-
PDF
- Split View
-
Views
-
Cite
Cite
Junji Furuse, Makoto Ueno, Masafumi Ikeda, Takuji Okusaka, Zhaoyang Teng, Momoko Furuya, Tatsuya Ioka, Liposomal irinotecan with fluorouracil and leucovorin after gemcitabine-based therapy in Japanese patients with metastatic pancreatic cancer: additional safety analysis of a randomized phase 2 trial, Japanese Journal of Clinical Oncology, Volume 53, Issue 2, February 2023, Pages 130–137, https://doi.org/10.1093/jjco/hyac177
Close - Share Icon Share
Abstract
Nanoliposomal irinotecan (nal-IRI) was recently authorized in Japan for unresectable pancreatic cancer after disease progression following chemotherapy. Physicians now consider certain aspects of nal-IRI safety profile as slightly different from conventional irinotecan. This report aims to explore additional aspects of the nal-IRI safety in Japanese phase 2 study.
We analyzed the incidence, time to first onset, and time to resolution for adverse events that require special attention and other selected toxicities in the nal-IRI combination group (n = 46).
Leukopenia/neutropenia (76.1%/71.7%), diarrhea (58.7%) and hepatic dysfunction (41.3%) were the most commonly reported treatment-emergent adverse events, with a median time to onset of 21.0 days (range: 8, 97), 9.0 days (1, 61) and 22.0 days (2, 325), respectively, and a median time to resolution of 8.0 days (95% confidence intervals: 8, 9), 4.0 days (4, 8) and 40.0 days (9, –), respectively. Eight patients experienced Grade ≥ 3 diarrhea and their symptoms were well controlled by dose modification except one patient who had drug withdrawal. The median time to resolution for Grade ≥ 3 and Grade ≤ 2 diarrhea was 17.5 days (95% confidence intervals: 1, 31) and 4 days (3, 7), respectively. Anorexia occurred in 28/46 patients (60.9%) with a median time to onset of 4.0 days (range: 2, 132) and a median time to resolution of 12.0 days (95% confidence intervals: 6, 26).
We explored safety profile of nal-IRI combination regimen recognized as effective and tolerable treatment for Japanese unresectable pancreatic cancer patients. Although the treatment-emergent adverse events occurred were controllable, patients with prolonged toxicities should be closely managed.
Introduction
Pancreatic cancer is the eighth most common cancer and the fourth leading cause of cancer-related deaths in Japan. In 2018, 37 000 deaths were reported among the 43 000 newly diagnosed cases of pancreatic cancer (1). The incidence rate of this disease among adults aged >50 years in Japan is the highest globally, and the crude mortality rates for patients with pancreatic cancer are also the highest in Japan compared with selected Asian and Western countries (2). Importantly, only ~20% of all patients have resectable disease at diagnosis, which explains the poor prognosis of this cancer.
Several studies have shown that combination regimens comprising gemcitabine (GEM) plus nab-paclitaxel (nab-PTX) or bolus and continuous infusion of 5-fluorouracil (5-FU), leucovorin (LV), oxaliplatin and irinotecan (FOLFIRINOX) provide a clear survival benefit over GEM monotherapy in the first-line treatment of metastatic pancreatic cancer (3–8). GEM plus nab-PTX is more commonly used than FOLFIRINOX for first-line chemotherapy in many countries, including Japan, which is consistent with the guidelines defined by the National Comprehensive Cancer Networks (NCCN), the European Society of Medical Oncology (ESMO) and the Japanese Clinical Practice Guidelines for Pancreatic Cancer (9–11).
Patients with metastatic pancreatic cancer (mPAC) whose disease progresses after chemotherapy are candidates for second-line chemotherapy. However, there had not been any recommended second-line treatment until an international, multicenter, phase 3 NAPOLI-1 study (NCT01494506) demonstrated the survival benefit of liposomal irinotecan (nal-IRI) plus 5-fluorouracil and leucovorin (5-FU/LV) over 5-FU/LV. Since then, the nal-IRI regimen has been widely approved as an effective second-line treatment, and is now recommended in clinical practice guidelines (12,13). Nal-IRI plus fluorouracil/calcium levofolinate hydrate (5-FU/l-LV) combination was approved by the Japanese regulatory agency, PMDA (pharmaceuticals and medical devices agency), in March 2020 for the treatment of mPAC patients with prior systemic chemotherapy based on the findings of the NAPOLI-1 study (14) and the local phase 2 study (331501 study, NCT02697058) (15). When referring to global clinical trial data for Japanese patients, differences among ethnic groups are considered a concern because the pharmacokinetic profiles of anticancer agents are different between Asian and non-Asian patients. A previous report (16) showed that Asian patients presented a significantly higher mean maximum plasma concentration (Cmax) of unencapsulated SN-38 and a lower Cmax of total irinotecan after nal-IRI therapy compared with Caucasian patients, which was associated with increased Grade 3 or 4 neutropenia and decreased Grade 3 or 4 diarrhea in Asian patients. The Japanese authority also concluded that Japanese patients treated with the nal-IRI combination tended to have a higher incidence of hematological adverse events, such as neutropenia and leukopenia compared with non-Japanese patients. Although there is not enough evidence to understand the safety profile specific to nal-IRI in Japanese patients, all common adverse events observed in the study were known adverse events of conventional irinotecan. As there was no tendency for a higher incidence of fatal and serious adverse events in Japanese patients compared with non-Japanese patients, nal-IRI combination therapy was accepted as tolerable. The Japanese Pancreas Society also recommends this combination therapy in patients with mPAC as a second-line treatment (17).
