-
PDF
- Split View
-
Views
-
Cite
Cite
Shumpei Yamamoto, Hiromitsu Kanzaki, Chihiro Sakaguchi, Hirokazu Mouri, Takao Tsuzuki, Junichiro Nasu, Sayo Kobayashi, Tatsuya Toyokawa, Yuka Obayashi, Masafumi Inoue, Ryo Kato, Minoru Matsubara, Masahide Kita, Hiroyuki Okada, Current prognostic factors of advanced gastric cancer patients treated with chemotherapy: real world data from a Japanese 12 institutions, Japanese Journal of Clinical Oncology, Volume 53, Issue 10, October 2023, Pages 928–935, https://doi.org/10.1093/jjco/hyad091
Close - Share Icon Share
Abstract
Understanding the prognostic factors of advanced gastric cancer before starting chemotherapy is important to determine personalized treatment strategies. However, the details of chemotherapy and the prognosis of advanced gastric cancer patients have changed with the time and environment. The aim of this study was to understand the current reality of chemotherapy and to estimate the prognostic factors of advanced gastric cancer patients before starting chemotherapy at multiple centers. This includes specialized cancer hospitals and community hospitals, with the latest data under the Japanese insurance system.
We evaluated the clinical parameters and treatment details of 1025 patients who received systemic chemotherapy for unresectable advanced gastric cancer from 2012 to 2018 at 12 institutions in Japan. Prognostic factors were analyzed using the Cox proportional hazards regression model.
As of April 2021, 953 (93%) patients had died, while 72 (7%) patients survived. The median overall survival and progression-free survival of first-line chemotherapy was 11.8 months (95% confidence interval, 10.8–12.3 months) and 6.3 months (95% confidence interval, 5.9–6.9 months), respectively. Multivariate analysis revealed eight prognostic factors: age < 40 years, performance status ≥2, no gastrectomy, diffuse histological type, albumin <3.6, alkaline phosphatase ≥300, creatinine ≥1.0 and neutrophil-to-lymphocyte ratio > 3.0. Patients using trastuzumab showed better survival than patients without (16.1 months vs. 11.1 months; P = 0.0005).
We identified eight prognostic factors for patients with advanced gastric cancer undergoing Japanese standard chemotherapy. Our results will help clinicians develop treatment strategies for every patient.
Introduction
Gastric cancer is the fifth most common and third leading cause of cancer-related deaths worldwide (1), despite the steady decrease in the mortality rate in recent years. Up to two-thirds of gastric cancer patients are diagnosed with advanced gastric cancer (AGC) with an unresectable stage at the time of diagnosis. For these patients, systemic chemotherapy is the standard treatment to prolong the prognosis (2). Many new drugs are currently available for treating AGC. Trastuzumab has been used in these patients since 2011, ramucirumab in 2015 and nivolumab in 2017 under the Japanese insurance system based on promising clinical trial data (3–7). Accordingly, the recommended chemotherapy by the Japanese cancer treatment guideline has changed over time (8–11), and clinicians are sometimes needed to consider more than fourth-line treatment to improve the prognosis of patients with AGC.
Before initiating chemotherapy, understanding the prognostic factors of AGC is extremely important for estimating the prognosis for each patient and determining personalized treatment strategies (12). Thus, several previous studies identified prognosticators for AGC (13–17). Factors, such as performance status (PS), no gastrectomy and elevated alkaline phosphatase (ALP), are identified as important prognosticators (13,15,18). However, several prognostic factors were inconsistent between studies. This may be due to differences in the patients’ condition and environment or the era of each study. Many of these reports were derived from sub-analyses of clinical trials or retrospective studies in a single specialized cancer hospital. This may not reflect the actual clinical situation of a general hospital. Clinicians are sometimes required to initiate chemotherapy even for patients categorized under the ‘Eastern Cooperative Oncology Group’ PS 3 or have renal dysfunction that were not recommended in guidelines and excluded in clinical trials. Furthermore, advancements in chemotherapy have improved the prognosis of AGC patients yearly, and the prognosticators are continuously revised. For instance, the neutrophil-to-lymphocyte ratio (NLR) in peripheral blood, which is a good prognostic factor for various cancers, has been considered reliable for gastric cancer (19–21). Some studies have also shown that AGC in young adults may be a poor prognostic factor (22–24).
Given this background, this study aimed to estimate the current prognostic factors of AGC before chemotherapy using latest real-world data from multiple centers, including specialized cancer hospitals and community hospitals in Japan.
