-
PDF
- Split View
-
Views
-
Cite
Cite
Daisuke Kawauchi, Makoto Ohno, Yasuji Miyakita, Masamichi Takahashi, Shunsuke Yanagisawa, Takaki Omura, Akihiko Yoshida, Yuko Kubo, Hiroshi Igaki, Koichi Ichimura, Yoshitaka Narita, Consulting a neurosurgeon upon initial medical assessment reduces the time to the first surgery and potentially contributes to improved prognosis for glioblastoma patients, Japanese Journal of Clinical Oncology, Volume 53, Issue 11, November 2023, Pages 1027–1033, https://doi.org/10.1093/jjco/hyad093
Close - Share Icon Share
Abstract
The neurological status of glioblastoma patients rapidly deteriorates. We recently demonstrated that early diagnosis and surgery within 3 weeks from the initial symptoms are associated with improved survival. While glioblastoma is a semi-urgent disease, the prehospital behaviors and clinical outcomes of glioblastoma patients are poorly understood. We aimed to disclose how prehospital patient behavior influences the clinical outcomes of glioblastoma patients.
Isocitrate dehydrogenase-wildtype glioblastoma patients treated at our institution between January 2010 and December 2019 were reviewed. Patients were divided into two groups, neurosurgeon and non-neurosurgeon groups, based on the primary doctor whom patients sought for an initial evaluation. Patient demographics and prognoses were examined.
Of 170 patients, 109 and 61 were classified into the neurosurgeon and non-neurosurgeon groups, respectively. The median age of neurosurgeon group was significantly younger than the non-neurosurgeon group (61 vs. 69 years old, P = 0.019) and in better performance status (preoperative Karnofsky performance status scores |$\ge$|80: 72.5 vs. 55.7%, P = 0.027). The neurosurgeon group exhibited a significantly shorter duration from the first hospital visit to the first surgery than the non-neurosurgeon group (18 vs. 29 days, P < 0.0001). Furthermore, the overall survival of the neurosurgeon group was significantly more prolonged than that of the non-neurosurgeon group (22.9 vs. 14.0 months, P = 0.038).
Seeking an initial evaluation by a neurosurgeon was potentially associated with prolonged survival in glioblastoma patients. A short duration from the first hospital visit to the first surgery is essential in enhancing glioblastoma patient prognosis.
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor among adults. The malignancy of GBM is attributed mainly to its aggressively fast-growing nature. Its expected volume doubling time of 49.6 days (1) is shorter than that of other cancers, such as small-cell lung cancer (86.3 days) (2), breast cancer (103–127 days for triple-negative) (3,4), cutaneous melanoma (144 days) (5) or colon cancer (146.5–398.5 days) (6). Because a larger preoperative tumor volume was associated with poor preoperative Karnofsky performance status (KPS) score and worse clinical outcome (7,8), diagnosis and treatment timing largely influence patient prognosis. We recently reported that early diagnosis and surgical intervention within 3 weeks from initial symptoms are associated with prolonged survival of patients with GBM (9). We also demonstrated that patients with asymptomatic, incidentally discovered GBM exhibited longer survival (10). These results clearly emphasized that early diagnosis and surgical intervention play a vital role in enhancing GBM patient prognosis.
GBM is characterized by rapid neurological deterioration in a short period. Half of the patients have a KPS 70 or less before the first surgery (11). To facilitate early diagnosis and surgical intervention, we need to understand the presurgical patient behavior and discover a major bottleneck that harnesses early GBM treatment. However, prehospital and presurgical patient behavior and clinical outcomes are poorly understood. This study aimed to disclose how prehospital patient behavior influences the clinical outcomes in GBM patients and propose potential actions to further improve clinical outcomes in GBM patients.
Patients and methods
Patient and tumor characteristics
This study was a single-center retrospective analysis of a consecutive series of patients with isocitrate dehydrogenase (IDH)-wildtype GBM. We identified adult GBM patients (20 years old or older) that were newly diagnosed and treated at our institution between January 2010 and December 2019. This study included patients who received their first surgery in other hospitals and were treated with adjuvant therapy at our hospital. We reviewed patient data including age, sex, clinical history, presurgical physical assessment, radiological images, surgical reports and postsurgical clinical courses. Patients were classified into two groups: the neurosurgeon group and the non-neurosurgeon group, based on the primary doctor whom patients sought for the first evaluation following the onset of the initial symptoms. Histological diagnosis of GBM was certified based on the World Health Organization (WHO) classification 2007/2016 of tumors of the central nervous system. Furthermore, we followed the WHO classification 2021 and included only IDH-wildtype GBM cases (12). We defined overall survival (OS) as the interval between initial surgery and death. Patients with unknown survival were censored at the last follow-up date.
Tumor volume was calculated by multiplying the maximum diameter of the tumor along the axial, coronal and sagittal planes. The measurements were obtained from the initial MRI scan used for the clinical diagnosis of the brain tumor. In most cases, GBM tumors appeared as gadolinium-enhanced lesions on T1-weighted images. However, there were some tumors that initially presented as small FLAIR-hyperintense lesions. For these cases, patients underwent frequent monitoring through follow-up MRI scans. Once tumor growth or the presence of an enhanced lesion was confirmed, the tumor was considered a semi-emergency and appropriate treatment was initiated.
