-
PDF
- Split View
-
Views
-
Cite
Cite
Takahiro Osawa, Keita Sasaki, Ryunosuke Machida, Takashi Matsumoto, Yoshiyuki Matsui, Hiroshi Kitamura, Hiroyuki Nishiyama, Real-world treatment trends for patients with advanced prostate cancer and renal cell carcinoma and their cost—a survey in Japan, Japanese Journal of Clinical Oncology, Volume 54, Issue 10, October 2024, Pages 1062–1070, https://doi.org/10.1093/jjco/hyae045
Close - Share Icon Share
Abstract
Advanced (Stage IV) prostate and renal cancer have poor prognosis, and several therapies have been developed, but many are very costly. This study investigated drug regimens used in patients with untreated Stage IV prostate cancer and renal cell carcinoma and calculated the monthly cost of each.
We surveyed first-line drugs administered to patients with untreated Stage IV prostate cancer and renal cancer at Japan Clinical Oncology Group affiliated centers from April 2022 to March 2023. Drug costs were calculated according to drug prices in September 2023. Individual drug costs were calculated or converted to 28-day costs.
A total of 700 patients with untreated Stage IV prostate cancer were surveyed. Androgen deprivation therapy + androgen receptor signaling inhibitor was the most common regimen (56%). The cost of androgen deprivation therapy + androgen receptor signaling inhibitor was 10.6–30.8-fold compared with conventional treatments. A total of 399 patients with Stage IV renal cancer were surveyed. Among them, 91% of patients received immune-oncology drug-based regimen. All patients received treatments with a monthly cost of ≥500 000 Japanese yen, and 80.4% of patients received treatments with a monthly cost of ≥1 million Japanese yen, of combination treatments. The cost of immune-oncology drug-based regimen was 1.2–3.1-fold that of TKI alone.
To the best of our knowledge, this is the first report of a survey of first-line drug therapy in untreated Stage IV prostate cancer and renal cell carcinoma stratified by age and treatment costs. Our results show that most Japanese patients received state-of-the-art, effective treatments with high financial burden.
Introduction
Japan is facing an unprecedented super-aging society (1). Prostate cancer and renal cell carcinoma (RCC) are the most common urologic cancers among older adults. In Japan, the incidence of prostate cancer is ~15 per 10 000 population, and it was the leading cancer in men in 2019 (2). The average age of patients with prostate cancer is 71.3 years, and the 10-year cancer-specific survival proportion for Stages I–III and IV (14.6% of all patients) are ~90% (good prognosis) and 36.9% (poor prognosis), respectively. By contrast, the average age of patients with RCC is 65.5 years. Similar to prostate cancer, the 10-year cancer-specific survival proportion for Stages I and II and IV (16.2% of all patients) are ~80% (good prognosis) and 7.3% (poor prognosis), respectively (3). Both prostate cancer and RCC have extremely poor prognoses when diagnosed at Stage IV when metastasis has occurred.
The standard treatment for Stage IV prostate cancer was androgen deprivation therapy (ADT) (4). However, metastatic prostate cancer that initially responded to ADT became resistant to ADT and progressed to metastatic castration-resistant prostate cancer within 2–3 years (5). Subsequently, new therapies were developed, and docetaxel, abiraterone, enzalutamide and apalutamide, in combination with ADT, impaired disease progression and improved overall survival (OS) in randomized clinical trials (6–15). This is now the current standard of care.
The standard of care for Stage IV RCC has reached a turning point: inhibitors of the programmed cell death receptor pathway (immune-oncology drug: IO) in combination with a tyrosine kinase inhibitor (TKI) (IO–TKI combination) or in combination with other IO agents (IO–IO combination) were compared with the TKI sunitinib alone. IO–TKI and IO–IO combination therapy showed improved response rates, progression-free survival and OS compared with TKI sunitinib alone (16–26). Thus, with the development of new therapeutic approaches, improvement in prognosis has been achieved, but both TKIs and IOs are expensive treatments.
Although the development of new therapies has improved prognosis, the cost of new drug therapies for cancer treatment has skyrocketed in recent years, which has become a problem worldwide (27–29). For example, high drug costs threaten healthcare budgets and limit funding for other areas such as public investment. In countries that, unlike Japan, do not have universal health insurance, the high cost of prescription drugs can lead to high out-of-pocket costs for individual patients, making drugs unaffordable for those who need them. Recently, higher drug costs have been widely reported across new therapies, particularly for patients with prostate cancer and RCC (30,31). In this study, we surveyed patients with untreated Stage IV prostate cancer and RCC in Japan to determine the currently used drugs. We also calculated the proportion of new treatments in the total drug treatment and the monthly cost per month for each treatment. This study was carried out under the leadership of the Japan Clinical Oncology Group (JCOG) Health Economic Committee.
Patients and methods
A survey was conducted among physicians at JCOG-affiliated centers regarding the initial drug therapy given to patients with untreated Stage IV prostate cancer and RCC. The patients were first diagnosed with advanced cancer at JCOG institutions between April 2022 and March 2023. The number of patients in different age categories (<75 and ≥75 years) were collected separately. A Google Form questionnaire was used, and no personal patient information was collected; the survey investigated the initial treatment given.
The prostate cancer drug regimens included ADT alone (goserelin, leuprorelin or degarelix), ADT + antiandrogen (bicalutamide or flutamide), ADT + docetaxel and ADT + androgen receptor signaling inhibitor (ARSI; abiraterone, apalutamide or enzalutamide). For RCC drug therapy, the study included patients who received TKI (sunitinib, pazopanib, cabozantinib or sorafenib), IO–IO (nivolumab + ipilimumab) and IO–TKI (pembrolizumab + axitinib, avelumab + axitinib, nivolumab + cabozantinib or pembrolizumab + lenvatinib) regimens.
Drug costs were calculated according to drug prices as of September 2023. For docetaxel, avelumab and ipilimumab doses, a body weight of 59 kg and a body surface area of 1.68 m2 were used as the standard for Japanese patients. For nivolumab + ipilimumab, the monthly cost was calculated after calculating the 12-month drug cost because the cost of treatment in the first 3 months and beyond varies. We defined high-cost and very high-cost treatments as those that cost ≥500 000 and ≥1 000 000 Japanese yen (JPY) per month, respectively, as per the definition prescribed by the JCOG Health Economics Committee.
Results
Among 44 JCOG participating centers, 38 and 36 centers provided information about the drugs they used to treat prostate cancer and RCC, respectively (Supplementary Table 1). A total of 700 patients with untreated Stage IV prostate cancer and 137 patients with Stage IV RCC were surveyed.