Patients and methods
Patients
We conducted a multicenter retrospective study at 12 institutions in Japan. We enrolled patients who started first-line systemic chemotherapy for unresectable AGC between January 2012 and December 2018. We collected data until April, 2021. The inclusion criteria were patients histologically diagnosed with adenocarcinoma and clinically diagnosed as primary gastric cancer. The included cases were obtained from the clinical records of each facility or department. The exclusion criteria were as follows: patients who continued chemotherapy after transferring to another hospital, with unknown progress and detailed data, without gastric adenocarcinoma, with more severe concurrent cancers and with suspected metastatic gastric cancer or invasion from other malignancies.
The methodologies were conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the ethics committees of the Okayama University Hospital (Number 1903-005) and affiliated hospitals, and informed consent was obtained using the opt-out method.
Evaluations
The clinical parameters of all patients were retrospectively reviewed and registered in a database dedicated to this study, including age, body mass index, sex, PS, disease status (unresectable/recurrence), site of primary cancer, histological type of cancer, human epidermal growth factor receptor 2 (HER2) status, location of metastasis (liver, peritoneum, lung and bone), number of distant metastatic sites, ascites, serum albumin, ALP, aspartate transaminase (AST), creatinine (Cre), hemoglobin (Hb), lactate dehydrogenase (LDH) and NLR at the initiation of first-line chemotherapy. Treatment details were also collected, such as the chemotherapy regimen, overall response and overall survival (OS), which was calculated as the time between when first-line chemotherapy was started and the date of death, and progression-free survival (PFS), which was calculated from when first-line chemotherapy was started to the date of evidence of progressive disease and death. All data were handled in an anonymous format.
Outcomes
The primary objective of this study was to elucidate the prognostic factors for AGC before starting first-line chemotherapy. The secondary outcomes were the treatment results, transition rates of second- and third-line chemotherapy, and background characteristics of patients who received systemic chemotherapy for unresectable gastric cancer.
Statistical analysis
OS and PFS were analyzed using the Kaplan–Meier method. Differences between survival curves were assessed using the log-rank test. Hazard ratios (HRs) were calculated using the Cox proportional hazard regression model in the univariate and multivariate analyses of survival. Factors included in the univariate analysis were age (<40), sex (male), PS (≥2), site of primary (upper-middle third of the stomach), no previous gastrectomy, histological type of cancer (diffuse), HER2 status (negative), liver metastasis, peritoneum metastasis, lung metastasis, bone metastasis, number of distant metastatic sites (≥2), presence of ascites, Alb <3.6 g/dl, ALP ≥300 U/l, AST >30 U/l, LDH ≥250 U/l, Hb <11 g/dl, Cre ≥1.0 mg/dl and NLR >3.0. The cut-off value of age (24–26) and laboratory variables (13,15,19,27), except for AST, LDH, Cre, were determined based on previous reports. The Cut-off values of AST and LDH were dichotomized at the limit of its normal range. Cre was dichotomized with the cut-off point at 1.0 with reference to grade 1 adverse events in the National Cancer Institute Common Toxicity Criteria (version 5.0). The cut-off ALP values (≥300 U/l) in this study were measured using the Japan Society Clinical Chemistry method, which has three times the measurement value compared with the International Federation of Clinical Chemistry and Laboratory Medicine method. Differences were considered significant at a P value <0.05. Statistical calculations were performed using the JMP Pro version 15 software (SAS Institute, NC, USA). Harrell’s concordance index (C-index) to assess the discriminate ability of a prognostic classification was evaluated by STATA software version 18 (Stata Corp, TX, USA).
Result
Patient characteristics
We included 1075 patients with AGC. After exclusion of 50 AGC patients (28 patients who continued chemotherapy after transferring to another hospital, 12 patients with unknown progress and detailed data, eight patients without gastric adenocarcinoma and two patients with more severe concurrent cancers), 1025 AGC patients from 12 institutions were analyzed in this study. As of April 2021, 953 (93%) patients had died, while 72 (7%) patients survived. The patient characteristics are listed in Table 1. The median age was 68 years (range 24–89), and the proportion of men was 68.9%. The ratio of PS 0, 1, 2, 3 and 4 was 53.2%, 37.6%, 7.3%, 1.7% and 0.2%, respectively. A total of 19% of the patients underwent gastrectomy before and after first-line chemotherapy. A total of ⁓60.3% were diffuse histological type and 18.5% was HER2-positive case. In total, 35% of the patients had ≥2 distant metastatic sites.