Molecular profiles of the tumors, including IDH, O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status, telomerase reverse transcriptase (TERT) promoter, serine/threonine-protein kinase B-raf (BRAF), and H3 histone, family 3A (H3F3A), were analyzed as previously described (13–15). MGMT promoter status was considered methylated when its mean level at the 16 CpG regions was greater than 16% (14,16). We determined the extent of resection based on the surgeon’s operative records and postoperative imaging studies, classified as either total (100%) resection, subtotal (95–99%) resection, partial (<94%) resection, or biopsy. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Statistical analysis
To compare continuous variables such as age, durations from initial symptom onset or first hospital visit to various clinical events, and tumor volumes, we utilized the Mann–Whitney U test. The chi-squared test was employed to assess the distribution of non-continuous variables, including gender, initial symptoms, preoperative KPS, tumor locations, molecular characteristics of tumors and the extent of tumor resection, between the two groups. OS was calculated using the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazards regression model was used to identify significant predictors of more prolonged survival. Statistical analyses and figure production were performed using GraphPad Prism 10 (GraphPad Software, Inc., La Jolla, California, USA). Statistical significance was defined as a P value of <0.05.
Characteristics and clinical results of neurosurgeon and non-neurosurgeon groups
| . | Neurosurgeon group N = 109 . | Non-neurosurgeon group N = 61 . | P value . |
|---|---|---|---|
| Male: Female | 70: 39 (64.2%) | 33: 28 (54.1%) | 0.20 |
| Age (years) | 61 (29–85) | 69 (27–87) | 0.019 |
| Duration from initial symptom onset to | |||
| First hospital visit (days) | 7 (0–461) | 9 (0–195) | 0.67 |
| Duration from the first hospital visit to | |||
| Radiological diagnosis (days) | 0 (0–339) | 1 (0–94) | <0.0001 |
| First surgery (days) | 18 (1–2646) | 29 (5–749) | <0.0001 |
| Initial symptoms | |||
| Headache | 23 (21.1%) | 12 (19.7%) | 0.83 |
| Paralysis | 21 (19.3%) | 16 (26.2%) | 0.29 |
| Seizure | 17 (15.6%) | 3 (4.9%) | 0.038 |
| Aphasia | 15 (13.8%) | 5 (8.2%) | 0.28 |
| Disorder of consciousness | 12 (11.0%) | 5 (8.2%) | 0.56 |
| Sensory disturbance | 7 (6.4%) | 3 (4.9%) | 0.69 |
| Memory disturbance | 6 (5.5%) | 11 (18.0%) | 0.009 |
| Anopsia | 3 (2.8%) | 0 | 0.19 |
| Incidental | 3 (2.8%) | 3 (4.9%) | 0.46 |
| Other | 0 | 3 (4.9%) | 0.020 |
| Unknown | 2 (1.8%) | 0 | 0.29 |
| Preoperative KPS | |||
| 100 or 90 | 41 (37.6%) | 18 (29.5%) | KPS ≥ 80 vs KPS < 80 0.027 |
| 80 | 38 (34.9%) | 16 (26.2%) | |
| 70 | 16 (14.7%) | 12 (19.7%) | |
| 60 or below | 14 (12.8%) | 15 (24.6%) | |
| Tumor volume | 20.4 cm3 (0.2–178.6) | 17.4 cm3 (0.3–161.3) | 0.62 |
| Single lobe | |||
| Frontal lobe | 37 (33.9%) | 20 (32.8%) | 0.88 |
| Parietal lobe | 27 (24.8%) | 14 (23.0%) | 0.79 |
| Temporal lobe | 21 (19.3%) | 8 (13.1%) | 0.31 |
| Occipital lobe | 2 (1.8%) | 1 (1.6%) | 0.93 |
| Multiple lobes | |||
| Frontoparietal lobes | 5 (4.6%) | 2 (3.3%) | 0.68 |
| Frontotemporal lobes | 1 (0.9%) | 3 (4.9%) | 0.10 |
| Temporoparietal lobes | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Temporooccipital lobes | 1 (0.9%) | 2 (3.3%) | 0.26 |
| Parietooccipital lobes | 2 (1.8%) | 0 | 0.29 |
| Infratentorial region | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Other | 9 (8.3%) | 7 (11.5%) | 0.49 |
| MGMT promoter | |||
| Methylated | 43 (39.4%) | 26 (42.6%) | 0.69 |
| Unmethylated | 66 (60.6%) | 35 (57.4%) | 0.69 |
| Unknown | 0 | 0 | |
| TERT promoter | |||