The main treatments that were introduced for metastatic hormone-sensitive prostate cancer (mCSPC) stratified by age and cost per month are shown in Fig. 1 and Table 1. ADT + ARSI was the most common (56%) regimen, and ADT + docetaxel was the least common (4.0%). Patients who were ≥75 years old were more likely to be treated with ADT alone than patients <75 years old (18.5% vs. 9.6%). Furthermore, patients ≥75 years old were less likely to be treated with ADT + apalutamide than those <75 years old (9.8% vs. 18.4%). Of note, ADT + ARSI was administered to 56% of all treated patients, with drug costs ranging from 272 874 to 424 746 JPY (Table 1). The cost of ADT + ARSI was 10.6–30.8-fold compared with ADT alone and ADT + antiandrogen, which are conventional treatments. Therefore, although no patients received high-cost treatments according to the definition used by this study, treatment costs for mCSPC have greatly increased. Details of drug costs for prostate cancer are described in Supplementary Table 2.
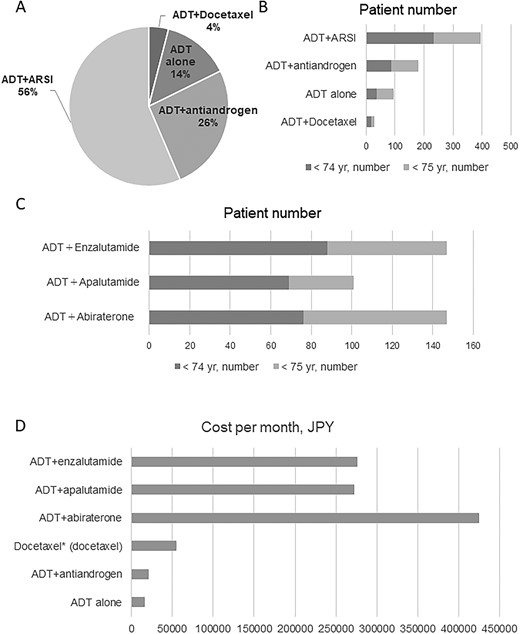
(A) Treatment selection for patients with untreated Stage IV prostate cancer. (B) Treatment selection (stratified by age) for patients with untreated Stage IV prostate cancer. (C) Number of patients stratified by age for each of the three ADT + ARSI treatments. (D) Treatment costs of different regimens. ADT, androgen deprivation therapy; ARSI, androgen receptor signaling inhibitor.
Number of patients by type of treatment, breakdown of patients by age, and monthly drug costs
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | . | Cost per month, JPY . |
|---|---|---|---|---|---|
| ADT alone | 36 (9.6%) | 60 (18.5%) | 96 (13.7%) | 16 383 | |
| ADT+ antiandrogen | 87 (23.2%) | 94 (28.9%) | 181 (25.9%) | 21 319 | |
| ADT+ docetaxel | 19 (5.1%) | 9 (2.8%) | 28 (4.0%) | 54 693 | |
| ADT+ abiraterone | 76 (20.3%) | 71 (21.8%) | 147 (21.0%) |  | 424 746 |
| ADT+ apalutamide | 69 (18.4%) | 32 (9.8%) | 101 (14.4%) | 272 874 | |
| ADT+ enzalutamide | 88 (23.5%) | 59 (18.2%) | 147 (21.0%) | 275 971 | |
| Total | 375 (100%) | 325 (100%) | 700 (100%) |
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | . | Cost per month, JPY . |
|---|---|---|---|---|---|
| ADT alone | 36 (9.6%) | 60 (18.5%) | 96 (13.7%) | 16 383 | |
| ADT+ antiandrogen | 87 (23.2%) | 94 (28.9%) | 181 (25.9%) | 21 319 | |
| ADT+ docetaxel | 19 (5.1%) | 9 (2.8%) | 28 (4.0%) | 54 693 | |
| ADT+ abiraterone | 76 (20.3%) | 71 (21.8%) | 147 (21.0%) |  | 424 746 |
| ADT+ apalutamide | 69 (18.4%) | 32 (9.8%) | 101 (14.4%) | 272 874 | |
| ADT+ enzalutamide | 88 (23.5%) | 59 (18.2%) | 147 (21.0%) | 275 971 | |
| Total | 375 (100%) | 325 (100%) | 700 (100%) |
ADT, androgen deprivation therapy.
Number of patients by type of treatment, breakdown of patients by age, and monthly drug costs
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | . | Cost per month, JPY . |
|---|---|---|---|---|---|
| ADT alone | 36 (9.6%) | 60 (18.5%) | 96 (13.7%) | 16 383 | |
| ADT+ antiandrogen | 87 (23.2%) | 94 (28.9%) | 181 (25.9%) | 21 319 | |
| ADT+ docetaxel | 19 (5.1%) | 9 (2.8%) | 28 (4.0%) | 54 693 | |
| ADT+ abiraterone | 76 (20.3%) | 71 (21.8%) | 147 (21.0%) |  | 424 746 |
| ADT+ apalutamide | 69 (18.4%) | 32 (9.8%) | 101 (14.4%) | 272 874 | |
| ADT+ enzalutamide | 88 (23.5%) | 59 (18.2%) | 147 (21.0%) | 275 971 | |
| Total | 375 (100%) | 325 (100%) | 700 (100%) |
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | . | Cost per month, JPY . |
|---|---|---|---|---|---|
| ADT alone | 36 (9.6%) | 60 (18.5%) | 96 (13.7%) | 16 383 | |
| ADT+ antiandrogen | 87 (23.2%) | 94 (28.9%) | 181 (25.9%) | 21 319 | |
| ADT+ docetaxel | 19 (5.1%) | 9 (2.8%) | 28 (4.0%) | 54 693 | |
| ADT+ abiraterone | 76 (20.3%) | 71 (21.8%) | 147 (21.0%) |  | 424 746 |
| ADT+ apalutamide | 69 (18.4%) | 32 (9.8%) | 101 (14.4%) | 272 874 | |
| ADT+ enzalutamide | 88 (23.5%) | 59 (18.2%) | 147 (21.0%) | 275 971 | |
| Total | 375 (100%) | 325 (100%) | 700 (100%) |
ADT, androgen deprivation therapy.