| Characteristics . | . | Total (n = 1025) . |
|---|---|---|
| Number of patients, (%) | ||
| Age (median age, range) | 68 (24–89) | |
| Sex | Male | 707 (69.0%) |
| Performance status | 0 | 543 (53.2%) |
| 1 | 384 (37.6%) | |
| 2 | 75 (7.3%) | |
| 3 | 17 (1.7%) | |
| 4 | 2 (0.2%) | |
| Disease status at first chemotherapy | Unresectable | 914 (89.2%) |
| Recurrence | 110 (10.7%) | |
| Unknown | 1 (0.1%) | |
| Time to recurrence ≥6 months | Yes | 67 (6.5%) |
| Adjuvant chemotherapy | Yes | 84 (8.2%) |
| Site of primary | Upper/middle third of the stomach | 808 (78.9%) |
| Lower third of the stomach | 193 (18.8%) | |
| unknown | 24 (2.3%) | |
| Gastrectomya | Yes | 195 (19%) |
| No | 830 (81%) | |
| Histological type | Intestinal | 397 (38.7%) |
| Diffuse | 602 (58.7%) | |
| Unknown | 26 (2.6%) | |
| HER2 status | Positive | 170 (16.6%) |
| Negative | 749 (73.1%) | |
| Unknown | 106 (10.3%) | |
| Location of metastasis | ||
| Liver | 377 (36.8%) | |
| Peritoneum | 471 (46.0%) | |
| Bone | 90 (8.8%) | |
| Number of distant metastasis | 2≧ | 362 (35.3%) |
| Ascites | Yes | 452 (47%) |
| Unknown | 2 (2.0%) | |
| Clinical laboratory data | ||
| Alb | median, (range) g/dl | 3.4 (1.0–5.9) |
| ALP | median, (range) U/l | 270 (116–12 234) |
| AST | median, (range) U/l | 37 (9.0–667) |
| Cre | median, (range) mg/dl | 0.74 (0.26–4.02) |
| Hb | median, (range) g/dl | 11.1(5.9–16.5) |
| LDH | median, (range) U/l | 204 (21–3511) |
| PLT | median, (range) 10^4/μl | 27 (1.7–434) |
| NLR | median, (range) | 3.5 (0.23–99.6) |
| CEA | median, (range) ng/ml | 5.3 (0.05–110 676) |
| CA19-9 | median, (range) U/ml | 23.4 (0–8 274 800) |
| Characteristics . | . | Total (n = 1025) . |
|---|---|---|
| Number of patients, (%) | ||
| Age (median age, range) | 68 (24–89) | |
| Sex | Male | 707 (69.0%) |
| Performance status | 0 | 543 (53.2%) |
| 1 | 384 (37.6%) | |
| 2 | 75 (7.3%) | |
| 3 | 17 (1.7%) | |
| 4 | 2 (0.2%) | |
| Disease status at first chemotherapy | Unresectable | 914 (89.2%) |
| Recurrence | 110 (10.7%) | |
| Unknown | 1 (0.1%) | |
| Time to recurrence ≥6 months | Yes | 67 (6.5%) |
| Adjuvant chemotherapy | Yes | 84 (8.2%) |
| Site of primary | Upper/middle third of the stomach | 808 (78.9%) |
| Lower third of the stomach | 193 (18.8%) | |
| unknown | 24 (2.3%) | |
| Gastrectomya | Yes | 195 (19%) |
| No | 830 (81%) | |
| Histological type | Intestinal | 397 (38.7%) |
| Diffuse | 602 (58.7%) | |
| Unknown | 26 (2.6%) | |
| HER2 status | Positive | 170 (16.6%) |
| Negative | 749 (73.1%) | |
| Unknown | 106 (10.3%) | |
| Location of metastasis | ||
| Liver | 377 (36.8%) | |
| Peritoneum | 471 (46.0%) | |
| Bone | 90 (8.8%) | |
| Number of distant metastasis | 2≧ | 362 (35.3%) |
| Ascites | Yes | 452 (47%) |
| Unknown | 2 (2.0%) | |
| Clinical laboratory data | ||
| Alb | median, (range) g/dl | 3.4 (1.0–5.9) |
| ALP | median, (range) U/l | 270 (116–12 234) |
| AST | median, (range) U/l | 37 (9.0–667) |
| Cre | median, (range) mg/dl | 0.74 (0.26–4.02) |
| Hb | median, (range) g/dl | 11.1(5.9–16.5) |
| LDH | median, (range) U/l | 204 (21–3511) |
| PLT | median, (range) 10^4/μl | 27 (1.7–434) |
| NLR | median, (range) | 3.5 (0.23–99.6) |
| CEA | median, (range) ng/ml | 5.3 (0.05–110 676) |
| CA19-9 | median, (range) U/ml | 23.4 (0–8 274 800) |
HER2, human epidermal growth factor receptor 2; Alb, albumin; ALP, alkaline phosphatase; AST, aspartate transaminase; Cre, creatinine; Hb, hemoglobin; LDH, lactate dehydrogenase; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
aGastrectomy, gastrectomy before and after first-line chemotherapy.