| WT | 36 (33.0%) | 25 (41.0%) | 0.30 |
| C228T | 45 (41.3%) | 25 (41.0%) | 0.97 |
| C250T | 17 (15.6%) | 4 (6.6%) | 0.086 |
| Unknown | 11 (10.1%) | 7 (11.5%) | 0.78 |
| BRAF | |||
| WT | 90 (82.6%) | 51 (83.6%) | 0.86 |
| V600E | 4 (3.7%) | 2 (3.3%) | 0.89 |
| Unknown | 15 (13.8%) | 8 (13.1%) | 0.91 |
| H3F3A | |||
| WT | 88 (80.7%) | 49 (80.3%) | 0.95 |
| K27M | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Unknown | 19 (17.4%) | 10 (16.4%) | 0.86 |
| Extent of resection | |||
| Total (100%) | 41 (37.6%) | 13 (21.3%) | Total vs. non-total 0.029 |
| Subtotal (95–99%) | 16 (14.7%) | 11 (18.0%) | |
| Partial (<94%) | 35 (32.1%) | 16 (26.2%) | |
| Biopsy | 17 (15.6%) | 21 (34.4%) | |
| . | Neurosurgeon group N = 109 . | Non-neurosurgeon group N = 61 . | P value . |
|---|---|---|---|
| Male: Female | 70: 39 (64.2%) | 33: 28 (54.1%) | 0.20 |
| Age (years) | 61 (29–85) | 69 (27–87) | 0.019 |
| Duration from initial symptom onset to | |||
| First hospital visit (days) | 7 (0–461) | 9 (0–195) | 0.67 |
| Duration from the first hospital visit to | |||
| Radiological diagnosis (days) | 0 (0–339) | 1 (0–94) | <0.0001 |
| First surgery (days) | 18 (1–2646) | 29 (5–749) | <0.0001 |
| Initial symptoms | |||
| Headache | 23 (21.1%) | 12 (19.7%) | 0.83 |
| Paralysis | 21 (19.3%) | 16 (26.2%) | 0.29 |
| Seizure | 17 (15.6%) | 3 (4.9%) | 0.038 |
| Aphasia | 15 (13.8%) | 5 (8.2%) | 0.28 |
| Disorder of consciousness | 12 (11.0%) | 5 (8.2%) | 0.56 |
| Sensory disturbance | 7 (6.4%) | 3 (4.9%) | 0.69 |
| Memory disturbance | 6 (5.5%) | 11 (18.0%) | 0.009 |
| Anopsia | 3 (2.8%) | 0 | 0.19 |
| Incidental | 3 (2.8%) | 3 (4.9%) | 0.46 |
| Other | 0 | 3 (4.9%) | 0.020 |
| Unknown | 2 (1.8%) | 0 | 0.29 |
| Preoperative KPS | |||
| 100 or 90 | 41 (37.6%) | 18 (29.5%) | KPS ≥ 80 vs KPS < 80 0.027 |
| 80 | 38 (34.9%) | 16 (26.2%) | |
| 70 | 16 (14.7%) | 12 (19.7%) | |
| 60 or below | 14 (12.8%) | 15 (24.6%) | |
| Tumor volume | 20.4 cm3 (0.2–178.6) | 17.4 cm3 (0.3–161.3) | 0.62 |
| Single lobe | |||
| Frontal lobe | 37 (33.9%) | 20 (32.8%) | 0.88 |
| Parietal lobe | 27 (24.8%) | 14 (23.0%) | 0.79 |
| Temporal lobe | 21 (19.3%) | 8 (13.1%) | 0.31 |
| Occipital lobe | 2 (1.8%) | 1 (1.6%) | 0.93 |
| Multiple lobes | |||
| Frontoparietal lobes | 5 (4.6%) | 2 (3.3%) | 0.68 |
| Frontotemporal lobes | 1 (0.9%) | 3 (4.9%) | 0.10 |
| Temporoparietal lobes | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Temporooccipital lobes | 1 (0.9%) | 2 (3.3%) | 0.26 |
| Parietooccipital lobes | 2 (1.8%) | 0 | 0.29 |
| Infratentorial region | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Other | 9 (8.3%) | 7 (11.5%) | 0.49 |
| MGMT promoter | |||
| Methylated | 43 (39.4%) | 26 (42.6%) | 0.69 |
| Unmethylated | 66 (60.6%) | 35 (57.4%) | 0.69 |
| Unknown | 0 | 0 | |
| TERT promoter | |||
| WT | 36 (33.0%) | 25 (41.0%) | 0.30 |
| C228T | 45 (41.3%) | 25 (41.0%) | 0.97 |
| C250T | 17 (15.6%) | 4 (6.6%) | 0.086 |
| Unknown | 11 (10.1%) | 7 (11.5%) | 0.78 |
| BRAF | |||
| WT | 90 (82.6%) | 51 (83.6%) | 0.86 |
| V600E | 4 (3.7%) | 2 (3.3%) | 0.89 |
| Unknown | 15 (13.8%) | 8 (13.1%) | 0.91 |
| H3F3A | |||
| WT | 88 (80.7%) | 49 (80.3%) | 0.95 |
| K27M | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Unknown | 19 (17.4%) | 10 (16.4%) | 0.86 |
| Extent of resection | |||
| Total (100%) | 41 (37.6%) | 13 (21.3%) | Total vs. non-total 0.029 |
| Subtotal (95–99%) | 16 (14.7%) | 11 (18.0%) | |
| Partial (<94%) | 35 (32.1%) | 16 (26.2%) | |
| Biopsy | 17 (15.6%) | 21 (34.4%) | |
BRAF, serine/threonine-protein kinase B-raf; H3F3A, H3 histone, family 3A; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA-methyltransferase; TERT, telomerase reverse transcriptase.