Table 2 lists the results of clinical trials on primary drug therapy for mCSPC. It summarizes the drug dose, OS, recurrence-free survival, older adult sample size and treatment duration of each clinical trial. The control treatment was ADT alone in all clinical trials except the ENZAMET trial. The LATITUDE trial included patients with high-risk mCSPC, while the other trials included all mCSPC patients. In each trial, >45% of patients were >70 years old and 20–30% were >75 years old. The previously reported median durations of treatment for ADT alone, ADT + antiandrogen, ADT + docetaxel, ADT + abiraterone, ADT + apalutamide and ADT + enzalutamide were 13.8–20.2, 13.8–20.2, 33, 25.8, 39.3 and 40.2 months, respectively (Table 2). ADT + enzalutamide had the longest median treatment duration, and the overall total treatment cost was 11 094 034 JPY. Since no head-to-head trials have compared these three ARSIs, clinicians are faced with the challenging task of choosing the most appropriate treatment for patients with mCSPC.
Results of pivotal trials investigating hormone therapies for metastatic prostate cancer
| . | Latitude . | Charrted . | Enzamet . | Arches . | Titan . |
|---|---|---|---|---|---|
| Author | Fizazi (2017, 2019) | Sweeney (2015) | Davis (2019) | Armstrong (2019) | Chi (2019, 2021) |
| Kyriakopoulos (2018) | Sweeney (2023) | Armstrong (2022) | |||
| New treatment | Abiraterone + ADT | Docetaxel + ADT | Enzalutamide + ADT | Enzalutamide + ADT | Apalutamide + ADT |
| Dosage | 1000 mg | 75 mg/m^2 | 160 mg | 160 mg | 240 mg |
| Control | Placebo + ADT | ADT | NSAA + ADT | Placebo + ADT | Placebo + ADT |
| Inclusion criteria | High-risk mHSPC | mHSPC | mHSPC | mHSPC | mHSPC |
| Gleason >8 | |||||
| Bone meta >3 | |||||
| Visceral meta | |||||
| N (total) | 1199 | 790 | 1125 | 1150 | 1152 |
| N (new treatment) | 597 | 397 | 563 | 574 | 525 |
| Age (new treatment), years, median (range) | 68 yr (38–89) | 64 yr (36–88) | 69 yr (63–74) | 70 yr (46–92) | 69 yr (45–94) |
| Elderly patients (new treatment), No. (%) | ≥75 yr, 123 (20.6%) | ≥70 yr, 178 (44.8%) | ≥70 yr, 257 (45.6%) | ≥75 yr, 170 (29.6%) | ≥75 yr, 133 (25.3%) |
| OS, months, median, New treatment/control | 53.3/36.5 | 57.6/47.2 | NR/NR | NR/NR | NR/52.2 |
| HR 0.66 (95%CI 0.56–0.78) | HR 0.72 (95%CI 0.59–0.89) | HR 0.70 (95%CI 0.58–0.84) | HR 0.66 (95%CI 0.53–0.81) | HR 0.66 (95% 0.53–0.79) | |
| PFS, months, median, New treatment/control | rPFS 33.1/14.7 | cPFS 33.0/19.8 | cPFS 81.0/25.0 | rPFS 49.8/38.9 | rPFS NR/22.1 |
| HR 0.46 (95%CI 0.39–0.54) | HR 0.62 (95%CI 0.51–0.75) | HR 0.45 (95%CI 0.39–0.53) | HR 0.63 (95%CI 0.52–0.76) | HR 0.48 (95% 0.39–0.60) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 25.8 (IQR: 12.3–49) | NA | NA | 40.2 (range 0.2–58.1) | 39.3 (range 0–55.7) |
| Control | 14.4 (IQR: 7.3–25.8) | NA | NA | 13.8 (range 0.2–27.6) | 20.2 (range 0.1–37.0) |
| . | Latitude . | Charrted . | Enzamet . | Arches . | Titan . |
|---|---|---|---|---|---|
| Author | Fizazi (2017, 2019) | Sweeney (2015) | Davis (2019) | Armstrong (2019) | Chi (2019, 2021) |
| Kyriakopoulos (2018) | Sweeney (2023) | Armstrong (2022) | |||
| New treatment | Abiraterone + ADT | Docetaxel + ADT | Enzalutamide + ADT | Enzalutamide + ADT | Apalutamide + ADT |
| Dosage | 1000 mg | 75 mg/m^2 | 160 mg | 160 mg | 240 mg |
| Control | Placebo + ADT | ADT | NSAA + ADT | Placebo + ADT | Placebo + ADT |
| Inclusion criteria | High-risk mHSPC | mHSPC | mHSPC | mHSPC | mHSPC |
| Gleason >8 | |||||
| Bone meta >3 | |||||
| Visceral meta | |||||
| N (total) | 1199 | 790 | 1125 | 1150 | 1152 |
| N (new treatment) | 597 | 397 | 563 | 574 | 525 |
| Age (new treatment), years, median (range) | 68 yr (38–89) | 64 yr (36–88) | 69 yr (63–74) | 70 yr (46–92) | 69 yr (45–94) |
| Elderly patients (new treatment), No. (%) | ≥75 yr, 123 (20.6%) | ≥70 yr, 178 (44.8%) | ≥70 yr, 257 (45.6%) | ≥75 yr, 170 (29.6%) | ≥75 yr, 133 (25.3%) |
| OS, months, median, New treatment/control | 53.3/36.5 | 57.6/47.2 | NR/NR | NR/NR | NR/52.2 |
| HR 0.66 (95%CI 0.56–0.78) | HR 0.72 (95%CI 0.59–0.89) | HR 0.70 (95%CI 0.58–0.84) | HR 0.66 (95%CI 0.53–0.81) | HR 0.66 (95% 0.53–0.79) | |
| PFS, months, median, New treatment/control | rPFS 33.1/14.7 | cPFS 33.0/19.8 | cPFS 81.0/25.0 | rPFS 49.8/38.9 | rPFS NR/22.1 |
| HR 0.46 (95%CI 0.39–0.54) | HR 0.62 (95%CI 0.51–0.75) | HR 0.45 (95%CI 0.39–0.53) | HR 0.63 (95%CI 0.52–0.76) | HR 0.48 (95% 0.39–0.60) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 25.8 (IQR: 12.3–49) | NA | NA | 40.2 (range 0.2–58.1) | 39.3 (range 0–55.7) |
| Control | 14.4 (IQR: 7.3–25.8) | NA | NA | 13.8 (range 0.2–27.6) | 20.2 (range 0.1–37.0) |
c, clinical; CI, confidence interval; meta, metastasis; HR, hazard ratio; NA, not available; NR, not reached; NSAA, non-steroidal antiandrogen; OS, overall survival; PC, prostate cancer; PFS, progression-free survival; r, radiographic.