Several adverse events are specified as important identified or potential risks of nal-IRI that require attention in the Risk Management Plan (RMP), a document that aims to assess risk management and pharmacovigilance activities to minimize the risk of this drug. Potential risks in the RMP include drug-related adverse events commonly noted during clinical trials and other adverse events known to conventional irinotecan. Considering that nal-IRI is a novel formulation of irinotecan encapsulated in circulating liposome-based nanoparticles, the safety profiles of both nal-IRI and conventional irinotecan were initially believed to be similar. However, these drugs may exhibit different characteristics since their drug delivery system and PK profile are not the same. In fact, some Japanese physicians believe that certain aspects of the nal-IRI safety profile are slightly different from the conventional irinotecan. The purpose of this report was to further explore the safety of nal-IRI in patients included in the Japanese phase II study, better understand the safety profile in Japanese patients and gain greater insight regarding the management of adverse events in clinical practice.
Patients and methods
Study design
331501 study was a prospective, open-label, randomized, multicenter phase 2 study that evaluated tolerability, safety and efficacy of nal-IRI + 5-FU/l-LV in Japanese patients with mPAC who progressed after gemcitabine-based therapy (Clinicaltrials.gov, identifier: NCT02697058) (15). This study was approved by the institutional review board or ethics committee of each participating institution, and it was conducted per the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent for participation was obtained from all patients. The details of the 331501 study protocol have been described in a previous report (15). Briefly, 101 patients aged ≥ 20 years with mPAC that had progressed or recurred following gemcitabine-based therapy were assessed for eligibility in 16 participating Japanese centers between 30 March 2016 and 31 January 2018. The patients randomized to the nal-IRI + 5-FU/l-LV arm received intravenous (IV) infusions of 80 mg/m2 nal-IRI (irinotecan hydrochloride trihydrate salt) followed by 200 mg/m2l-LV and 2400 mg/m2 5-FU every 2 weeks. The patients in the 5-FU/l-LV arm received IV infusions of 200 mg/m2l-LV followed by 2400 mg/m2 5-FU every 2 weeks.
All adverse events and laboratory results were coded to the preferred term and system-organ class using the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1, and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Treatment-emergent adverse events (TEAEs) were defined as adverse events reported from the time of the first administration of the study drug to 30 days after administration of the last dose. All TEAEs requiring dose reductions were considered treatment-related.
Patients or their caregivers also maintained a paper diary to record pain intensity and analgesic consumption throughout the study. Untoward events recorded in the diary were reported as adverse events according to the investigator’s discretion and clinical judgment.
Prophylactic treatments
All patients had initially received combinations of dexamethasone with other antiemetic premedication, such as 5-HT3 receptor antagonists. The antiemetic treatments were selected according the institutional standard for 5-FU administration, and the reasons for drug usage were recorded by the investigators (Supplementary Table 1). Also, prophylactic administration of atropine was allowed in patients who experienced cholinergic symptoms in the previous cycles.
Dose modification protocol for adverse events
In the 331501 study, dosing could be held for up to 3 weeks from when it was due to allow for recovery from treatment-related toxicity. If the time required for recovery from toxicity exceeded 3 weeks, the patient was discontinued from the study unless a clear therapeutic benefit was noted. In such cases, the investigator and the sponsor evaluated the related risks and benefits and subsequently decided whether the patient should continue this therapy or not. Dose reduction was not required for Grade 2 TEAEs. If a dose was reduced during the treatment owing to toxic effects, it was to remain reduced for the entire duration of the study. Dose re-escalation to an earlier dose was not permitted. Any patient who had two dose reductions and also experienced an adverse event that would require a third dose reduction had to be discontinued from the study treatment.
Post-hoc analyses
For this additional safety analysis, the safety population of the nal-IRI + 5-FU/l-LV arm included all patients who received ≥1 dose of nal-IRI, and were analyzed for the incidence, time to first onset, and time to resolution of TEAEs associated with 12 identified risks in the RMP (defined as ‘TEAEs of special interest’). Furthermore, this analysis included adverse events that tend to have a significant impact on the patients’ quality of life, such as anorexia, nausea, vomiting, malaise, fatigue, alopecia and peripheral (sensory) neuropathy (defined as selected TEAEs). The definitions of the 12 TEAEs of special interest are described in Table 1. Time to onset of TEAEs of special interest and time to onset of selected TEAEs were summarized descriptively, including median, min and max. The median (95% confidence intervals, CI) for time to resolution of TEAEs of special interest and time to resolution of selected TEAEs were calculated using the Kaplan–Meier method. The time to onset was calculated as the number of days from the first investigational product exposure to the first AE occurrence date including an additional day. For resolved events of all applicable cases, the time to resolution was calculated as a difference of end of AE date and the time to onset. For non-resolved AEs (censored 30 days after last dose) it was calculated as a difference between date of censor and time to onset.