Characteristics and clinical results of neurosurgeon and non-neurosurgeon groups
| . | Neurosurgeon group N = 109 . | Non-neurosurgeon group N = 61 . | P value . |
|---|---|---|---|
| Male: Female | 70: 39 (64.2%) | 33: 28 (54.1%) | 0.20 |
| Age (years) | 61 (29–85) | 69 (27–87) | 0.019 |
| Duration from initial symptom onset to | |||
| First hospital visit (days) | 7 (0–461) | 9 (0–195) | 0.67 |
| Duration from the first hospital visit to | |||
| Radiological diagnosis (days) | 0 (0–339) | 1 (0–94) | <0.0001 |
| First surgery (days) | 18 (1–2646) | 29 (5–749) | <0.0001 |
| Initial symptoms | |||
| Headache | 23 (21.1%) | 12 (19.7%) | 0.83 |
| Paralysis | 21 (19.3%) | 16 (26.2%) | 0.29 |
| Seizure | 17 (15.6%) | 3 (4.9%) | 0.038 |
| Aphasia | 15 (13.8%) | 5 (8.2%) | 0.28 |
| Disorder of consciousness | 12 (11.0%) | 5 (8.2%) | 0.56 |
| Sensory disturbance | 7 (6.4%) | 3 (4.9%) | 0.69 |
| Memory disturbance | 6 (5.5%) | 11 (18.0%) | 0.009 |
| Anopsia | 3 (2.8%) | 0 | 0.19 |
| Incidental | 3 (2.8%) | 3 (4.9%) | 0.46 |
| Other | 0 | 3 (4.9%) | 0.020 |
| Unknown | 2 (1.8%) | 0 | 0.29 |
| Preoperative KPS | |||
| 100 or 90 | 41 (37.6%) | 18 (29.5%) | KPS ≥ 80 vs KPS < 80 0.027 |
| 80 | 38 (34.9%) | 16 (26.2%) | |
| 70 | 16 (14.7%) | 12 (19.7%) | |
| 60 or below | 14 (12.8%) | 15 (24.6%) | |
| Tumor volume | 20.4 cm3 (0.2–178.6) | 17.4 cm3 (0.3–161.3) | 0.62 |
| Single lobe | |||
| Frontal lobe | 37 (33.9%) | 20 (32.8%) | 0.88 |
| Parietal lobe | 27 (24.8%) | 14 (23.0%) | 0.79 |
| Temporal lobe | 21 (19.3%) | 8 (13.1%) | 0.31 |
| Occipital lobe | 2 (1.8%) | 1 (1.6%) | 0.93 |
| Multiple lobes | |||
| Frontoparietal lobes | 5 (4.6%) | 2 (3.3%) | 0.68 |
| Frontotemporal lobes | 1 (0.9%) | 3 (4.9%) | 0.10 |
| Temporoparietal lobes | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Temporooccipital lobes | 1 (0.9%) | 2 (3.3%) | 0.26 |
| Parietooccipital lobes | 2 (1.8%) | 0 | 0.29 |
| Infratentorial region | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Other | 9 (8.3%) | 7 (11.5%) | 0.49 |
| MGMT promoter | |||
| Methylated | 43 (39.4%) | 26 (42.6%) | 0.69 |
| Unmethylated | 66 (60.6%) | 35 (57.4%) | 0.69 |
| Unknown | 0 | 0 | |
| TERT promoter | |||
| WT | 36 (33.0%) | 25 (41.0%) | 0.30 |
| C228T | 45 (41.3%) | 25 (41.0%) | 0.97 |
| C250T | 17 (15.6%) | 4 (6.6%) | 0.086 |
| Unknown | 11 (10.1%) | 7 (11.5%) | 0.78 |
| BRAF | |||
| WT | 90 (82.6%) | 51 (83.6%) | 0.86 |
| V600E | 4 (3.7%) | 2 (3.3%) | 0.89 |
| Unknown | 15 (13.8%) | 8 (13.1%) | 0.91 |
| H3F3A | |||
| WT | 88 (80.7%) | 49 (80.3%) | 0.95 |
| K27M | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Unknown | 19 (17.4%) | 10 (16.4%) | 0.86 |
| Extent of resection | |||
| Total (100%) | 41 (37.6%) | 13 (21.3%) | Total vs. non-total 0.029 |
| Subtotal (95–99%) | 16 (14.7%) | 11 (18.0%) | |
| Partial (<94%) | 35 (32.1%) | 16 (26.2%) | |
| Biopsy | 17 (15.6%) | 21 (34.4%) | |
| . | Neurosurgeon group N = 109 . | Non-neurosurgeon group N = 61 . | P value . |
|---|---|---|---|
| Male: Female | 70: 39 (64.2%) | 33: 28 (54.1%) | 0.20 |
| Age (years) | 61 (29–85) | 69 (27–87) | 0.019 |
| Duration from initial symptom onset to | |||
| First hospital visit (days) | 7 (0–461) | 9 (0–195) | 0.67 |
| Duration from the first hospital visit to | |||
| Radiological diagnosis (days) | 0 (0–339) | 1 (0–94) | <0.0001 |
| First surgery (days) | 18 (1–2646) | 29 (5–749) | <0.0001 |
| Initial symptoms | |||
| Headache | 23 (21.1%) | 12 (19.7%) | 0.83 |
| Paralysis | 21 (19.3%) | 16 (26.2%) | 0.29 |
| Seizure | 17 (15.6%) | 3 (4.9%) | 0.038 |
| Aphasia | 15 (13.8%) | 5 (8.2%) | 0.28 |
| Disorder of consciousness | 12 (11.0%) | 5 (8.2%) | 0.56 |
| Sensory disturbance | 7 (6.4%) | 3 (4.9%) | 0.69 |
| Memory disturbance | 6 (5.5%) | 11 (18.0%) | 0.009 |
| Anopsia | 3 (2.8%) | 0 | 0.19 |
| Incidental | 3 (2.8%) | 3 (4.9%) | 0.46 |
| Other | 0 | 3 (4.9%) | 0.020 |
| Unknown | 2 (1.8%) | 0 | 0.29 |
| Preoperative KPS | |||
| 100 or 90 | 41 (37.6%) | 18 (29.5%) | KPS ≥ 80 vs KPS < 80 0.027 |
| 80 | 38 (34.9%) | 16 (26.2%) | |
| 70 | 16 (14.7%) | 12 (19.7%) | |
| 60 or below | 14 (12.8%) | 15 (24.6%) | |
| Tumor volume | 20.4 cm3 (0.2–178.6) | 17.4 cm3 (0.3–161.3) | 0.62 |
| Single lobe | |||
| Frontal lobe | 37 (33.9%) | 20 (32.8%) | 0.88 |
| Parietal lobe | 27 (24.8%) | 14 (23.0%) | 0.79 |
| Temporal lobe | 21 (19.3%) | 8 (13.1%) | 0.31 |
| Occipital lobe | 2 (1.8%) | 1 (1.6%) | 0.93 |
| Multiple lobes | |||
| Frontoparietal lobes | 5 (4.6%) | 2 (3.3%) | 0.68 |
| Frontotemporal lobes | 1 (0.9%) | 3 (4.9%) | 0.10 |
| Temporoparietal lobes | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Temporooccipital lobes | 1 (0.9%) | 2 (3.3%) | 0.26 |
| Parietooccipital lobes | 2 (1.8%) | 0 | 0.29 |
| Infratentorial region | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Other | 9 (8.3%) | 7 (11.5%) | 0.49 |
| MGMT promoter | |||
| Methylated | 43 (39.4%) | 26 (42.6%) | 0.69 |
| Unmethylated | 66 (60.6%) | 35 (57.4%) | 0.69 |
| Unknown | 0 | 0 | |
| TERT promoter | |||