Results of pivotal trials investigating hormone therapies for metastatic prostate cancer
| . | Latitude . | Charrted . | Enzamet . | Arches . | Titan . |
|---|---|---|---|---|---|
| Author | Fizazi (2017, 2019) | Sweeney (2015) | Davis (2019) | Armstrong (2019) | Chi (2019, 2021) |
| Kyriakopoulos (2018) | Sweeney (2023) | Armstrong (2022) | |||
| New treatment | Abiraterone + ADT | Docetaxel + ADT | Enzalutamide + ADT | Enzalutamide + ADT | Apalutamide + ADT |
| Dosage | 1000 mg | 75 mg/m^2 | 160 mg | 160 mg | 240 mg |
| Control | Placebo + ADT | ADT | NSAA + ADT | Placebo + ADT | Placebo + ADT |
| Inclusion criteria | High-risk mHSPC | mHSPC | mHSPC | mHSPC | mHSPC |
| Gleason >8 | |||||
| Bone meta >3 | |||||
| Visceral meta | |||||
| N (total) | 1199 | 790 | 1125 | 1150 | 1152 |
| N (new treatment) | 597 | 397 | 563 | 574 | 525 |
| Age (new treatment), years, median (range) | 68 yr (38–89) | 64 yr (36–88) | 69 yr (63–74) | 70 yr (46–92) | 69 yr (45–94) |
| Elderly patients (new treatment), No. (%) | ≥75 yr, 123 (20.6%) | ≥70 yr, 178 (44.8%) | ≥70 yr, 257 (45.6%) | ≥75 yr, 170 (29.6%) | ≥75 yr, 133 (25.3%) |
| OS, months, median, New treatment/control | 53.3/36.5 | 57.6/47.2 | NR/NR | NR/NR | NR/52.2 |
| HR 0.66 (95%CI 0.56–0.78) | HR 0.72 (95%CI 0.59–0.89) | HR 0.70 (95%CI 0.58–0.84) | HR 0.66 (95%CI 0.53–0.81) | HR 0.66 (95% 0.53–0.79) | |
| PFS, months, median, New treatment/control | rPFS 33.1/14.7 | cPFS 33.0/19.8 | cPFS 81.0/25.0 | rPFS 49.8/38.9 | rPFS NR/22.1 |
| HR 0.46 (95%CI 0.39–0.54) | HR 0.62 (95%CI 0.51–0.75) | HR 0.45 (95%CI 0.39–0.53) | HR 0.63 (95%CI 0.52–0.76) | HR 0.48 (95% 0.39–0.60) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 25.8 (IQR: 12.3–49) | NA | NA | 40.2 (range 0.2–58.1) | 39.3 (range 0–55.7) |
| Control | 14.4 (IQR: 7.3–25.8) | NA | NA | 13.8 (range 0.2–27.6) | 20.2 (range 0.1–37.0) |
| . | Latitude . | Charrted . | Enzamet . | Arches . | Titan . |
|---|---|---|---|---|---|
| Author | Fizazi (2017, 2019) | Sweeney (2015) | Davis (2019) | Armstrong (2019) | Chi (2019, 2021) |
| Kyriakopoulos (2018) | Sweeney (2023) | Armstrong (2022) | |||
| New treatment | Abiraterone + ADT | Docetaxel + ADT | Enzalutamide + ADT | Enzalutamide + ADT | Apalutamide + ADT |
| Dosage | 1000 mg | 75 mg/m^2 | 160 mg | 160 mg | 240 mg |
| Control | Placebo + ADT | ADT | NSAA + ADT | Placebo + ADT | Placebo + ADT |
| Inclusion criteria | High-risk mHSPC | mHSPC | mHSPC | mHSPC | mHSPC |
| Gleason >8 | |||||
| Bone meta >3 | |||||
| Visceral meta | |||||
| N (total) | 1199 | 790 | 1125 | 1150 | 1152 |
| N (new treatment) | 597 | 397 | 563 | 574 | 525 |
| Age (new treatment), years, median (range) | 68 yr (38–89) | 64 yr (36–88) | 69 yr (63–74) | 70 yr (46–92) | 69 yr (45–94) |
| Elderly patients (new treatment), No. (%) | ≥75 yr, 123 (20.6%) | ≥70 yr, 178 (44.8%) | ≥70 yr, 257 (45.6%) | ≥75 yr, 170 (29.6%) | ≥75 yr, 133 (25.3%) |
| OS, months, median, New treatment/control | 53.3/36.5 | 57.6/47.2 | NR/NR | NR/NR | NR/52.2 |
| HR 0.66 (95%CI 0.56–0.78) | HR 0.72 (95%CI 0.59–0.89) | HR 0.70 (95%CI 0.58–0.84) | HR 0.66 (95%CI 0.53–0.81) | HR 0.66 (95% 0.53–0.79) | |
| PFS, months, median, New treatment/control | rPFS 33.1/14.7 | cPFS 33.0/19.8 | cPFS 81.0/25.0 | rPFS 49.8/38.9 | rPFS NR/22.1 |
| HR 0.46 (95%CI 0.39–0.54) | HR 0.62 (95%CI 0.51–0.75) | HR 0.45 (95%CI 0.39–0.53) | HR 0.63 (95%CI 0.52–0.76) | HR 0.48 (95% 0.39–0.60) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 25.8 (IQR: 12.3–49) | NA | NA | 40.2 (range 0.2–58.1) | 39.3 (range 0–55.7) |
| Control | 14.4 (IQR: 7.3–25.8) | NA | NA | 13.8 (range 0.2–27.6) | 20.2 (range 0.1–37.0) |
c, clinical; CI, confidence interval; meta, metastasis; HR, hazard ratio; NA, not available; NR, not reached; NSAA, non-steroidal antiandrogen; OS, overall survival; PC, prostate cancer; PFS, progression-free survival; r, radiographic.
Figure 2 and Table 3 show the treatments that were used for metastatic renal cancer stratified by age and cost per month: TKI alone, nivolumab + ipilimumab, pembrolizumab + axitinib, avelumab + axitinib, nivolumab + cabozantinib, pembrolizumab + lenvatinib were administered to 8.8, 19.3, 10.8, 9.3, 17.5 and 34.3% of patients. A higher proportion of patients >75 years of age were treated with TKIs alone than patients <75 years of age (13.5 vs. 6.9%). Moreover, patients ≥75 years of age were also more likely to receive avelumab + axitinib than those <75 (6.9 vs. 15.3%). Drug costs ranged from 623 243 to 1 616 042 JPY, all of which were high-cost treatments (>500 000 JPY). Four combination therapies were very expensive, costing >1 000 000 JPY/month, and the fifth did not strictly meet the definition of a very high-cost treatment, but was expensive, costing close to 1 000 000 JPY/month. According to the definition of this study, all patients received high-cost treatments as primary therapy for metastatic renal cancer. Furthermore, 80.4% of patients with metastatic renal cancer received very high-cost treatments, with breakdown of 81.3% of <75 years of age and 78.4% of ≥75 years of age. The details of drug costs for RCC are described in Supplementary Table 3.