| AE term . | PT lists and SMQ used . |
|---|---|
| Neutropenia | Neutropenia (Agranulocytosis, Band neutrophil count decreased, Band neutrophil percentage decreased, Cyclic neutropenia, Febrile neutropenia, Full blood count abnormal¸ Granulocyte count decreased, Granulocytes maturation arrest, Granulocytopenia, Idiopathic neutropenia, Metamyelocyte count decreased, Myeloblast count decreased, Myeloblast percentage decreased, Myelocyte count decreased, Myelocyte percentage decreased, Myeloid maturation arrest, Neutropenia, Neutropenia neonatal, Neutropenic infection, Neutropenic sepsis, Neutrophil count abnormal, Neutrophil count decreased, Neutrophil percentage decreased, Pancytopenia, Promyelocyte count decreased) |
| Leukopenia | Hematopoietic leukopenia (SMQ) |
| Thrombocytopenia | Hematopoietic thrombocytopenia (SMQ) |
| Anemia | Hematopoietic erythrogenic (SMQ) |
| Diarrhea | Non-infectious diarrhoea (SMQ) |
| Infection | Infection and Infestation, excluding Septic shock: Endotoxic shock, Septic shock, Toxic shock syndrome, Toxic shock syndrome staphylococcal and Toxic shock syndrome streptococcal |
| Hepatic dysfunction | Drug related hepatic disorders—comprehensive search (SMQ) |
| Infusion reactions | Hypersensitivity MedDRA SMQ (narrow) |
| Thromboembolism | Embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ) |
| Enteritis | Gastrointestinal non-specific inflammation (SMQ) |
| Intestinal obstruction | Gastrointestinal obstruction (SMQ) |
| Gastrointestinal hemorrhage | Gastrointestinal Hemorrhage |
| Disseminated intravascular Coagulation | Disseminated intravascular coagulation |
| Interstitial lung disease | Interstitial lung disease (SMQ) |
| Acute kidney injury | Acute renal failure (SMQ) |
| Myocardial infarction and angina pectoris | Myocardial Infarction and Other ischemic heart disease (SMQ) |
| Ventricular extrasystoles | Ventricular tachyarrhythmias SMQ |
| AE term . | PT lists and SMQ used . |
|---|---|
| Neutropenia | Neutropenia (Agranulocytosis, Band neutrophil count decreased, Band neutrophil percentage decreased, Cyclic neutropenia, Febrile neutropenia, Full blood count abnormal¸ Granulocyte count decreased, Granulocytes maturation arrest, Granulocytopenia, Idiopathic neutropenia, Metamyelocyte count decreased, Myeloblast count decreased, Myeloblast percentage decreased, Myelocyte count decreased, Myelocyte percentage decreased, Myeloid maturation arrest, Neutropenia, Neutropenia neonatal, Neutropenic infection, Neutropenic sepsis, Neutrophil count abnormal, Neutrophil count decreased, Neutrophil percentage decreased, Pancytopenia, Promyelocyte count decreased) |
| Leukopenia | Hematopoietic leukopenia (SMQ) |
| Thrombocytopenia | Hematopoietic thrombocytopenia (SMQ) |
| Anemia | Hematopoietic erythrogenic (SMQ) |
| Diarrhea | Non-infectious diarrhoea (SMQ) |
| Infection | Infection and Infestation, excluding Septic shock: Endotoxic shock, Septic shock, Toxic shock syndrome, Toxic shock syndrome staphylococcal and Toxic shock syndrome streptococcal |
| Hepatic dysfunction | Drug related hepatic disorders—comprehensive search (SMQ) |
| Infusion reactions | Hypersensitivity MedDRA SMQ (narrow) |
| Thromboembolism | Embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ) |
| Enteritis | Gastrointestinal non-specific inflammation (SMQ) |
| Intestinal obstruction | Gastrointestinal obstruction (SMQ) |
| Gastrointestinal hemorrhage | Gastrointestinal Hemorrhage |
| Disseminated intravascular Coagulation | Disseminated intravascular coagulation |
| Interstitial lung disease | Interstitial lung disease (SMQ) |
| Acute kidney injury | Acute renal failure (SMQ) |
| Myocardial infarction and angina pectoris | Myocardial Infarction and Other ischemic heart disease (SMQ) |
| Ventricular extrasystoles | Ventricular tachyarrhythmias SMQ |
MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SMQ, standardized MedDRA queries.
| AE term . | PT lists and SMQ used . |
|---|---|
| Neutropenia | Neutropenia (Agranulocytosis, Band neutrophil count decreased, Band neutrophil percentage decreased, Cyclic neutropenia, Febrile neutropenia, Full blood count abnormal¸ Granulocyte count decreased, Granulocytes maturation arrest, Granulocytopenia, Idiopathic neutropenia, Metamyelocyte count decreased, Myeloblast count decreased, Myeloblast percentage decreased, Myelocyte count decreased, Myelocyte percentage decreased, Myeloid maturation arrest, Neutropenia, Neutropenia neonatal, Neutropenic infection, Neutropenic sepsis, Neutrophil count abnormal, Neutrophil count decreased, Neutrophil percentage decreased, Pancytopenia, Promyelocyte count decreased) |
| Leukopenia | Hematopoietic leukopenia (SMQ) |
| Thrombocytopenia | Hematopoietic thrombocytopenia (SMQ) |
| Anemia | Hematopoietic erythrogenic (SMQ) |
| Diarrhea | Non-infectious diarrhoea (SMQ) |
| Infection | Infection and Infestation, excluding Septic shock: Endotoxic shock, Septic shock, Toxic shock syndrome, Toxic shock syndrome staphylococcal and Toxic shock syndrome streptococcal |
| Hepatic dysfunction | Drug related hepatic disorders—comprehensive search (SMQ) |
| Infusion reactions | Hypersensitivity MedDRA SMQ (narrow) |
| Thromboembolism | Embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ) |
| Enteritis | Gastrointestinal non-specific inflammation (SMQ) |
| Intestinal obstruction | Gastrointestinal obstruction (SMQ) |
| Gastrointestinal hemorrhage | Gastrointestinal Hemorrhage |
| Disseminated intravascular Coagulation | Disseminated intravascular coagulation |
| Interstitial lung disease | Interstitial lung disease (SMQ) |
| Acute kidney injury | Acute renal failure (SMQ) |
| Myocardial infarction and angina pectoris | Myocardial Infarction and Other ischemic heart disease (SMQ) |
| Ventricular extrasystoles | Ventricular tachyarrhythmias SMQ |