| WT | 36 (33.0%) | 25 (41.0%) | 0.30 |
| C228T | 45 (41.3%) | 25 (41.0%) | 0.97 |
| C250T | 17 (15.6%) | 4 (6.6%) | 0.086 |
| Unknown | 11 (10.1%) | 7 (11.5%) | 0.78 |
| BRAF | |||
| WT | 90 (82.6%) | 51 (83.6%) | 0.86 |
| V600E | 4 (3.7%) | 2 (3.3%) | 0.89 |
| Unknown | 15 (13.8%) | 8 (13.1%) | 0.91 |
| H3F3A | |||
| WT | 88 (80.7%) | 49 (80.3%) | 0.95 |
| K27M | 2 (1.8%) | 2 (3.3%) | 0.55 |
| Unknown | 19 (17.4%) | 10 (16.4%) | 0.86 |
| Extent of resection | |||
| Total (100%) | 41 (37.6%) | 13 (21.3%) | Total vs. non-total 0.029 |
| Subtotal (95–99%) | 16 (14.7%) | 11 (18.0%) | |
| Partial (<94%) | 35 (32.1%) | 16 (26.2%) | |
| Biopsy | 17 (15.6%) | 21 (34.4%) | |
BRAF, serine/threonine-protein kinase B-raf; H3F3A, H3 histone, family 3A; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA-methyltransferase; TERT, telomerase reverse transcriptase.
Results
Patient demographics
One hundred seventy newly diagnosed IDH-wildtype GBM patients were treated at our institution between January 2010 and December 2019. 109 and 61 patients were classified into the neurosurgeon and non-neurosurgeon groups, respectively.
The patient demographics of the neurosurgeon and non-neurosurgeon groups are summarized in Table 1. Male patients composed 64.2% and 54.1% in the neurosurgeon and non-neurosurgeon groups, respectively (P = 0.20). The median age of the neurosurgeon group was significantly younger than the non-neurosurgeon group (61 vs. 69 years old, P = 0.019, respectively). There was no significant difference in the median duration from the onset of symptoms to the clinic or hospital visit (7 vs. 9 days, P = 0.67) between the neurosurgeon and non-neurosurgeon groups. In contrast, the neurosurgeon group demonstrated a significantly shorter median duration from the first hospital visit to the radiological diagnosis of a tumor (0 vs. 1 day, P < 0.0001) and to the first surgery (18 vs. 29 days, P < 0.0001) than the non-neurosurgeon group.
Subgroups of the non-neurosurgeon group and the clinical time course of patients from the initial symptom onset to the first surgery in neurosurgeon and non-neurosurgeon groups are summarized in Supplementary Table S1. The majority of primary doctors in the non-neurosurgeon group comprised neurologists (n = 21) and general physicians (n = 19). The median duration from the initial symptom onset to the first visit was 6, 7 and 10 days in the neurologist, neurosurgeon and general physician group, respectively. The median duration from the first hospital visit to the radiological diagnosis was 0 days in the neurosurgeon and general physician groups and 1 day in the neurologist group. The median duration from the first hospital visit to first surgery in the neurosurgeon, neurologist and general physician groups were 18, 26 and 32 days, respectively.
Initial symptoms and KPS
The initial symptoms of the patients in the two groups are summarized in Table 1. Headache and paralysis were common initial symptoms in both neurosurgeon and non-neurosurgeon groups. Significantly more patients in the neurosurgeon group present neurologically specific symptoms such as seizure (n = 17, 15.6%, P = 0.038). In contrast, neurologically unspecific symptoms, such as memory disturbance, was some of the most frequent symptoms observed in the non-neurosurgeon group (n = 11, 18.0%, P = 0.009).
In addition, significantly more patients with preoperative KPS scores |$\ge$|80 were found in the neurosurgeon group (n = 79, 72.5%) than in the non-neurosurgeon group (n = 34, 55.7%, P = 0.027, Fig. 1A). Within the non-neurosurgeon group, we observed a notable disparity in the proportion of patients with low preoperative KPS (<70) between different primary doctors. Specifically, the general physician (47.4%, P = 0.029) and psychiatrist group (33.3%, P = 0.041) exhibited a significantly higher proportion of patients with low preoperative KPS compared with the neurosurgeon group (72.5%, Supplementary Table S2). This discrepancy in preoperative KPS can be attributed to the considerably longer duration from the first hospital visit to the first surgery in the general physician group (32 days, P = 0.0052) and psychiatrist group (34.5 days, P = 0.011) compared with the neurosurgeon group (18 days), as outlined in Supplementary Table S1. These findings indicate that a longer duration between the first visit and the first surgery is potentially associated with a higher likelihood of having a lower preoperative KPS.

Distribution of patients with high (|$\ge$|80) and low (|$\le$|70) Karnofsky performance status (KPS) scores and with total and not total resection in the neurosurgeon and non-neurosurgeon groups. (A) The percentage of patients with high preoperative KPS scores (|$\ge$|80) was 72.5% (n = 79) in the neurosurgeon group and 55.7% (n = 34) in the non-neurosurgeon group (P = 0.027) (B) The percentage of patients with total tumor resection was 37.6% (n = 41) in the neurosurgeon group and 21.3% (n = 13) in the non-neurosurgeon group (P = 0.029).