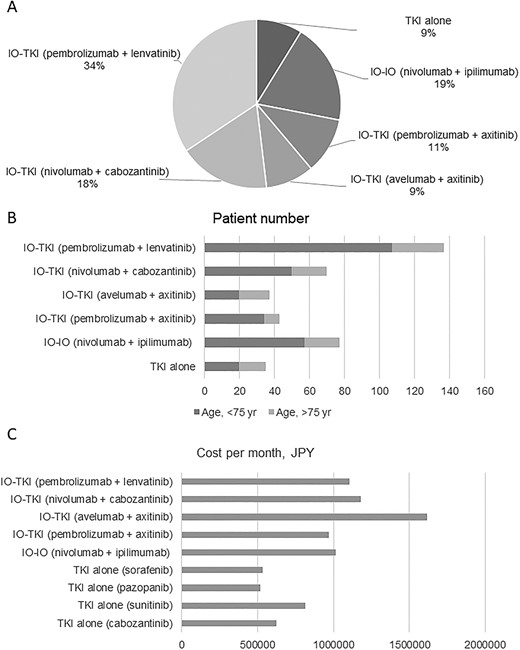
(A) Treatment selection for patients with untreated Stage IV renal cell carcinoma (RCC). (B) Treatment selection stratified by age for patients with untreated Stage IV RCC. TKI, tyrosine kinase inhibitor; IO, immune-oncology drug; JPY, Japanese yen.
Number of patients by type of treatment, breakdown of patients by age, and monthly drug costs
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | Cost per month, JPY . |
|---|---|---|---|---|
| TKI alone | 20 (6.9%) | 15 (13.5%) | 35 (8.8%) | 623 243 |
| IO–IO (nivolumab + ipilimumab) | 57 (19.8%) | 20 (18%) | 77 (19.3%) | 1 012 535 |
| IO–TKI (pembrolizumab + axitinib) | 34 (11.8%) | 9 (8.1%) | 43 (10.8%) | 971 919 |
| IO–TKI (avelumab + axitinib) | 20 (6.9%) | 17 (15.3%) | 37 (9.3%) | 1 616 042 |
| IO–TKI (nivolumab + cabozantinib) | 50 (17.4%) | 20 (18%) | 70 (17.5%) | 1 181 236 |
| IO–TKI (pembrolizumab + lenvatinib) | 107 (37.2%) | 30 (27%) | 137 (34.3%) | 1 104 981 |
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | Cost per month, JPY . |
|---|---|---|---|---|
| TKI alone | 20 (6.9%) | 15 (13.5%) | 35 (8.8%) | 623 243 |
| IO–IO (nivolumab + ipilimumab) | 57 (19.8%) | 20 (18%) | 77 (19.3%) | 1 012 535 |
| IO–TKI (pembrolizumab + axitinib) | 34 (11.8%) | 9 (8.1%) | 43 (10.8%) | 971 919 |
| IO–TKI (avelumab + axitinib) | 20 (6.9%) | 17 (15.3%) | 37 (9.3%) | 1 616 042 |
| IO–TKI (nivolumab + cabozantinib) | 50 (17.4%) | 20 (18%) | 70 (17.5%) | 1 181 236 |
| IO–TKI (pembrolizumab + lenvatinib) | 107 (37.2%) | 30 (27%) | 137 (34.3%) | 1 104 981 |
TKI, tyrosine kinase inhibitor; IO, immune-oncology drug; JPY, Japanese yen
Number of patients by type of treatment, breakdown of patients by age, and monthly drug costs
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | Cost per month, JPY . |
|---|---|---|---|---|
| TKI alone | 20 (6.9%) | 15 (13.5%) | 35 (8.8%) | 623 243 |
| IO–IO (nivolumab + ipilimumab) | 57 (19.8%) | 20 (18%) | 77 (19.3%) | 1 012 535 |
| IO–TKI (pembrolizumab + axitinib) | 34 (11.8%) | 9 (8.1%) | 43 (10.8%) | 971 919 |
| IO–TKI (avelumab + axitinib) | 20 (6.9%) | 17 (15.3%) | 37 (9.3%) | 1 616 042 |
| IO–TKI (nivolumab + cabozantinib) | 50 (17.4%) | 20 (18%) | 70 (17.5%) | 1 181 236 |
| IO–TKI (pembrolizumab + lenvatinib) | 107 (37.2%) | 30 (27%) | 137 (34.3%) | 1 104 981 |
| Treatment . | Age, <75 yr . | Age, ≥75 yr . | Total . | Cost per month, JPY . |
|---|---|---|---|---|
| TKI alone | 20 (6.9%) | 15 (13.5%) | 35 (8.8%) | 623 243 |
| IO–IO (nivolumab + ipilimumab) | 57 (19.8%) | 20 (18%) | 77 (19.3%) | 1 012 535 |
| IO–TKI (pembrolizumab + axitinib) | 34 (11.8%) | 9 (8.1%) | 43 (10.8%) | 971 919 |
| IO–TKI (avelumab + axitinib) | 20 (6.9%) | 17 (15.3%) | 37 (9.3%) | 1 616 042 |
| IO–TKI (nivolumab + cabozantinib) | 50 (17.4%) | 20 (18%) | 70 (17.5%) | 1 181 236 |
| IO–TKI (pembrolizumab + lenvatinib) | 107 (37.2%) | 30 (27%) | 137 (34.3%) | 1 104 981 |
TKI, tyrosine kinase inhibitor; IO, immune-oncology drug; JPY, Japanese yen
Table 4 lists the results of clinical trials of metastatic RCC treatments. In all trials, the control was sunitinib alone. Four trials included ~40% of patients aged ≥65 years. In the nivolumab + ipilimumab trial, only 8.2% of the patients were aged ≥75 years. The duration of TKI alone, nivolumab + ipilimumab, pembrolizumab + axitinib, avelumab + axitinib and nivolumab + cabozantinib and pembrolizumab + lenvatinib treatments were reported as 7.3–11, 7.9, 10.4, 8.6–9.0, 14.3 and 17 months of treatment, respectively (Table 4). The pembrolizumab + lenvatinib regimen had the longest median treatment duration, and the overall total treatment cost was calculated to be 18 784 677 JPY. The lack of head-to-head trials has made it difficult for clinicians to select the first-line treatment for patients with metastatic RCC.