| Characteristics . | . | Total (n = 1025) . |
|---|---|---|
| Number of patients, (%) | ||
| Age (median age, range) | 68 (24–89) | |
| Sex | Male | 707 (69.0%) |
| Performance status | 0 | 543 (53.2%) |
| 1 | 384 (37.6%) | |
| 2 | 75 (7.3%) | |
| 3 | 17 (1.7%) | |
| 4 | 2 (0.2%) | |
| Disease status at first chemotherapy | Unresectable | 914 (89.2%) |
| Recurrence | 110 (10.7%) | |
| Unknown | 1 (0.1%) | |
| Time to recurrence ≥6 months | Yes | 67 (6.5%) |
| Adjuvant chemotherapy | Yes | 84 (8.2%) |
| Site of primary | Upper/middle third of the stomach | 808 (78.9%) |
| Lower third of the stomach | 193 (18.8%) | |
| unknown | 24 (2.3%) | |
| Gastrectomya | Yes | 195 (19%) |
| No | 830 (81%) | |
| Histological type | Intestinal | 397 (38.7%) |
| Diffuse | 602 (58.7%) | |
| Unknown | 26 (2.6%) | |
| HER2 status | Positive | 170 (16.6%) |
| Negative | 749 (73.1%) | |
| Unknown | 106 (10.3%) | |
| Location of metastasis | ||
| Liver | 377 (36.8%) | |
| Peritoneum | 471 (46.0%) | |
| Bone | 90 (8.8%) | |
| Number of distant metastasis | 2≧ | 362 (35.3%) |
| Ascites | Yes | 452 (47%) |
| Unknown | 2 (2.0%) | |
| Clinical laboratory data | ||
| Alb | median, (range) g/dl | 3.4 (1.0–5.9) |
| ALP | median, (range) U/l | 270 (116–12 234) |
| AST | median, (range) U/l | 37 (9.0–667) |
| Cre | median, (range) mg/dl | 0.74 (0.26–4.02) |
| Hb | median, (range) g/dl | 11.1(5.9–16.5) |
| LDH | median, (range) U/l | 204 (21–3511) |
| PLT | median, (range) 10^4/μl | 27 (1.7–434) |
| NLR | median, (range) | 3.5 (0.23–99.6) |
| CEA | median, (range) ng/ml | 5.3 (0.05–110 676) |
| CA19-9 | median, (range) U/ml | 23.4 (0–8 274 800) |
| Characteristics . | . | Total (n = 1025) . |
|---|---|---|
| Number of patients, (%) | ||
| Age (median age, range) | 68 (24–89) | |
| Sex | Male | 707 (69.0%) |
| Performance status | 0 | 543 (53.2%) |
| 1 | 384 (37.6%) | |
| 2 | 75 (7.3%) | |
| 3 | 17 (1.7%) | |
| 4 | 2 (0.2%) | |
| Disease status at first chemotherapy | Unresectable | 914 (89.2%) |
| Recurrence | 110 (10.7%) | |
| Unknown | 1 (0.1%) | |
| Time to recurrence ≥6 months | Yes | 67 (6.5%) |
| Adjuvant chemotherapy | Yes | 84 (8.2%) |
| Site of primary | Upper/middle third of the stomach | 808 (78.9%) |
| Lower third of the stomach | 193 (18.8%) | |
| unknown | 24 (2.3%) | |
| Gastrectomya | Yes | 195 (19%) |
| No | 830 (81%) | |
| Histological type | Intestinal | 397 (38.7%) |
| Diffuse | 602 (58.7%) | |
| Unknown | 26 (2.6%) | |
| HER2 status | Positive | 170 (16.6%) |
| Negative | 749 (73.1%) | |
| Unknown | 106 (10.3%) | |
| Location of metastasis | ||
| Liver | 377 (36.8%) | |
| Peritoneum | 471 (46.0%) | |
| Bone | 90 (8.8%) | |
| Number of distant metastasis | 2≧ | 362 (35.3%) |
| Ascites | Yes | 452 (47%) |
| Unknown | 2 (2.0%) | |
| Clinical laboratory data | ||
| Alb | median, (range) g/dl | 3.4 (1.0–5.9) |
| ALP | median, (range) U/l | 270 (116–12 234) |
| AST | median, (range) U/l | 37 (9.0–667) |
| Cre | median, (range) mg/dl | 0.74 (0.26–4.02) |
| Hb | median, (range) g/dl | 11.1(5.9–16.5) |
| LDH | median, (range) U/l | 204 (21–3511) |
| PLT | median, (range) 10^4/μl | 27 (1.7–434) |
| NLR | median, (range) | 3.5 (0.23–99.6) |
| CEA | median, (range) ng/ml | 5.3 (0.05–110 676) |
| CA19-9 | median, (range) U/ml | 23.4 (0–8 274 800) |
HER2, human epidermal growth factor receptor 2; Alb, albumin; ALP, alkaline phosphatase; AST, aspartate transaminase; Cre, creatinine; Hb, hemoglobin; LDH, lactate dehydrogenase; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
aGastrectomy, gastrectomy before and after first-line chemotherapy.