Tumor characteristics and clinical outcomes
The tumor characteristics and clinical outcomes of patients are summarized in Table 1. There was no significant difference in median tumor volumes between the neurosurgeon and non-neurosurgeon groups (20.4 vs. 17.4 cm3, P = 0.62, respectively). The tumor locations and molecular characteristics of tumors, including MGMT promoter methylation status, TERT promoter mutation, BRAF mutation and H3F3A mutation, were similar in both groups.
Regarding clinical outcomes, we observed more totally resected tumors in the neurosurgeon group (n = 41, 37.6%) than in the non-neurosurgeon group (n = 13, 21.3%, P = 0.029, Fig. 1B). Furthermore, the median OS of the neurosurgeon group (22.9 months) was significantly more enhanced than that of the non-neurosurgeon group (14.0 months, P = 0.038, Fig. 2).
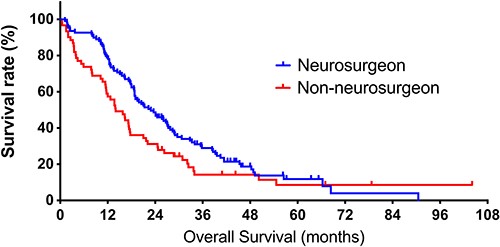
Kaplan–Meier curves of overall survival (OS) in the neurosurgeon and non-neurosurgeon groups. The neurosurgeon group includes patients who sought their first evaluation by a neurosurgeon, and the non-neurosurgeon group comprises patients who sought their first evaluation by a non-neurosurgeon. The median OS of patients in the neurosurgeon and non-neurosurgeon groups is 22.9 and 14.0 months (P = 0.038).
Knowing that there is a significant difference in duration from the first hospital visit to the first surgery between the neurosurgeon and non-neurosurgeon groups, we assessed if this time difference affects the prognosis of GBM patients. We divided patients into two groups; the rapid surgery group (n = 85) included patients who received the first surgery within 20 days of the first hospital visit, and the delayed surgery group (n = 85) included patients who received the first surgery 21 days or later. As a result, we observed that the patients in the rapid surgery group exhibited significantly prolonged survival over those in the delayed surgery group (24.5 vs. 16.2 months, P = 0.016, respectively, Fig. 3).
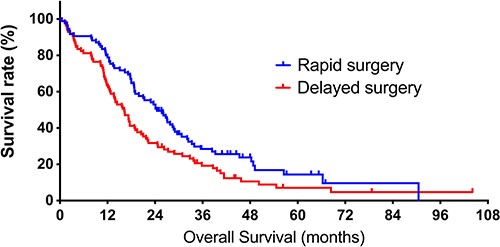
Kaplan–Meier curves of overall survival (OS) in the rapid and delayed surgery groups. The rapid surgery group includes patients who received the first surgery within 20 days of the first hospital visit. The delayed surgery group includes patients who received the first surgery 21 days or later. The median OS of patients in rapid and delayed surgery groups is 24.5 and 16.2 months (P = 0.016).
In addition, we examined whether the availability of CT or MRI at the initial visit hospital had an impact on patient prognosis. Our analysis revealed that the availability of CT did not significantly affect patient prognosis (19.0 vs. 22.1 months, P = 0.078, Supplementary Fig. S1). In contrast, the availability of MRI was associated with an improved prognosis (19.9 vs. 13.4 months, P = 0.0086, Supplementary Fig. S2). The reason for the enhanced patient prognosis in the group of patients who visited a hospital where MRI was available was attributed to a significantly shorter duration from the initial hospital visit to the first surgical intervention (19.0 vs. 26.5 days, P = 0.048, Supplementary Fig. S3). This finding highlights the importance of timely access to MRI for prompt surgical interventions, ultimately leading to improved patient outcomes.
We also classified GBM patients based on their initial symptoms and examined the impact of their initial visit being with a neurosurgeon or a non-neurosurgeon. Of 170 patients included in the analysis, 37 (21.8%) presented with paralysis as their initial symptom, while 35 (20.5%) presented with headaches (Supplementary Table S3). When comparing patients with an initial symptom of paralysis, we did not find a significant difference in prognosis between the neurosurgeon and non-neurosurgeon groups (15.6 vs. 13.7 months, P = 0.86, Supplementary Fig. S4). In contrast, the results were different for patients with an initial symptom of headache. In this subgroup, those who initially consulted with a neurosurgeon experienced significantly improved prognosis compared with those who visited a non-neurosurgeon (29.6 vs. 16.9 months, P = 0.0049, Supplementary Fig. S5). These findings suggest that GBM patients with an initial symptom of headache particularly benefit from visiting a neurosurgeon for their initial consultation.
Furthermore, we examined the prognostic differences between patients who underwent their first surgery at the same institution where they had their initial evaluation and those who had their first surgery at a different institution. Our results revealed that patients who had their first surgery at the same institution experienced a significantly shorter duration from the initial evaluation to the first surgery (11.5 vs. 23.5 days, P < 0.0001, Supplementary Fig. S6) and showed an improved prognosis compared with those who had their first surgery at a different institution (27.0 vs. 17.8 months, P = 0.030, Supplementary Fig. S7). These findings highlight the critical impact of rapid surgical intervention on patient outcomes.