Results of pivotal trials investigating combination therapies for metastatic renal cell carcinoma
| . | CheckMate 214 . | JAVELIN Renal 101 . | KEYNOTE-426 . | CheckMate 9ER . | CLEAR . |
|---|---|---|---|---|---|
| Author | Motzer (2018, 2019) | Motzer (2019) | Rini (2019) | Choueiri (2021) | Motzer (2021) |
| Albiges (2020) | Choueiri (2020) | Powles (2020) | Motzer (2022) | Choueiri (2023) | |
| New treatment | Ipilimumab +Nivolumab | Avelumab + Axitinib | Pembrolizumab +Axitinib | Nivolumab + Cabozantinib | Pembrolizumab +Lenvatinib |
| Dosage | Nivolumab (3 mg per kilogram), Ipilimumab (1 mg per kilogram) | Avelumab (a dose of 10 mg per kilogram of body weight), axitinib (5 mg twice daily) | Pembrolizumab was (a dose of 200 mg once every 3 weeks), Axitinib (a dose of 5 mg twice daily) | Nivolumab (a dose of 240 mg), cabozantinib (a dose of 40 mg once daily) | Lenvatinib (a dose of 20 mg orally once daily), pembrolizumab (a dose of 200 mg) |
| Control | Sunitinib | Sunitinib | Sunitinib | Sunitinib | Sunitinib |
| Inclusion criteria | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component |
| IMDC risk | Intermediate/poor | All risk | All risk | All risk | All risk |
| N (total) | 847 | 886 | 861 | 651 | 1069 |
| N (new treatment) | 425 | 442 | 432 | 323 | 355 |
| Age (treatment), years, median (range) | 62 yr (26–85) | 62 yr (29–83) | 62 yr (30–89) | 62 yr (29–90) | 64 yr (34–88) |
| Elderly patients (treatment), No. (%) | ≥75 yr, 35 (8.2%) | ≥65 yr, 171 (38.7%) | ≥65 yr, 172 (39.8%) | ≥65 yr, 132 (40.9%) | ≥65 yr, 161 (45.4%) |
| OS, months, median New treatment/control | 48.1/26.6 | NR/NR | NR/35.7 | 37.7/34.3 | NR/NR |
| HR 0.65, 95%CI (0.54–0.78) | 0.80, 95% CI (0.62–1.03 | HR 0.68, 95%CI (0.55–0.85) | HR 0.70, 95%CI (0.55–0.90) | HR 0.72 95%CI (0.55–0.93) | |
| PFS, months, median New treatment/control | 11.2/8.3 | 13.3/8.0 | 15.4/11.1 | 16.6/8.3 | 23.3/9.2 |
| HR 0.74 (95%CI 0.62–0.88) | HR 0.69 (95% CI 0.57–0.83) | HR 0.71 (95%CI 0.60–0.84) | HR 0.56 (95%CI 0.46–0.68) | HR 0.42 (95%CI 0.34–0.52) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 7.9 (2.1–21.8) | 8.6 (0.5–25.3) for avelumab, 9.0 (0.02–24.9) for axitinib | 10.4 (0.03–21.2) | 14.3 (0.2–27.3) | 17.0 (0.1–39.1) |
| Control | 7.8 (3.5–19.6) | 7.3 (0.2–23) | 7.8 (0.07–20.5) | 9.2 (0.8–27.6) | 11.0 (0.1–40.0) |
| . | CheckMate 214 . | JAVELIN Renal 101 . | KEYNOTE-426 . | CheckMate 9ER . | CLEAR . |
|---|---|---|---|---|---|
| Author | Motzer (2018, 2019) | Motzer (2019) | Rini (2019) | Choueiri (2021) | Motzer (2021) |
| Albiges (2020) | Choueiri (2020) | Powles (2020) | Motzer (2022) | Choueiri (2023) | |
| New treatment | Ipilimumab +Nivolumab | Avelumab + Axitinib | Pembrolizumab +Axitinib | Nivolumab + Cabozantinib | Pembrolizumab +Lenvatinib |
| Dosage | Nivolumab (3 mg per kilogram), Ipilimumab (1 mg per kilogram) | Avelumab (a dose of 10 mg per kilogram of body weight), axitinib (5 mg twice daily) | Pembrolizumab was (a dose of 200 mg once every 3 weeks), Axitinib (a dose of 5 mg twice daily) | Nivolumab (a dose of 240 mg), cabozantinib (a dose of 40 mg once daily) | Lenvatinib (a dose of 20 mg orally once daily), pembrolizumab (a dose of 200 mg) |
| Control | Sunitinib | Sunitinib | Sunitinib | Sunitinib | Sunitinib |
| Inclusion criteria | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component |
| IMDC risk | Intermediate/poor | All risk | All risk | All risk | All risk |
| N (total) | 847 | 886 | 861 | 651 | 1069 |
| N (new treatment) | 425 | 442 | 432 | 323 | 355 |
| Age (treatment), years, median (range) | 62 yr (26–85) | 62 yr (29–83) | 62 yr (30–89) | 62 yr (29–90) | 64 yr (34–88) |
| Elderly patients (treatment), No. (%) | ≥75 yr, 35 (8.2%) | ≥65 yr, 171 (38.7%) | ≥65 yr, 172 (39.8%) | ≥65 yr, 132 (40.9%) | ≥65 yr, 161 (45.4%) |
| OS, months, median New treatment/control | 48.1/26.6 | NR/NR | NR/35.7 | 37.7/34.3 | NR/NR |
| HR 0.65, 95%CI (0.54–0.78) | 0.80, 95% CI (0.62–1.03 | HR 0.68, 95%CI (0.55–0.85) | HR 0.70, 95%CI (0.55–0.90) | HR 0.72 95%CI (0.55–0.93) | |
| PFS, months, median New treatment/control | 11.2/8.3 | 13.3/8.0 | 15.4/11.1 | 16.6/8.3 | 23.3/9.2 |
| HR 0.74 (95%CI 0.62–0.88) | HR 0.69 (95% CI 0.57–0.83) | HR 0.71 (95%CI 0.60–0.84) | HR 0.56 (95%CI 0.46–0.68) | HR 0.42 (95%CI 0.34–0.52) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 7.9 (2.1–21.8) | 8.6 (0.5–25.3) for avelumab, 9.0 (0.02–24.9) for axitinib | 10.4 (0.03–21.2) | 14.3 (0.2–27.3) | 17.0 (0.1–39.1) |
| Control | 7.8 (3.5–19.6) | 7.3 (0.2–23) | 7.8 (0.07–20.5) | 9.2 (0.8–27.6) | 11.0 (0.1–40.0) |
Results of pivotal trials investigating combination therapies for metastatic renal cell carcinoma
| . | CheckMate 214 . | JAVELIN Renal 101 . | KEYNOTE-426 . | CheckMate 9ER . | CLEAR . |
|---|---|---|---|---|---|