Approximately half of the patients received combination chemotherapy with cisplatin whereas a quarter of the patients received oxaliplatin (Table 2). Second-line chemotherapy was administered to 65.7% of patients, while 33.2% received third-line chemotherapy. The use of trastuzumab, ramucirumab and nivolumab during all periods of chemotherapy was 14.1%, 30.7% and 16.5%, respectively.
| . | . | Number of patients, (%) . |
|---|---|---|
| The regimen of 1st treatment | Cisplatin base | 521 (50.8%) |
| Oxaliplatin base | 245 (23.9%) | |
| Taxane base | 41 (4.0%) | |
| Fluoropyrimidine monotherapy | 198 (19.3%) | |
| Others | 20 (2.0%) | |
| Number of patients | Receiving 2nd chemotherapy | 673 (65.7%) |
| Receiving 3rd chemotherapy | 340 (33.2%) | |
| Receiving more than 4th chemotherapy | 126 (12.3%) | |
| Number of patients | Using Trastuzumab | 145 (14.1%) |
| Using Nivolumab | 169 (16.5%) | |
| Using Ramucirumab | 315 (30.7%) |
| . | . | Number of patients, (%) . |
|---|---|---|
| The regimen of 1st treatment | Cisplatin base | 521 (50.8%) |
| Oxaliplatin base | 245 (23.9%) | |
| Taxane base | 41 (4.0%) | |
| Fluoropyrimidine monotherapy | 198 (19.3%) | |
| Others | 20 (2.0%) | |
| Number of patients | Receiving 2nd chemotherapy | 673 (65.7%) |
| Receiving 3rd chemotherapy | 340 (33.2%) | |
| Receiving more than 4th chemotherapy | 126 (12.3%) | |
| Number of patients | Using Trastuzumab | 145 (14.1%) |
| Using Nivolumab | 169 (16.5%) | |
| Using Ramucirumab | 315 (30.7%) |
| . | . | Number of patients, (%) . |
|---|---|---|
| The regimen of 1st treatment | Cisplatin base | 521 (50.8%) |
| Oxaliplatin base | 245 (23.9%) | |
| Taxane base | 41 (4.0%) | |
| Fluoropyrimidine monotherapy | 198 (19.3%) | |
| Others | 20 (2.0%) | |
| Number of patients | Receiving 2nd chemotherapy | 673 (65.7%) |
| Receiving 3rd chemotherapy | 340 (33.2%) | |
| Receiving more than 4th chemotherapy | 126 (12.3%) | |
| Number of patients | Using Trastuzumab | 145 (14.1%) |
| Using Nivolumab | 169 (16.5%) | |
| Using Ramucirumab | 315 (30.7%) |
| . | . | Number of patients, (%) . |
|---|---|---|
| The regimen of 1st treatment | Cisplatin base | 521 (50.8%) |
| Oxaliplatin base | 245 (23.9%) | |
| Taxane base | 41 (4.0%) | |
| Fluoropyrimidine monotherapy | 198 (19.3%) | |
| Others | 20 (2.0%) | |
| Number of patients | Receiving 2nd chemotherapy | 673 (65.7%) |
| Receiving 3rd chemotherapy | 340 (33.2%) | |
| Receiving more than 4th chemotherapy | 126 (12.3%) | |
| Number of patients | Using Trastuzumab | 145 (14.1%) |
| Using Nivolumab | 169 (16.5%) | |
| Using Ramucirumab | 315 (30.7%) |
Complete and partial responses to first-line chemotherapy were achieved in 1.6% and 35.2% of patients, respectively. The overall response rate was 36.8%. The median OS and PFS of first line chemotherapy was 11.8 months [95% confidence interval (CI), 10.8–12.3 months] and 6.3 months (95% CI, 5.9–6.9 months), respectively.
