA 1007 PET/CT (E, arrowhead). Gleason score is 4 + 3, similarly (F).
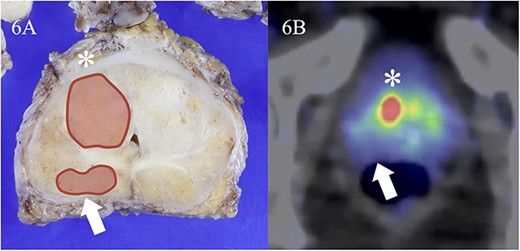
False negative finding identified on 18F-PSMA 1007 PET/CT. Tumor lesion is in the right ventral segment of the midgland on macroscopic observation (A, asterisk). The largest diameter is 18 mm and Gleason score is 3 + 3. Significant uptake is found in the same area on 18F-PSMA 1007 PET/CT (B, asterisk). The SUVmax is 44.2. In contrast, tumor in the right dorsal segment of the midgland (FA, arrow) representing maximal 10 mm and Gleason score 3 + 4 can’t be detected on 18F-PSMA 1007 PET/CT (B, arrow).
Diagnostic performance of 18F-PSMA 1007 PET/CT in patients in the Part B Cohort 1
| Uptake time . | Sensitivity . | Specificity . | Positive predictive value . | Negative predictive value . | Accuracy . |
|---|---|---|---|---|---|
| 60 min | 80.0% | 96.5% | 91.4% | 91.2% | 91.3% |
| (32/40) | (83/86) | (32/35) | (83/91) | (115/126) | |
| 90 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) | |
| 180 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) |
| Uptake time . | Sensitivity . | Specificity . | Positive predictive value . | Negative predictive value . | Accuracy . |
|---|---|---|---|---|---|
| 60 min | 80.0% | 96.5% | 91.4% | 91.2% | 91.3% |
| (32/40) | (83/86) | (32/35) | (83/91) | (115/126) | |
| 90 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) | |
| 180 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) |
Note.- The numbers in parenthesis are numbers of prostatic segments.
Diagnostic performance of 18F-PSMA 1007 PET/CT in patients in the Part B Cohort 1
| Uptake time . | Sensitivity . | Specificity . | Positive predictive value . | Negative predictive value . | Accuracy . |
|---|---|---|---|---|---|
| 60 min | 80.0% | 96.5% | 91.4% | 91.2% | 91.3% |
| (32/40) | (83/86) | (32/35) | (83/91) | (115/126) | |
| 90 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) | |
| 180 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) |
| Uptake time . | Sensitivity . | Specificity . | Positive predictive value . | Negative predictive value . | Accuracy . |
|---|---|---|---|---|---|
| 60 min | 80.0% | 96.5% | 91.4% | 91.2% | 91.3% |
| (32/40) | (83/86) | (32/35) | (83/91) | (115/126) | |
| 90 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) | |
| 180 min | 85.0% | 96.5% | 91.9% | 93.3% | 92.9% |
| (34/40) | (83/86) | (34/37) | (83/89) | (117/126) |
Note.- The numbers in parenthesis are numbers of prostatic segments.
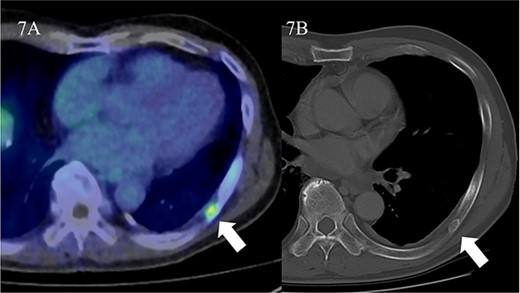
False positive finding resulted from a benign rib tumor. Focal uptake is identified in the left rib on 18F-PSMA 1007 PET/CT (A, arrow). The SUVmax is 2.8. Corresponded CT showed an osteolytic but slightly sclerotic rim lesion. No significant change was found in CT appearance of rib lesion for 3 years (B, arrow). This patient was diagnosed as having recurrent tumor within prostate after IMRT.