The univariate analysis revealed several significant predictors for an improved prognosis in patients with glioblastoma, including initial evaluation by a neurosurgeon, total extent of tumor resection, good preoperative KPS (≥80), methylated MGMT promoter, and a shorter duration (within 20 days) from the first hospital visit to the first surgery. Furthermore, the multivariate analysis confirmed that total tumor resection, good preoperative KPS (≥80), methylated MGMT promoter, and a shorter duration (0 to 20 days) from the first hospital visit to the first surgery were independent predictors of prolonged survival. These variables retained their significance even after adjusting for other factors. The findings from both the univariate and multivariate analyses have been summarized in Table 2, highlighting the key predictors associated with enhanced prognosis in patients with GBM.
Univariate and multivariate predictors of survival in 170 patients with glioblastoma
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Variable . | HR Estimate (95% CI) . | P value . | HR Estimate (95% CI) . | P value . |
| Age (yrs) | ||||
| 50 or older | 1 | 1 | ||
| 49 or younger | 0.89 (0.59–1.31) | 0.56 | 0.70 (0.45–1.07) | 0.11 |
| Initial evaluation | ||||
| Non-neurosurgeon | 1 | 1 | ||
| Neurosurgeon | 0.70 (0.50–0.99) | 0.039 | 0.81 (0.56–1.16) | 0.24 |
| Extent of resection of the tumor | ||||
| 99% or less | 1 | 1 | ||
| Total | 0.64 (0.44–0.91) | 0.014 | 0.65 (0.45–0.94) | 0.024 |
| Preoperative KPS | ||||
| 70 or below | 1 | 1 | ||
| 80 or above | 0.44 (0.31–0.62) | <0.0001 | 0.42 (0.30–0.60) | <0.0001 |
| MGMT promoter | ||||
| Unmethlylated | 1 | 1 | ||
| Methylated | 0.48 (0.34–0.68) | <0.0001 | 0.38 (0.26–0.55) | <0.0001 |
| Duration from the first hospital visit to the first surgery | ||||
| 21 days or later | 1 | 1 | ||
| 0–20 days | 0.67 (0.48–0.93) | 0.017 | 0.70 (0.49–0.99) | 0.046 |
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Variable . | HR Estimate (95% CI) . | P value . | HR Estimate (95% CI) . | P value . |
| Age (yrs) | ||||
| 50 or older | 1 | 1 | ||
| 49 or younger | 0.89 (0.59–1.31) | 0.56 | 0.70 (0.45–1.07) | 0.11 |
| Initial evaluation | ||||
| Non-neurosurgeon | 1 | 1 | ||
| Neurosurgeon | 0.70 (0.50–0.99) | 0.039 | 0.81 (0.56–1.16) | 0.24 |
| Extent of resection of the tumor | ||||
| 99% or less | 1 | 1 | ||
| Total | 0.64 (0.44–0.91) | 0.014 | 0.65 (0.45–0.94) | 0.024 |
| Preoperative KPS | ||||
| 70 or below | 1 | 1 | ||
| 80 or above | 0.44 (0.31–0.62) | <0.0001 | 0.42 (0.30–0.60) | <0.0001 |
| MGMT promoter | ||||
| Unmethlylated | 1 | 1 | ||
| Methylated | 0.48 (0.34–0.68) | <0.0001 | 0.38 (0.26–0.55) | <0.0001 |
| Duration from the first hospital visit to the first surgery | ||||
| 21 days or later | 1 | 1 | ||
| 0–20 days | 0.67 (0.48–0.93) | 0.017 | 0.70 (0.49–0.99) | 0.046 |
HR, hazard ratio; CI, confidence interval; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA-methyltransferase
Univariate and multivariate predictors of survival in 170 patients with glioblastoma
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Variable . | HR Estimate (95% CI) . | P value . | HR Estimate (95% CI) . | P value . |
| Age (yrs) | ||||
| 50 or older | 1 | 1 | ||
| 49 or younger | 0.89 (0.59–1.31) | 0.56 | 0.70 (0.45–1.07) | 0.11 |
| Initial evaluation | ||||
| Non-neurosurgeon | 1 | 1 | ||
| Neurosurgeon | 0.70 (0.50–0.99) | 0.039 | 0.81 (0.56–1.16) | 0.24 |
| Extent of resection of the tumor | ||||
| 99% or less | 1 | 1 | ||
| Total | 0.64 (0.44–0.91) | 0.014 | 0.65 (0.45–0.94) | 0.024 |
| Preoperative KPS | ||||
| 70 or below | 1 | 1 | ||
| 80 or above | 0.44 (0.31–0.62) | <0.0001 | 0.42 (0.30–0.60) | <0.0001 |
| MGMT promoter | ||||
| Unmethlylated | 1 | 1 | ||
| Methylated | 0.48 (0.34–0.68) | <0.0001 | 0.38 (0.26–0.55) | <0.0001 |
| Duration from the first hospital visit to the first surgery | ||||
| 21 days or later | 1 | 1 | ||
| 0–20 days | 0.67 (0.48–0.93) | 0.017 | 0.70 (0.49–0.99) | 0.046 |
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Variable . | HR Estimate (95% CI) . | P value . | HR Estimate (95% CI) . | P value . |
| Age (yrs) | ||||
| 50 or older | 1 | 1 | ||
| 49 or younger | 0.89 (0.59–1.31) | 0.56 | 0.70 (0.45–1.07) | 0.11 |
| Initial evaluation | ||||
| Non-neurosurgeon | 1 | 1 | ||
| Neurosurgeon | 0.70 (0.50–0.99) | 0.039 | 0.81 (0.56–1.16) | 0.24 |
| Extent of resection of the tumor | ||||
| 99% or less | 1 | 1 | ||
| Total | 0.64 (0.44–0.91) | 0.014 | 0.65 (0.45–0.94) | 0.024 |
| Preoperative KPS | ||||
| 70 or below | 1 | 1 | ||
| 80 or above | 0.44 (0.31–0.62) | <0.0001 | 0.42 (0.30–0.60) | <0.0001 |
| MGMT promoter | ||||
| Unmethlylated | 1 | 1 | ||
| Methylated | 0.48 (0.34–0.68) | <0.0001 | 0.38 (0.26–0.55) | <0.0001 |
| Duration from the first hospital visit to the first surgery | ||||
| 21 days or later | 1 | 1 | ||
| 0–20 days | 0.67 (0.48–0.93) | 0.017 | 0.70 (0.49–0.99) | 0.046 |
HR, hazard ratio; CI, confidence interval; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA-methyltransferase
Discussion