| AE term . | PT lists and SMQ used . |
|---|---|
| Neutropenia | Neutropenia (Agranulocytosis, Band neutrophil count decreased, Band neutrophil percentage decreased, Cyclic neutropenia, Febrile neutropenia, Full blood count abnormal¸ Granulocyte count decreased, Granulocytes maturation arrest, Granulocytopenia, Idiopathic neutropenia, Metamyelocyte count decreased, Myeloblast count decreased, Myeloblast percentage decreased, Myelocyte count decreased, Myelocyte percentage decreased, Myeloid maturation arrest, Neutropenia, Neutropenia neonatal, Neutropenic infection, Neutropenic sepsis, Neutrophil count abnormal, Neutrophil count decreased, Neutrophil percentage decreased, Pancytopenia, Promyelocyte count decreased) |
| Leukopenia | Hematopoietic leukopenia (SMQ) |
| Thrombocytopenia | Hematopoietic thrombocytopenia (SMQ) |
| Anemia | Hematopoietic erythrogenic (SMQ) |
| Diarrhea | Non-infectious diarrhoea (SMQ) |
| Infection | Infection and Infestation, excluding Septic shock: Endotoxic shock, Septic shock, Toxic shock syndrome, Toxic shock syndrome staphylococcal and Toxic shock syndrome streptococcal |
| Hepatic dysfunction | Drug related hepatic disorders—comprehensive search (SMQ) |
| Infusion reactions | Hypersensitivity MedDRA SMQ (narrow) |
| Thromboembolism | Embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ) |
| Enteritis | Gastrointestinal non-specific inflammation (SMQ) |
| Intestinal obstruction | Gastrointestinal obstruction (SMQ) |
| Gastrointestinal hemorrhage | Gastrointestinal Hemorrhage |
| Disseminated intravascular Coagulation | Disseminated intravascular coagulation |
| Interstitial lung disease | Interstitial lung disease (SMQ) |
| Acute kidney injury | Acute renal failure (SMQ) |
| Myocardial infarction and angina pectoris | Myocardial Infarction and Other ischemic heart disease (SMQ) |
| Ventricular extrasystoles | Ventricular tachyarrhythmias SMQ |
MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SMQ, standardized MedDRA queries.
Results
The most common (≥20%) TEAEs associated with the 12 identified risks in RMP (TEAEs of special interest) that were observed in the nal-IRI + 5-FU/l-LV arm (n = 46) were, leukopenia (76.1%), neutropenia (71.7%), diarrhea (58.7%), hepatic dysfunction (41.3%), infection (23.9%) and anemia (21.7%; Table 2).
| Grouped term (n = 46) . | All grade TEAE n (%) . | TEAE with Grade 3 or 4 n (%) . | Treatment related TEAE n (%) . | TEAE leading to dose reduction n (%) . | TEAE leading to dose interruption n (%) . | TEAE leading to dose discontinuation n (%) . |
|---|---|---|---|---|---|---|
| Leukopenia | 35 (76.1) | 21 (45.7) | 35 (76.1) | 15 (32.6) | 26 (56.5) | 2 (4.3) |
| Neutropenia | 33 (71.7) | 21 (45.7) | 33 (71.7) | 15 (32.6) | 24 (52.2) | 2 (4.3) |
| Anemia | 10 (21.7) | 3 (6.5) | 9 (19.6) | 2 (4.3) | 0 (0) | 1 (2.2) |
| Thrombocytopenia | 4 (8.7) | 0 (0) | 4 (8.7) | 0 (0) | 1 (2.2) | 0 (0) |
| Diarrhea | 27 (58.7) | 8(17.4) | 26 (56.5) | 8 (17.4) | 5 (10.9) | 1 (2.2) |
| Hepatic dysfunction | 19 (41.3) | 10 (21.7) | 9 (19.6) | 1 (2.2) | 4 (8.7) | 0 (0) |
| Infection | 11 (23.9) | 3 (6.5) | 5 (10.9) | 0 (0) | 2 (4.3) | 1 (2.2) |
| Infusion reaction | 5 (10.9) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Acute kidney injury | 3 (6.5) | 0 (0) | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) |
| Thromboembolism | 2 (4.3) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 1 (2.2) |
| Intestinal obstruction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enteritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal hemorrhage | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Disseminated intravascular coagulation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Interstitial lung disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myocardial infarction and angina Pectoris | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Ventricular extrasystoles | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grouped term (n = 46) . | All grade TEAE n (%) . | TEAE with Grade 3 or 4 n (%) . | Treatment related TEAE n (%) . | TEAE leading to dose reduction n (%) . | TEAE leading to dose interruption n (%) . | TEAE leading to dose discontinuation n (%) . |
|---|---|---|---|---|---|---|
| Leukopenia | 35 (76.1) | 21 (45.7) | 35 (76.1) | 15 (32.6) | 26 (56.5) | 2 (4.3) |
| Neutropenia | 33 (71.7) | 21 (45.7) | 33 (71.7) | 15 (32.6) | 24 (52.2) | 2 (4.3) |
| Anemia | 10 (21.7) | 3 (6.5) | 9 (19.6) | 2 (4.3) | 0 (0) | 1 (2.2) |
| Thrombocytopenia | 4 (8.7) | 0 (0) | 4 (8.7) | 0 (0) | 1 (2.2) | 0 (0) |
| Diarrhea | 27 (58.7) | 8(17.4) | 26 (56.5) | 8 (17.4) | 5 (10.9) | 1 (2.2) |
| Hepatic dysfunction | 19 (41.3) | 10 (21.7) | 9 (19.6) | 1 (2.2) | 4 (8.7) | 0 (0) |
| Infection | 11 (23.9) | 3 (6.5) | 5 (10.9) | 0 (0) | 2 (4.3) | 1 (2.2) |
| Infusion reaction | 5 (10.9) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Acute kidney injury | 3 (6.5) | 0 (0) | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) |
| Thromboembolism | 2 (4.3) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 1 (2.2) |
| Intestinal obstruction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enteritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal hemorrhage | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Disseminated intravascular coagulation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Interstitial lung disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myocardial infarction and angina Pectoris | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Ventricular extrasystoles | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Percentages are based on n (safety analysis set); Adverse events were coded using MedDRA 18.1
GT, grouped term; TEAEs, treatment-emergent adverse events; nal-IRI + 5-FU/l-LV, Liposomal irinotectan with 5- fluorouracil and leucovorin.