Discussion
The purpose of the present study was to evaluate clinical safety, whole-body biodistribution, and internal radiation dosimetry. 18F-PSMA 1007 was found to be safe, well tolerated, and not associated with any AEs. Radiation burden of 3.7 Mbq/kg injection translates to the effective dose of 4.97 ± 0.53 mSv in all subjects, which was within the range of 4.4 to 5.5 mSv reported by the previous study (9). Rapid washout of 18F-PSMA 1007 activity from the blood was observed with minimal urinary excretion. High hepatobiliary clearance was identified with a slow washout eventually appearing in the gastrointestinal tract.
We observed that 18F-PSMA 1007 PET/CT had a high accuracy of diagnosis, 91.3–92.9%, of pathologically proven prostatic cancer without dependence on 18F-PSMA 1007 uptake time. Sensitivity was highest between the 90 and 180 min image groups compared with the 60 min image group, despite representing the highest specificity among all image groups. First, it is tempting to speculate that this reflects the difficulty of correctly detecting prostatic cancer foci with diameters between 5 and 8 mm when uptake time is set at 60 min, as opposed to when the uptake time is set at 90 or 180 min and counting of subcentimetric foci is improved. However, we did not find a statistically significant difference of sensitivity between these groups. Given the greater difficulties of visualizing prostatic cancer foci <5 mm in the largest diameter, the scan interpreters would be expected to underestimate counts on images from the current PET/CT system equipped with semi-conductor detector.
Comparison of the diagnostic performance characteristics of 18F-PSMA 1007 PET/CT at three different acquisition times revealed similar trends. At any uptake time, the positive predictive value for detecting prostate cancer was sufficiently high (over 91%) to permit definitive interpretation of a positive PET/CT examination. Similarly, the negative predictive value for detecting prostate cancer was high enough (over 91%) at each uptake time. Given that the sensitivities of 18F-PSMA 1007 PET/CT at three different uptake times were comparable, the observed consistency reflects minimal differences in image quality between acquisition times. We documented the undercounting of only six to eight prostate cancer foci within 40 prostatic segments on 18F-PSMA 1007 PET/CT. Our findings showed that an uptake time of at least 60 min is preferable for detecting primary foci >5 mm in the largest diameter on 18F-PSMA 1007 PET/CT.
In contrast to previous studies, our study focused on radiologic–pathologic correlation using prostatectomy specimens (11,16–19). A total of 23 cancer foci were identified within the prostate in seven patients who received prostatectomy. Of these, 15 cancer foci were >5 mm in the largest diameter and 8 cancer foci were < or = 5 mm on microscopic examination. We documented that there were three false negative lesions. It is likely that the causes of the false negative results for small tumor foci were incomplete recovery of counts and peripheral location of the specific tumor within posterior elements of the prostatic lobe. We speculate that small cancer foci of the posterior prostatic lobe might be easily affected by CT based attenuation correction (CTAC) error due to misregistration of rectal air. Negative artifacts caused by CTAC error from 0 Hounsfield units of rectal air density can lead to the underestimation of accumulations of posterior elements adjacent to the rectum. Interestingly, all three false negative lesions were identifiable on MR images obtained preoperatively. Possibly, artifacts related to misregistration of rectal air lead to underestimation of lesion number regardless of imaging modality.
We observed one false positive lesion on a rib of one patient in Cohort 2. This lesion was osteolytic, had a slightly sclerotic rim on CT, and could be identified on routine CT images obtained three years before. Despite the high sensitivity of detection for bone metastasis on PET/CT (20,21), uptake of 18F-PSMA 1007 within ribs was reported to be non-specific. The reasons for this apparent paradox are not immediately clear. Watabe et al.(22) described the phenomenon of non-specific rib uptake on 18F-PSMA 1007 PET/CT in a large series of cases. Since this phenomenon is hardly ever observed in bones other than ribs, it might be that F-18 PSMA 1007 has an affinity for areas of pathologic damage in asymptomatic cases of rib injury. In addition, the one false positive lesion in the rib hadn’t changed in 3 years and was identified as a non-specific benign bone tumor in our study. Given the greater difficulties in interpreting rib uptake on 18F-PSMA 1007 PET/CT in patients with prostate cancer, the observers may be biased in favor of detecting bone metastasis more selectively. This interpretation might be restricted to those patients at high risk for bone metastasis development.