Prognostic factors
Table 3 shows the results of the univariate and multivariate analyses for survival using patient characteristics and clinical laboratory data at the first-line chemotherapy. Univariate analysis showed a significant association between survival and age < 40 years, PS ≥2, site of primary tumor (UM), no gastrectomy, diffuse histological type, presence of liver/peritoneum/bone/lung metastasis, number of distant metastases ≥2, presence of ascites, Alb <3.6, ALP ≥300, AST ≥30, Cre ≥1.0, LDH ≥250, Hb <11 and NLR >3.0. On the other hand, the HER2-positive status was not. Multivariate analysis was performed, including the 18 variables that were identified by univariate analysis. Prognostic factors for poor survival by multivariate analysis included these eight factors; age < 40 (P = 0.032; HR 1.60; 95% CI,1.04–2.49), PS ≥2 (P < 0.001; HR 2.43; 95% CI, 1.83–3.14), no gastrectomy (P < 0.001; HR 1.75; 95% CI, 1.42–2.17), diffuse histological type (P < 0.001; HR 1.53; 95% CI, 1.30–1.80), Alb <3.6 (P = 0.034; HR 1.21; 95% CI, 1.01–1.44), ALP ≥300 (P = 0.048; HR 1.20; 95% CI, 1.00–1.44), Cre ≥1.0 (P < 0.001; HR 1.48; 95% CI, 1.20–1.81) and NLR >3.0 (P < 0.001; HR 1.33; 95% CI, 1.13–1.56).
| . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|
| Factors . | Category . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age | <40 (vs. ≥40) | 1.83 | 1.25–2.7 | 0.002 | 1.6 | 1.04–2.49 | 0.032 |
| Sex | Male (vs. Female) | 1 | 0.87–1.14 | 0.94 | |||
| Performance Status | 2–4 (vs. 0–1) | 2.96 | 2.39–3.67 | <0.001 | 2.4 | 1.83–3.14 | <0.001 |
| Site of primary | UM (vs. L) | 1.23 | 1.04–1.45 | 0.015 | 1.2 | 0.99–1.45 | 0.058 |
| Gastrectomya | No (vs. Yes) | 2.21 | 1.85–2.63 | <0.001 | 1.75 | 1.42–2.17 | <0.001 |
| Histological type | Diffuse (vs. Intestinal) | 1.34 | 1.18–1.53 | <0.001 | 1.53 | 1.30–1.80 | <0.001 |
| Liver metastasis | Yes (vs. No) | 1.35 | 1.18–1.54 | <0.001 | 1.12 | 0.92–1.38 | 0.26 |
| Peritoneum metastasis | Yes (vs. No) | 1.33 | 1.17–1.52 | <0.001 | 1.16 | 0.96–1.40 | 0.12 |
| Bone metastasis | Yes (vs. No) | 1.39 | 1.11–1.73 | 0.004 | 0.93 | 0.69–1.26 | 0.65 |
| Lung metastasis | Yes (vs. No) | 1.31 | 1.05–1.64 | 0.018 | 1.07 | 0.81–1.42 | 0.62 |
| Number of metastasis | ≧2 (vs. <2) | 1.69 | 1.47–1.93 | <0.001 | 1.08 | 0.88–1.33 | 0.43 |
| Ascites | Yes (vs. No) | 1.58 | 1.38–1.81 | <0.001 | 1.06 | 0.90–1.25 | 0.43 |
| HER2 status | Positive (vs. Negative) | 0.88 | 0.74–1.05 | 0.171 | |||
| Alb | <3.6 (vs. ≧3.6) | 1.7 | 1.47–1.97 | <0.001 | 1.21 | 1.01–1.44 | 0.034 |
| ALP | ≧300 (vs. <300) | 1.46 | 1.27–1.66 | <0.001 | 1.2 | 1.00–1.44 | 0.048 |