| Grouped term (n = 46) . | All grade TEAE n (%) . | TEAE with Grade 3 or 4 n (%) . | Treatment related TEAE n (%) . | TEAE leading to dose reduction n (%) . | TEAE leading to dose interruption n (%) . | TEAE leading to dose discontinuation n (%) . |
|---|---|---|---|---|---|---|
| Leukopenia | 35 (76.1) | 21 (45.7) | 35 (76.1) | 15 (32.6) | 26 (56.5) | 2 (4.3) |
| Neutropenia | 33 (71.7) | 21 (45.7) | 33 (71.7) | 15 (32.6) | 24 (52.2) | 2 (4.3) |
| Anemia | 10 (21.7) | 3 (6.5) | 9 (19.6) | 2 (4.3) | 0 (0) | 1 (2.2) |
| Thrombocytopenia | 4 (8.7) | 0 (0) | 4 (8.7) | 0 (0) | 1 (2.2) | 0 (0) |
| Diarrhea | 27 (58.7) | 8(17.4) | 26 (56.5) | 8 (17.4) | 5 (10.9) | 1 (2.2) |
| Hepatic dysfunction | 19 (41.3) | 10 (21.7) | 9 (19.6) | 1 (2.2) | 4 (8.7) | 0 (0) |
| Infection | 11 (23.9) | 3 (6.5) | 5 (10.9) | 0 (0) | 2 (4.3) | 1 (2.2) |
| Infusion reaction | 5 (10.9) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Acute kidney injury | 3 (6.5) | 0 (0) | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) |
| Thromboembolism | 2 (4.3) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 1 (2.2) |
| Intestinal obstruction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enteritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal hemorrhage | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Disseminated intravascular coagulation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Interstitial lung disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myocardial infarction and angina Pectoris | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Ventricular extrasystoles | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grouped term (n = 46) . | All grade TEAE n (%) . | TEAE with Grade 3 or 4 n (%) . | Treatment related TEAE n (%) . | TEAE leading to dose reduction n (%) . | TEAE leading to dose interruption n (%) . | TEAE leading to dose discontinuation n (%) . |
|---|---|---|---|---|---|---|
| Leukopenia | 35 (76.1) | 21 (45.7) | 35 (76.1) | 15 (32.6) | 26 (56.5) | 2 (4.3) |
| Neutropenia | 33 (71.7) | 21 (45.7) | 33 (71.7) | 15 (32.6) | 24 (52.2) | 2 (4.3) |
| Anemia | 10 (21.7) | 3 (6.5) | 9 (19.6) | 2 (4.3) | 0 (0) | 1 (2.2) |
| Thrombocytopenia | 4 (8.7) | 0 (0) | 4 (8.7) | 0 (0) | 1 (2.2) | 0 (0) |
| Diarrhea | 27 (58.7) | 8(17.4) | 26 (56.5) | 8 (17.4) | 5 (10.9) | 1 (2.2) |
| Hepatic dysfunction | 19 (41.3) | 10 (21.7) | 9 (19.6) | 1 (2.2) | 4 (8.7) | 0 (0) |
| Infection | 11 (23.9) | 3 (6.5) | 5 (10.9) | 0 (0) | 2 (4.3) | 1 (2.2) |
| Infusion reaction | 5 (10.9) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Acute kidney injury | 3 (6.5) | 0 (0) | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) |
| Thromboembolism | 2 (4.3) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 1 (2.2) |
| Intestinal obstruction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enteritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal hemorrhage | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Disseminated intravascular coagulation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Interstitial lung disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myocardial infarction and angina Pectoris | 1 (2.2) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) | 0 (0) |
| Ventricular extrasystoles | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Percentages are based on n (safety analysis set); Adverse events were coded using MedDRA 18.1
GT, grouped term; TEAEs, treatment-emergent adverse events; nal-IRI + 5-FU/l-LV, Liposomal irinotectan with 5- fluorouracil and leucovorin.
Intestinal obstruction, enteritis, disseminated intravascular coagulation, interstitial lung disease and ventricular extrasystoles were not observed, although these are known adverse events for conventional irinotecan (11).
For the Grade ≥3 TEAEs of special interest, leukopenia (45.7%), neutropenia (45.7%) and hepatic dysfunction (21.7%) were most commonly reported (Table 2). Four patients received G-CSF for the treatment of neutropenia (n = 2), febrile neutropenia (n = 1) or neutrophil count decreased (n = 1), and none of them had Grade ≥ 3 neutropenia recurrence afterwards (data not shown). As previously reported, a total of four deaths were reported in the nal-IRI + 5-FU/l-LV arm, and none of them was treatment-related.