Given the limitations of 18F-PSMA 1007, the ultimate role of 18F-PSMA 1007 in screening patients with BCR is to detect small foci of recurrent or metastatic disease not easily detected in routine imaging studies. Using a cutoff of 5 mm as the primary target lesion diameter, we estimated that specificity was high but the sensitivity with preferable positive predictive value was relatively high in our study. Although the status of primary foci and that of recurrent or metastatic lesions are different, lesions <5 mm must be interpreted with caution, as it can lead to false-negative findings. When precise definition of a focal positive lymph node on 18F-PSMA 1007 PET/CT is critical, such as when the assessment is conducted prior to radical prostatectomy, reference to initial PSA level should be considered for high-risk patients. The presence of focal positive lymph nodes in patients with elevated initial PSA appears to reliably point to the presence of metastasis and therefore to obviate the need for lymph node sampling or nodal dissection.
One potential limitation of our study was the small number of cases with BCR in Cohort B. Giesel et al (9) reported on 10 patients who underwent 18F-PSMA 1007 PET/CT prior to radical prostatectomy and lymph node dissection. Metastases were pathologically proven in seven of the 10 patients. Of these metastases, 18 lymph node metastases were identified with median diameter of 5 mm. Notably, none of the patients in our study had nodal metastasis, but we included patients with newly diagnosed low- to middle-risk prostate cancer. However, we evaluated 15 primary cancer foci >5 mm in the largest diameter. Considering the spatial resolution of the current PET/CT system, both primary and nodal metastatic prostate lesions around 5 mm can almost be detected.
In conclusion, the results of our study revealed that 18F-PSMA 1007 is a safe and well tolerated tracer that is not associated with any AEs. Analyzing the data on a lesion-by-lesion basis and using an uptake time of at least 60 min, we showed the high accuracy of using 18F-PSMA 1007 PET/CT to diagnose prostate cancer lesions >5 mm in the largest diameter. This non-invasive technique demonstrates reliable diagnostic accuracy in patients at high risk for prostate cancer, and it has a great potential to impact the management of prostate cancer patients.
Acknowledgements
We thank Dr. Jens Cardinale and Dr. Frederik L. Giesel, Department of Nuclear Medicine, University Hospital Duesseldorf, Medical Faculty, Heinrich-Heine-University, Duesseldorf, Germany for medical advice, Dr. Towako Taguchi, Department of Comprehensive Pathology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University for pathologic material, Sadahiro Naka, Radiopharmacist, Osaka University Hospital, and Takehito Okamoto, Ph. D., Kazuma Ishibashi, Imaging Science Department of Micron, Inc. for data processing. We also thank the patients and their families, and the participating study teams for making this study possible.
Author Contributions
Study management (Ukihide Tateishi), Manuscript edit (Ukihide Tateishi, Koichiro Kimura, Tadashi Watabe, Soichiro Yoshida, Yasuhisa Fujii), Study operation (Ukihide Tateishi, Koichiro Kimura, Junichi Tsuchiya, Daisuke Kano, Tadashi Watabe, Norio Nonomura, Katsuhiko Saito, Kota Yokoyama, Ken Yamagiwa, Takuya Adachi, Yuji Kojima, Soichiro Yoshida, Yasuhisa Fujii), Data collection (Koichiro Kimura, Junichi Tsuchiya, Kota Yokoyama, Ken Yamagiwa, Takuya Adachi, Yuji Kojima).
Funding
Japan Agency for Medical Research and Development (AMED; Nos. 20ck0106577, 21ck0106577 and 22ck0106577).
Conflict of Interest
Tokyo Medical and Dental University received synthesizer, QC equipment, materials and precursor (ABX, PS–101) from Sumitomo Heavy Industries. Ltd., Tokyo, Japan.
Ethics Statement
Approval of the research protocol by an Institutional Reviewer Board.
Informed Consent
A written informed consent was obtained from each subject.
Registry and the Registration No. of the study/trial: jRCT2032210711