In this study, we found that GBM patients who sought an initial evaluation directly from a neurosurgeon showed a potential association with prolonged survival. We presume that this prognostic advantage in the neurosurgeon group is primarily attributed to biases in patient backgrounds. For example, patients in the neurosurgeon group are significantly younger and in better performance status than those in the non-neurosurgeon group. The Japan Medical Association Research Institute survey reported that 55.2% of the Japanese population have a primary care doctor, and this rate increases to 76.5% in those 70 years of age or older (17). Also, ‘healthy’ people are less likely to have primary care doctors (49.8%) than ‘unhealthy’ people (85.0%) (18). (The classification of ‘healthy’ and ‘unhealthy’ is based on the surveyees’ self-judgment.) Furthermore, young patients or those who do not have a chronic disease seek health information on the internet more frequently than older patients or those with a chronic disease (19). These data indicate that young patients and those with better performance statuses are more likely to compose the neurosurgeon group by seeking and consulting a specific doctor to evaluate their initial symptoms. As a result, these patients in neurosurgeon tend to have more favorable prognoses, which aligns with the well-established prognostic factors for GBM patients (20,21).
We also observed a significantly higher total tumor resection rate in the neurosurgeon group than in the non-neurosurgeon group. This difference may similarly be attributed to the younger median age of patients in the neurosurgeon group, because young age is associated with a higher chance of total resection in the general GBM population (22).
Regardless of the difference in patient backgrounds, it is worth noting that patients in the neurosurgeon group have a significantly shorter duration from the first hospital visit to the radiological diagnosis of the tumor and the first surgery than the non-neurosurgeon group. This time difference is presumably due to the additional time spent for patients in the non-neurosurgeon group for professional assessment, medical referral, and transfer to a neurosurgeon. For example, patients who initially visited orthopedic surgeons spent 38 days on average from the first hospital visit to the radiological diagnosis of GBM. We assume that the orthopedic surgeons prioritized the assessment of the spinal cord or peripheral nerves before intracranial evaluations, based on the patient’s chief complaint. Also, patients in the ophthalmologist group spent an average of 131 days from the first hospital visit to the first surgery. For the record, ophthalmologists typically requested a head MRI and obtained a radiological diagnosis of GBM relatively rapidly. However, patient referral to the neurosurgical department was often critically delayed, presumably because patients in the ophthalmologist group experienced relatively mild initial symptoms, such as visual field defect or double vision. Since our data clearly shows that patients who received the delayed surgery suffered a worse prognosis, we believe this delay is an obstacle we must overcome for the benefit of GBM patients.
Practically, it is not feasible for neurosurgeons to cover all symptoms caused by a brain tumor. Patients experiencing neurologically nonspecific symptoms, such as memory disturbance (which ranks as the third most common symptom in the non-neurosurgeon group), often seek medical evaluation from departments specializing in neurology, internal medicine, or psychiatry. For the benefit of GBM patients, we need to establish stronger and more prompt cooperation between all physicians and neurosurgeons. With a common understanding that GBM is a time-dependent and semi-urgent disease, physicians of the non-neurosurgical department should rapidly refer patients with a suspicion of GBM to a neurosurgeon. Neurosurgeons must also diagnose and treat patients as soon as possible. Furthermore, we need to intensify the social awareness of brain diseases, including GBM, to prompt immediate consultation after initial symptoms to improve patient prognosis.
This study had several limitations. Given that this study relied on a retrospective analysis that included patients who underwent their initial surgery at a different medical facility, it was not feasible to obtain comprehensive comorbidity information, despite its potential impact on perioperative management. Also, the initial hospital visit and first surgery dates at other hospitals were identified based on referral letters, so some of the dates may not be accurate. Furthermore, there is uncertainty regarding whether the initial symptoms experienced by the patients were definitively caused by GBM. Additionally, incidental tumor discoveries were made in some cases. As a result, certain patients had an extended follow-up period from their initial hospital visit to the first surgery, such as 2646 days in the neurosurgeon group and 749 days in the non-neurosurgeon group. We made a compromise to include a diverse range of patients in order to portray a more realistic clinical scenario. However, it is crucial to acknowledge that some patient data may not align with the standard clinical course of GBM patients. Lastly, there have been many advances in therapeutic strategies, including awake surgery, intraoperative MRI and fluorescence guidance, bevacizumab and tumor treatment fields (23) between 2010 and 2019. These clinical advances may reduce or expand the advantages of rapid surgical intervention.
In summary, seeking an initial evaluation by a neurosurgeon was potentially associated with prolonged survival in GBM patients. We also found that a short duration from the first hospital visit to the first surgery was associated with enhanced prognosis. The neurological status of GBM patients rapidly deteriorates in a short period. Therefore, direct cooperation between all physicians and neurosurgeon is vital in GBM treatment. Finally, we need to bolster social awareness of brain diseases, including GBM, to prompt immediate consultation after initial symptoms to improve patient prognosis.
Acknowledgments
We thank Philip Pham, BS, for English language editing.
Conflict of interest
There are no conflicts of interest to declare.
References