With respect to the TEAEs that usually have a significant impact on the patients’ quality of life (selected TEAEs), nausea (78.3%) and anorexia (60.9%) were the most frequently observed. All of the selected TEAEs were not severe and rarely required dose modification (Table 3).
| Preferred term (n = 46) . | All grade TEAE . | Grade 3 or 4 TEAE . | Treatment-related TEAE . | TEAE leading to dose reduction . | TEAE leading to dose interruption . | TEAE leading to dose discontinuation . |
|---|---|---|---|---|---|---|
| Anorexia (decreased appetite) | 28 (60.9) | 0 (0) | 28 (60.9) | 1 (2.2) | 1 (2.2) | |
| Nausea | 36 (78.3) | 1 (2.2) | 36 (78.3) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 12 (26.1) | 0 (0) | 11 (23.9) | 0 (0) | 0 (0) | 0 (0) |
| Malaise | 13 (28.3) | 0 (0) | 12 (26.1) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 11 (23.9) | 1 (2.2) | 11 (23.9) | 1 (2.2) | 0 (0) | 0 (0) |
| Alopecia | 3 (6.5) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral sensory neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Preferred term (n = 46) . | All grade TEAE . | Grade 3 or 4 TEAE . | Treatment-related TEAE . | TEAE leading to dose reduction . | TEAE leading to dose interruption . | TEAE leading to dose discontinuation . |
|---|---|---|---|---|---|---|
| Anorexia (decreased appetite) | 28 (60.9) | 0 (0) | 28 (60.9) | 1 (2.2) | 1 (2.2) | |
| Nausea | 36 (78.3) | 1 (2.2) | 36 (78.3) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 12 (26.1) | 0 (0) | 11 (23.9) | 0 (0) | 0 (0) | 0 (0) |
| Malaise | 13 (28.3) | 0 (0) | 12 (26.1) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 11 (23.9) | 1 (2.2) | 11 (23.9) | 1 (2.2) | 0 (0) | 0 (0) |
| Alopecia | 3 (6.5) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral sensory neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
Percentages are based on n (safety analysis set); adverse events were coded using MedDRA 18.1.
| Preferred term (n = 46) . | All grade TEAE . | Grade 3 or 4 TEAE . | Treatment-related TEAE . | TEAE leading to dose reduction . | TEAE leading to dose interruption . | TEAE leading to dose discontinuation . |
|---|---|---|---|---|---|---|
| Anorexia (decreased appetite) | 28 (60.9) | 0 (0) | 28 (60.9) | 1 (2.2) | 1 (2.2) | |
| Nausea | 36 (78.3) | 1 (2.2) | 36 (78.3) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 12 (26.1) | 0 (0) | 11 (23.9) | 0 (0) | 0 (0) | 0 (0) |
| Malaise | 13 (28.3) | 0 (0) | 12 (26.1) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 11 (23.9) | 1 (2.2) | 11 (23.9) | 1 (2.2) | 0 (0) | 0 (0) |
| Alopecia | 3 (6.5) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral sensory neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Preferred term (n = 46) . | All grade TEAE . | Grade 3 or 4 TEAE . | Treatment-related TEAE . | TEAE leading to dose reduction . | TEAE leading to dose interruption . | TEAE leading to dose discontinuation . |
|---|---|---|---|---|---|---|
| Anorexia (decreased appetite) | 28 (60.9) | 0 (0) | 28 (60.9) | 1 (2.2) | 1 (2.2) | |
| Nausea | 36 (78.3) | 1 (2.2) | 36 (78.3) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 12 (26.1) | 0 (0) | 11 (23.9) | 0 (0) | 0 (0) | 0 (0) |
| Malaise | 13 (28.3) | 0 (0) | 12 (26.1) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 11 (23.9) | 1 (2.2) | 11 (23.9) | 1 (2.2) | 0 (0) | 0 (0) |
| Alopecia | 3 (6.5) | 0 (0) | 3 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral sensory neuropathy | 1 (2.2) | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
Percentages are based on n (safety analysis set); adverse events were coded using MedDRA 18.1.
Dose modification and discontinuation for TEAEs of special interest
Leukopenia (32.6%) and neutropenia (32.6%) were the most common reasons for dose reduction (Table 2). Of the eight patients who had Grade ≥3 diarrhea, two patients with Grade 4 diarrhea had a recurrence of Grade 1 diarrhea after dose reduction and one patient had recurring Grade 3 diarrhea within a few weeks after dose reduction for the first Grade 3 diarrhea and the second episode led to treatment discontinuation. The remaining five patients did not have recurrence of Grade 3 or higher diarrhea after dose modification (data not shown). All patients with Grade ≥3 diarrhea received an appropriate intervention. The medications used are listed in the Supplementary Table 2.
In addition, leukopenia (56.5%) and neutropenia (52.2%) were the most common reasons for dose interruption. Of note, some patients with Grade ≤2 leukopenia and neutropenia had dose interruption at the investigator’s discretion, although the protocol did not require dose modification for Grade ≤2 adverse events.
As for the selected TEAEs, one patient with Grade ≥3 nausea did not have any dose modification. In contrast, two patients with Grade 2 anorexia required dose reduction or dose interruption. Furthermore, one patient with Grade 3 fatigue had dose reduction (Table 3).
Time to onset
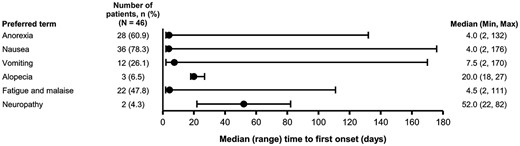
In most cases, the first occurrences of hematological toxicities, diarrhea and hepatic dysfunction occurred during the first 3 weeks from starting the nal-IRI combination treatment, as opposed to 106.0 days for infusion-related reactions (Fig. 1). With respect to selected TEAEs, the median time to first onset for anorexia, nausea, vomiting, fatigue and malaise was within 1 week, as opposed to 20.0 days for alopecia and 52.0 days for neuropathy (Fig. 2).
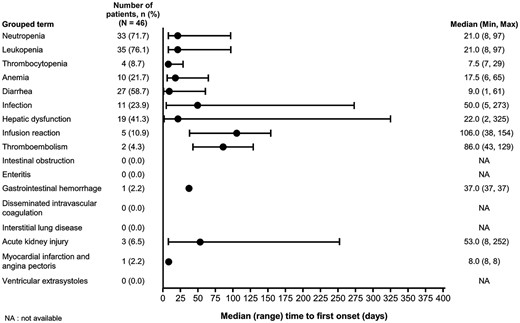
Time to onset of TEAEs of special interest (all grades). NA, data not available; TEAEs, treatment emergent adverse events.