| Author | Motzer (2018, 2019) | Motzer (2019) | Rini (2019) | Choueiri (2021) | Motzer (2021) |
| Albiges (2020) | Choueiri (2020) | Powles (2020) | Motzer (2022) | Choueiri (2023) | |
| New treatment | Ipilimumab +Nivolumab | Avelumab + Axitinib | Pembrolizumab +Axitinib | Nivolumab + Cabozantinib | Pembrolizumab +Lenvatinib |
| Dosage | Nivolumab (3 mg per kilogram), Ipilimumab (1 mg per kilogram) | Avelumab (a dose of 10 mg per kilogram of body weight), axitinib (5 mg twice daily) | Pembrolizumab was (a dose of 200 mg once every 3 weeks), Axitinib (a dose of 5 mg twice daily) | Nivolumab (a dose of 240 mg), cabozantinib (a dose of 40 mg once daily) | Lenvatinib (a dose of 20 mg orally once daily), pembrolizumab (a dose of 200 mg) |
| Control | Sunitinib | Sunitinib | Sunitinib | Sunitinib | Sunitinib |
| Inclusion criteria | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component |
| IMDC risk | Intermediate/poor | All risk | All risk | All risk | All risk |
| N (total) | 847 | 886 | 861 | 651 | 1069 |
| N (new treatment) | 425 | 442 | 432 | 323 | 355 |
| Age (treatment), years, median (range) | 62 yr (26–85) | 62 yr (29–83) | 62 yr (30–89) | 62 yr (29–90) | 64 yr (34–88) |
| Elderly patients (treatment), No. (%) | ≥75 yr, 35 (8.2%) | ≥65 yr, 171 (38.7%) | ≥65 yr, 172 (39.8%) | ≥65 yr, 132 (40.9%) | ≥65 yr, 161 (45.4%) |
| OS, months, median New treatment/control | 48.1/26.6 | NR/NR | NR/35.7 | 37.7/34.3 | NR/NR |
| HR 0.65, 95%CI (0.54–0.78) | 0.80, 95% CI (0.62–1.03 | HR 0.68, 95%CI (0.55–0.85) | HR 0.70, 95%CI (0.55–0.90) | HR 0.72 95%CI (0.55–0.93) | |
| PFS, months, median New treatment/control | 11.2/8.3 | 13.3/8.0 | 15.4/11.1 | 16.6/8.3 | 23.3/9.2 |
| HR 0.74 (95%CI 0.62–0.88) | HR 0.69 (95% CI 0.57–0.83) | HR 0.71 (95%CI 0.60–0.84) | HR 0.56 (95%CI 0.46–0.68) | HR 0.42 (95%CI 0.34–0.52) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 7.9 (2.1–21.8) | 8.6 (0.5–25.3) for avelumab, 9.0 (0.02–24.9) for axitinib | 10.4 (0.03–21.2) | 14.3 (0.2–27.3) | 17.0 (0.1–39.1) |
| Control | 7.8 (3.5–19.6) | 7.3 (0.2–23) | 7.8 (0.07–20.5) | 9.2 (0.8–27.6) | 11.0 (0.1–40.0) |
| . | CheckMate 214 . | JAVELIN Renal 101 . | KEYNOTE-426 . | CheckMate 9ER . | CLEAR . |
|---|---|---|---|---|---|
| Author | Motzer (2018, 2019) | Motzer (2019) | Rini (2019) | Choueiri (2021) | Motzer (2021) |
| Albiges (2020) | Choueiri (2020) | Powles (2020) | Motzer (2022) | Choueiri (2023) | |
| New treatment | Ipilimumab +Nivolumab | Avelumab + Axitinib | Pembrolizumab +Axitinib | Nivolumab + Cabozantinib | Pembrolizumab +Lenvatinib |
| Dosage | Nivolumab (3 mg per kilogram), Ipilimumab (1 mg per kilogram) | Avelumab (a dose of 10 mg per kilogram of body weight), axitinib (5 mg twice daily) | Pembrolizumab was (a dose of 200 mg once every 3 weeks), Axitinib (a dose of 5 mg twice daily) | Nivolumab (a dose of 240 mg), cabozantinib (a dose of 40 mg once daily) | Lenvatinib (a dose of 20 mg orally once daily), pembrolizumab (a dose of 200 mg) |
| Control | Sunitinib | Sunitinib | Sunitinib | Sunitinib | Sunitinib |
| Inclusion criteria | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component | Untreated advanced renal-cell carcinoma with a clear-cell component |
| IMDC risk | Intermediate/poor | All risk | All risk | All risk | All risk |
| N (total) | 847 | 886 | 861 | 651 | 1069 |
| N (new treatment) | 425 | 442 | 432 | 323 | 355 |
| Age (treatment), years, median (range) | 62 yr (26–85) | 62 yr (29–83) | 62 yr (30–89) | 62 yr (29–90) | 64 yr (34–88) |
| Elderly patients (treatment), No. (%) | ≥75 yr, 35 (8.2%) | ≥65 yr, 171 (38.7%) | ≥65 yr, 172 (39.8%) | ≥65 yr, 132 (40.9%) | ≥65 yr, 161 (45.4%) |
| OS, months, median New treatment/control | 48.1/26.6 | NR/NR | NR/35.7 | 37.7/34.3 | NR/NR |
| HR 0.65, 95%CI (0.54–0.78) | 0.80, 95% CI (0.62–1.03 | HR 0.68, 95%CI (0.55–0.85) | HR 0.70, 95%CI (0.55–0.90) | HR 0.72 95%CI (0.55–0.93) | |
| PFS, months, median New treatment/control | 11.2/8.3 | 13.3/8.0 | 15.4/11.1 | 16.6/8.3 | 23.3/9.2 |
| HR 0.74 (95%CI 0.62–0.88) | HR 0.69 (95% CI 0.57–0.83) | HR 0.71 (95%CI 0.60–0.84) | HR 0.56 (95%CI 0.46–0.68) | HR 0.42 (95%CI 0.34–0.52) | |
| Treatment duration, month, median (range) | |||||
| New treatment | 7.9 (2.1–21.8) | 8.6 (0.5–25.3) for avelumab, 9.0 (0.02–24.9) for axitinib | 10.4 (0.03–21.2) | 14.3 (0.2–27.3) | 17.0 (0.1–39.1) |
| Control | 7.8 (3.5–19.6) | 7.3 (0.2–23) | 7.8 (0.07–20.5) | 9.2 (0.8–27.6) | 11.0 (0.1–40.0) |
Discussion
Until 2015, ADT alone was the common treatment for mCSPC. However, in recent years, novel hormone therapy and chemotherapy drugs have been developed based on ADT, and clinical trials have shown their efficacy and safety. The 2023 Japan Urological Association guidelines also weakly recommend the use of ADT + ARSI as a primary hormone therapy for mCSPC (32).