Time to resolution
Of the most commonly reported TEAEs of special interest, the median time to resolution for neutropenia, leukopenia and diarrhea was around 1 week whereas it was 40.0 days for hepatic dysfunction, 13 days for infection and 16 days for anemia (Fig. 3). When analyzed by grade, the median time to resolution of Grade ≥3 diarrhea was 17.5 days (95% CI: 1, 31) compared with Grades 1 and 2 diarrhea, which was 4.0 days (95% CI: 3, 7; Supplementary Table 3).
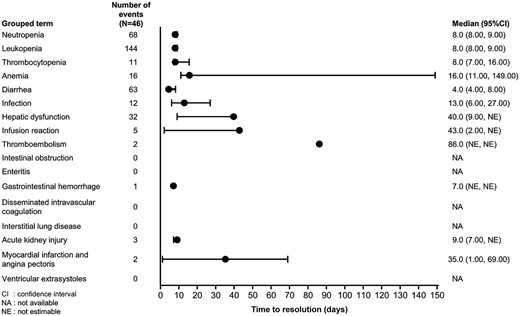
Time to resolution of TEAEs of special interest (all grades). CI, confidence intervals; NA, not available; NE, not estimable.
As for the time to resolution of selected TEAEs, the median time to resolution for anorexia, nausea, vomiting, fatigue and malaise was within 2 weeks (Fig. 4), and 24.0 days for neuropathy. There were no data available for alopecia because all three patients did not recover.
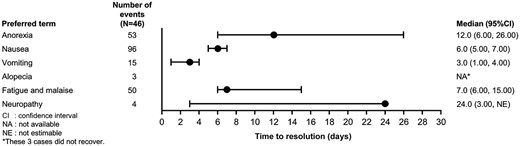
Overall, anemia, anorexia and Grade ≥3 diarrhea showed extended time to resolution in some patients compared with other toxicities.
Discussion
Our study is the first post-hoc safety analysis of Japanese mPAC patients treated with nal-IRI + 5FU/l-LV that reports on the time-to-onset and time-to-resolution for TEAEs of special interest associated with the 12 risks in RMP and selected TEAEs that can negatively affect patients’ quality of life in general. The Japanese phase 2 study (331501) had demonstrated that nal-IRI + 5-FU/l-LV was a safe and tolerable treatment with no new, unique or unexpected safety signals identified among the Japanese mPAC patients. Briefly, the most commonly observed Grade 3 or 4 TEAEs in the nal-IRI + 5-FU/l-LV arm of 331501 study were decreased neutrophil count (37%), decreased white blood cell count (20%) and diarrhea (17%). TEAEs leading to dose modifications, including dose reductions or delays, occurred in 76% of patients treated with nal-IRI + 5-FU/l-LV, with decreased white blood cell count (46%), decreased neutrophil count (44%) and diarrhea (11%) being the most common (15,18).
The safety profile of nal-IRI + 5-FU/l-LV observed in this study was consistent with the global phase 3 study (NAPOLI-1), suggesting that the toxicities were well-controlled by the protocol specified AE management strategies, including prophylaxis and dose modifications. The results of the NAPOLI-1 post-hoc analyses showed that an early (within 6 weeks) dose reduction or dose delay in the nal-IRI + 5-FU/LV group did not significantly impact OS or PFS compared with patients who did not require early dose modifications (19). This post-hoc analysis proposes that the implementation of a strategy that focuses on early dose reduction or delay for managing toxicities associated with nal-IRI combination therapy may not adversely affect clinical outcomes. Therefore, nal-IRI combination therapy should be managed by recommended dose modifications and other AE management strategies (19–21).
Adverse events of nal-IRI are usually managed in accordance with conventional irinotecan. However, it is not fully understood whether any differences in safety profiles exist between these two drugs. After 1 year of real-world experience with nal-IRI combination treatment, a few concerns have been raised that some of the safety profiles of nal-IRI are slightly different from conventional irinotecan. In our experience, some patients appear to have longer time to resolution than conventional irinotecan of some TEAEs, such as anorexia. Our results also show that anorexia and Grade ≥ 3 diarrhea tend to have a prolonged time to resolution with nal-IRI treatment in some patients. These prolonged adverse events can be due to the unique PK profile of nal-IRI due to the use of liposomal technology. A unique PK profile has been reported compared with conventional irinotecan, such that the therapeutic plasma concentrations of active metabolites SN-38 following nal-IRI administration are persistent for over 80 h. In contrast, SN-38 of conventional irinotecan in the plasma is rapidly cleared from the circulation within 24 h from the injection (18). However, the characteristics of patients who experienced prolonged GI-toxicities remain unknown. Further studies are needed to explore other GI toxicities that have a prolonged resolution time due to the PK profile of nal-IRI. In this study, prolonged GI-toxicities were well controlled with recommended dose modifications and usual AE management strategies. However, careful follow-up is essential in these patients because prolonged GI toxicities can deteriorate their mental and physical conditions.
Although the number of patients analyzed in this report was small, our analysis obtained valuable information for the median days to first onset and time to resolution of important TEAEs specific to nal-IRI combination treatment. A similar analysis was conducted previously by Gayle et al. in patients receiving nal-IRI + 5-FU/LV in the NAPOLI-1 study. This analysis revealed that the first occurrences of severe neutropenia, diarrhea, nausea and vomiting were observed within the first 6 weeks of treatment (22). To note, the results of our analysis showed that the median time to resolution of infusion-related reactions was 106 days. This can be explained by the fact that infusion-related reactions assessed in this study included hypersensitivity and delayed allergic reactions.