In the current survey, ADT + ARSI was introduced in 56% of patients in Japan. Three ADT + ARSI regimens are available, but the number of patients treated with ADT + apalutamide, which has been on the insurance list for only a short time, was less than that of the other two regimens. The low percentage of older patients administered ADT + apalutamide may be due to inexperience with administration of this combination therapy. By contrast, 34% of patients receive ADT + ARSI in the USA and Europe (33,34). This indicates that the use of ARSI is more widespread in Japan than in the West possibly due to Japan’s universal health insurance system, which makes the drugs available to many patients even if they are expensive. Although there was no treatment among the initial drug therapies for mCSPC in which the monthly cost exceeded 500 000 JPY, the total cost of ADT + ARSI is likely to be notably higher than other options because of the long treatment period involved.
Patients ≥75 years used ADT + antiandrogen more frequently than patients <75 (47.4 vs. 32.8%), whereas ADT + ARSI was used less frequently (49.8 vs. 62.2%). This suggests that clinicians balance efficacy and safety when choosing systemic treatment for mCSPC, considering the patient’s age and medical condition. Of note, prospective data are limited due to the lack of enrollment of patients aged ≥75 years in pivotal clinical trials. However, retrospective real-world data indicate that caution is needed regarding adverse events specific to older adults, such as falls, but appropriately adjusted doses are well tolerated and provide oncologic benefits similar to those observed in younger adults (35–37). Among the three ARSIs, ADT + Abiraterone is ~1.5-fold compared with the other two ARSIs. However, no cost-effectiveness analysis has been performed to evaluate the relative merits of these three ARSIs.
The treatment of metastatic renal cancer has reached a turning point with the development of novel therapies combining immune checkpoint inhibitors and TKIs, resulting in increased therapeutic response rates and survival. The Society for Immunotherapy of Cancer guidelines state that all patients without contraindications to immunotherapy should receive a first-line IO-based regimen (38). Due to the lack of a cost-effectiveness analysis, clinicians need to choose the best IO-based regimen based on evidence from clinical trial data, pathological findings, patient compliance, personal belief and regulatory approval.
In this survey, TKI (only), IO–IO and IO–TKI regimens were administered to 9, 19 and 72% of the patients, respectively. By contrast, a report based on a US database showed that between 2019 and 2022, TKI monotherapy decreased from 33.8 to 8.4%, while IO–IO increased from 52.8 to 57.7% and IO–TKI also increased from 13.3 to 33.9% (39,40). This indicates that the use of immune-combination therapy is more widespread in Japan than in the West due to drug availability, even for RCC drug therapy.
Notably, avelumab + axitinib therapy has the highest cost, >1.5 million JPY per month, while the costs of the other four immune-combination therapies are ~1 million JPY per month. All of the immune-combination therapies are expensive, but there are differences in cost among them; the relative differences between the therapies are small, but the absolute differences are appreciable. The most expensive drug among the TKI monotherapy options was sunitinib, but the monthly drug costs for both TKI monotherapy options exceeded 500 000 JPY. Because conventional TKI therapy is also expensive, the cost ratio of the new therapy compared with conventional therapy for RCC is not as obvious as that of prostate cancer, but the overall cost is larger (41).
While avelumab + axitinib was prescribed the least frequently, it was prescribed the most frequently to the elderly among first-line combined immunotherapies. This may be partly due to the drug characteristics (avelumab is a PDL1 antibody) and safety profile. This combination has the lowest incidence of immune-related adverse events, hence, the increased probability of prescribing it to older adults (20). The most frequently prescribed combined immunotherapy was pembrolizumab + lenvatinib, but the frequency of prescriptions for older adults was low. This is due to the higher incidence of adverse events in prospective studies compared with other immunocomplex therapies (42), resulting in physicians being cautious about administering the therapy.
There are clinical trials that have focused on reducing the cost of expensive drugs. One of them is the low-dose abiraterone trial in which the dose of abiraterone can be reduced by taking it after a meal with non-inferior effect to the standard dose (43,44). Although no Phase III trials have been conducted, the NCCN guidelines suggest taking a quarter of the usual dose of abiraterone with a low-fat diet as an alternative when circumstances preclude taking the typical dose (45,46). The other clinical trial is related to IO-based treatment. Considering the long-lasting effect of IO-based treatment, discontinuation of IO-based therapy may help reduce side effects and the financial burden of taking these drugs. There are two ongoing prospective trials that aim to confirm the non-inferiority of discontinuation versus continuation of IO-based treatment (47,48).
This study had limitations. First, the survey was conducted over a short period of 1 year at limited JCOG participating centers and did not capture the actual treatment duration. Second, the cost of each treatment was calculated based on 1 year of treatment at the usual dose, and thus, does not consider cases in which the dose was reduced or discontinued in actual practice. However, to the best of our knowledge, this is the first report of a survey of first-line drug regimens for patients with untreated Stage IV prostate cancer and RCC patients in Japan stratified by age and treatment costs. The survey results can be used to plan future health economic studies that examine cost-effectiveness and promote the efficient use of limited resources to ensure better patient outcomes.
Conclusion
This study reports on prescription preferences and respective drug costs in Japan based on a questionnaire survey of first-line drug therapy for untreated Stage IV prostate cancer and RCC. Most Japanese patients with urologic cancers receive state-of-the-art, effective treatments, but the costs of these treatments are very high and rapidly increasing.
Funding
This work was supported by the Research Fund of National Federation of Health Insurance Societies and in part by the National Cancer Center Research and Development Funds (2023-J-03).
Conflict of interest statement
Takahiro Osawa has received honoraria from Ono, MSD and Takeda. Hiroshi Kitamura has received honoraria from Astellas, AstraZeneca, Bristol-Myers Squibb, Janssen, MSD and Sanof and has received research expenses from AstraZeneca, Bristol-Myers Squibb and MSD. Hiroyuki Nishiyama received honoraria from Astellas, AstraZeneca, Bristol-Myers Squibb, Ono, MSD and Merck, donations for education and research from Bayer and consultant fees from Janssen, MSD, One and AstraZeneca. The other authors have no conflicts of interest.
Author contributions
T.O., K.S. and R.M. designed the research. T.O., K.S. and R.M. collected the data. T.O. analyzed the data. T.M., Y.M., H.K., and H.N supervised the project. T.O. wrote the paper. All authors read and approved the final manuscript.