In the 331501 study, a higher incidence of Grade ≥3 neutropenia and a decreased white blood cell count were reported in patients receiving nal-IRI + 5-FU/l-LV with UGT1A1 polymorphism. Consequently, initial dose reduction is necessary for these patients when starting treatment with nal-IRI. There is a clinical question on whether patients with either UGT1A1*28 or UGT1A1*6 heterozygous alleles need initial dose reduction to prevent Grade ≥3 neutropenia, decreased white blood cell count or diarrhea. There is no nal-IRI specific data to answer this question since these patients started with normal dose in the clinical trials and no safety analysis on this population has been conducted.
Several other clinical questions remain unanswered with respect to the practical use of nal-IRI combination therapy in Japanese mPAC patients, such as treating patients with renal or hepatic dysfunction, elderly patients and patients with ECOG PS ≥2. Usually, patients with underlying hepatic or renal disease and/or poor condition (ECOG PS ≥2) are excluded from clinical trials, including this study (15). As for elderly patients, the median age of the nal-IRI + 5FU/l-LV group in the 331501 study was 67 years (range: 39, 83). The evidence is still too scarce to prove that this combination treatment is tolerable for elderly and frail patients. Accumulation of reports considering the safety of nal-IRI combination treatment in these populations is awaited.
Limitations of the current analysis include its post-hoc nature and the small number of patients. The analysis focused on each TEAE parameter at once, therefore, it is difficult to grasp complex AE management as a whole. This analysis could not further explore the safety profile and efficacy of patients who had dose modifications. Although future research is necessary to answer the clinical questions listed above, this analysis can provide useful information to healthcare professionals regarding the effective support of mPAC patients receiving nal-IRI combination treatment and the management of potential adverse events.
Conclusions
In this analysis, we explored details of the safety profile specific to the nal-IRI plus 5-FU/l-LV regimen, which is recognized as an effective, safe and tolerable treatment for Japanese mPAC patients who progressed after systemic chemotherapy. Although the TEAEs occurring with nal-IRI + 5-FU/l-LV were well-controlled by AE management strategies per protocol, some adverse events with prolonged toxicities, such as diarrhea and anorexia, should be carefully managed in clinical practice.
Acknowledgements
The authors would like to thank the patients, their families, all investigators and support staff who participated in this study. The authors would also like to thank Ritu Guglani, MD, and Raghuraj Puthige, PhD, from Enago Life Sciences Pvt. Ltd. India for medical writing and editorial assistance.
Funding
Study 331501 (ClinicalTrials.gov Identifier: NCT02697058) was sponsored by Servier (Suresnes, France). This analysis was sponsored by Servier; rights for nal-IRI now reside with Ipsen in the USA (April 2017); PharmaEngine, Inc. holds the rights in Taiwan; Servier holds rights in the rest of the world.
Conflicts of interest
Momoko Furuya is employee of Nihon Servier, Tokyo, Japan. Zhaoyang Teng is employee of Sevier Pharmaceuticals Boston, USA. Takuji Okusaka is member of Data Safety Monitoring Board for AstraZeneca PLC, Incyte Japan, Ono Pharmaceutical, Pfizer Japan Inc., and reports grants from research funding from AstraZeneca PLC, Bristol-Myers Squibb Company, MSD, K.K., Syneos JF, reports consulting fee from Meiji Seika Pharma, Takara Bio Inc., and reports honoraria from MSD, K.K, AstraZeneca PLC, Incyte Japan, Eisai, Ono Pharmaceutical Co, Ltd, Teijin Pharma Ltd, Taiho Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd. Junji Furuse is member of Data Safety Monitoring for Onco Therapy Science, Taiho Pharmaceutical, reports received funding for this study from Servier Japan, reports grants from research funding from Ono Pharmaceutical Co, MSD, Merck Bio, J-Pharma, Taiho Pharmaceutical, Takeda, Chugai Pharmaceutical Co, Astra Zeneca PLC, Yakult Honsha, Eisai, Daiichi Sankyo, Mochida, Sanofi, Sumitomo Dainippon Bayer, Astellas, Incyte Japan. reports consulting fee from Fuji film, Mudi Pharma, Onco Therapy Science, Merck Bio, Ono Pharmaceutical Co, MSD, Taiho Pharmaceutical, Chugai Pharmaceutical Co, Astellas, Astra Zeneca PLC, Takara bio, Delta-Fly-Pharma, Incyte Japan and reports honoraria from Ono Pharmaceutical Co, Bayer, Eisai, Eli Lilly Japan, MSD, Yakult Honsha, Chugai Pharma, Novartis Pharma, Astra Zeneca, Pfizer, Takeda, Taiho Pharmaceutical, Sanofi, Mylan EPD, EA Pharma, Kyowa Hakko Kirin, Daiichi Sankyo, Teijin pharma, Servier Japan, Incyte Japan. Masafumi Ikeda is member of Data Safety Monitoring for Nihon Sevier, reports grant from research funding from Ono Pharmaceutical Co, Bristol Myers Squibb, Yakult, Delta-Fly Pharma, Nihon Sevier, Novartis and reports honoraria from Ono Pharmaceutical Co, Yakult, Nihon Sevier, MSD, Taiho. The authors report no other conflicts of interest in this work.
Authors’ contributions
JF, MU, MI, TO, AM and TI contributed to study design. ZT planned and performed data analysis. JF provided consultation on developing outline and writing the manuscript. MF devised the project, the main conceptual ideas, took the lead in proof outline and writing the manuscript with input from all authors. All authors critically reviewed and commented on drafts of the manuscript and approved the final report.