| AST | >30 (vs. ≤30) | 1.42 | 1.23–1.63 | <0.001 | 1.09 | 0.90–1.32 | 0.4 |
| Cre | ≧1.0 (vs. <1.0) | 1.43 | 1.18–1.72 | <0.001 | 1.48 | 1.20–1.81 | <0.001 |
| LDH | ≧250 (vs. <250) | 1.46 | 1.27–1.67 | <0.001 | 1.06 | 0.87–1.23 | 0.56 |
| Hb | <11 (vs. ≥11) | 1.27 | 1.11–1.44 | 0.004 | 1.05 | 0.90–1.22 | 0.52 |
| NLR | >3.0 (vs. ≤ 3.0) | 1.69 | 1.47–1.93 | <0.001 | 1.33 | 1.13–1.56 | <0.001 |
| . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|
| Factors . | Category . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age | <40 (vs. ≥40) | 1.83 | 1.25–2.7 | 0.002 | 1.6 | 1.04–2.49 | 0.032 |
| Sex | Male (vs. Female) | 1 | 0.87–1.14 | 0.94 | |||
| Performance Status | 2–4 (vs. 0–1) | 2.96 | 2.39–3.67 | <0.001 | 2.4 | 1.83–3.14 | <0.001 |
| Site of primary | UM (vs. L) | 1.23 | 1.04–1.45 | 0.015 | 1.2 | 0.99–1.45 | 0.058 |
| Gastrectomya | No (vs. Yes) | 2.21 | 1.85–2.63 | <0.001 | 1.75 | 1.42–2.17 | <0.001 |
| Histological type | Diffuse (vs. Intestinal) | 1.34 | 1.18–1.53 | <0.001 | 1.53 | 1.30–1.80 | <0.001 |
| Liver metastasis | Yes (vs. No) | 1.35 | 1.18–1.54 | <0.001 | 1.12 | 0.92–1.38 | 0.26 |
| Peritoneum metastasis | Yes (vs. No) | 1.33 | 1.17–1.52 | <0.001 | 1.16 | 0.96–1.40 | 0.12 |
| Bone metastasis | Yes (vs. No) | 1.39 | 1.11–1.73 | 0.004 | 0.93 | 0.69–1.26 | 0.65 |
| Lung metastasis | Yes (vs. No) | 1.31 | 1.05–1.64 | 0.018 | 1.07 | 0.81–1.42 | 0.62 |
| Number of metastasis | ≧2 (vs. <2) | 1.69 | 1.47–1.93 | <0.001 | 1.08 | 0.88–1.33 | 0.43 |
| Ascites | Yes (vs. No) | 1.58 | 1.38–1.81 | <0.001 | 1.06 | 0.90–1.25 | 0.43 |
| HER2 status | Positive (vs. Negative) | 0.88 | 0.74–1.05 | 0.171 | |||
| Alb | <3.6 (vs. ≧3.6) | 1.7 | 1.47–1.97 | <0.001 | 1.21 | 1.01–1.44 | 0.034 |
| ALP | ≧300 (vs. <300) | 1.46 | 1.27–1.66 | <0.001 | 1.2 | 1.00–1.44 | 0.048 |
| AST | >30 (vs. ≤30) | 1.42 | 1.23–1.63 | <0.001 | 1.09 | 0.90–1.32 | 0.4 |
| Cre | ≧1.0 (vs. <1.0) | 1.43 | 1.18–1.72 | <0.001 | 1.48 | 1.20–1.81 | <0.001 |
| LDH | ≧250 (vs. <250) | 1.46 | 1.27–1.67 | <0.001 | 1.06 | 0.87–1.23 | 0.56 |
| Hb | <11 (vs. ≥11) | 1.27 | 1.11–1.44 | 0.004 | 1.05 | 0.90–1.22 | 0.52 |
| NLR | >3.0 (vs. ≤ 3.0) | 1.69 | 1.47–1.93 | <0.001 | 1.33 | 1.13–1.56 | <0.001 |
UM, upper/middle third of the stomach; L, lower third of the stomach; HER2, human epidermal growth factor receptor 2; Alb, albumin; ALP, alkaline phosphatase; AST, aspartate transaminase; Cre, creatinine; Hb, hemoglobin; LDH, lactate dehydrogenase; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; 95% CI, 95% confidence interval.
aGastrectomy, gastrectomy before and after first-line chemotherapy.
| . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|
| Factors . | Category . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age | <40 (vs. ≥40) | 1.83 | 1.25–2.7 | 0.002 | 1.6 | 1.04–2.49 | 0.032 |
| Sex | Male (vs. Female) | 1 | 0.87–1.14 | 0.94 | |||
| Performance Status | 2–4 (vs. 0–1) | 2.96 | 2.39–3.67 | <0.001 | 2.4 | 1.83–3.14 | <0.001 |
| Site of primary | UM (vs. L) | 1.23 | 1.04–1.45 | 0.015 | 1.2 | 0.99–1.45 | 0.058 |
| Gastrectomya | No (vs. Yes) | 2.21 | 1.85–2.63 | <0.001 | 1.75 | 1.42–2.17 | <0.001 |
| Histological type | Diffuse (vs. Intestinal) | 1.34 | 1.18–1.53 | <0.001 | 1.53 | 1.30–1.80 | <0.001 |
| Liver metastasis | Yes (vs. No) | 1.35 | 1.18–1.54 | <0.001 | 1.12 | 0.92–1.38 | 0.26 |
| Peritoneum metastasis | Yes (vs. No) | 1.33 | 1.17–1.52 | <0.001 | 1.16 | 0.96–1.40 | 0.12 |
| Bone metastasis | Yes (vs. No) | 1.39 | 1.11–1.73 | 0.004 | 0.93 | 0.69–1.26 | 0.65 |
| Lung metastasis | Yes (vs. No) | 1.31 | 1.05–1.64 | 0.018 | 1.07 | 0.81–1.42 | 0.62 |
| Number of metastasis | ≧2 (vs. <2) | 1.69 | 1.47–1.93 | <0.001 | 1.08 | 0.88–1.33 | 0.43 |
| Ascites | Yes (vs. No) | 1.58 | 1.38–1.81 | <0.001 | 1.06 | 0.90–1.25 | 0.43 |
| HER2 status | Positive (vs. Negative) | 0.88 | 0.74–1.05 | 0.171 | |||
| Alb | <3.6 (vs. ≧3.6) | 1.7 | 1.47–1.97 | <0.001 | 1.21 | 1.01–1.44 | 0.034 |
| ALP | ≧300 (vs. <300) | 1.46 | 1.27–1.66 | <0.001 | 1.2 | 1.00–1.44 | 0.048 |
| AST | >30 (vs. ≤30) | 1.42 | 1.23–1.63 | <0.001 | 1.09 | 0.90–1.32 | 0.4 |
| Cre | ≧1.0 (vs. <1.0) | 1.43 | 1.18–1.72 | <0.001 | 1.48 | 1.20–1.81 | <0.001 |
| LDH | ≧250 (vs. <250) | 1.46 | 1.27–1.67 | <0.001 | 1.06 | 0.87–1.23 | 0.56 |
| Hb | <11 (vs. ≥11) | 1.27 | 1.11–1.44 | 0.004 | 1.05 | 0.90–1.22 | 0.52 |
| NLR | >3.0 (vs. ≤ 3.0) | 1.69 | 1.47–1.93 | <0.001 | 1.33 | 1.13–1.56 | <0.001 |
| . | . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|---|
| Factors . | Category . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age | <40 (vs. ≥40) | 1.83 | 1.25–2.7 | 0.002 | 1.6 | 1.04–2.49 | 0.032 |
| Sex | Male (vs. Female) | 1 | 0.87–1.14 | 0.94 | |||
| Performance Status | 2–4 (vs. 0–1) | 2.96 | 2.39–3.67 | <0.001 | 2.4 | 1.83–3.14 | <0.001 |
| Site of primary | UM (vs. L) | 1.23 | 1.04–1.45 | 0.015 | 1.2 | 0.99–1.45 | 0.058 |
| Gastrectomya | No (vs. Yes) | 2.21 | 1.85–2.63 | <0.001 | 1.75 | 1.42–2.17 | <0.001 |
| Histological type | Diffuse (vs. Intestinal) | 1.34 | 1.18–1.53 | <0.001 | 1.53 | 1.30–1.80 | <0.001 |
| Liver metastasis | Yes (vs. No) | 1.35 | 1.18–1.54 | <0.001 | 1.12 | 0.92–1.38 | 0.26 |
| Peritoneum metastasis | Yes (vs. No) | 1.33 | 1.17–1.52 | <0.001 | 1.16 | 0.96–1.40 | 0.12 |
| Bone metastasis | Yes (vs. No) | 1.39 | 1.11–1.73 | 0.004 | 0.93 | 0.69–1.26 | 0.65 |
| Lung metastasis | Yes (vs. No) | 1.31 | 1.05–1.64 | 0.018 | 1.07 | 0.81–1.42 | 0.62 |
| Number of metastasis | ≧2 (vs. <2) | 1.69 | 1.47–1.93 | <0.001 | 1.08 | 0.88–1.33 | 0.43 |
| Ascites | Yes (vs. No) | 1.58 | 1.38–1.81 | <0.001 | 1.06 | 0.90–1.25 | 0.43 |
| HER2 status | Positive (vs. Negative) | 0.88 | 0.74–1.05 | 0.171 | |||
| Alb | <3.6 (vs. ≧3.6) | 1.7 | 1.47–1.97 | <0.001 | 1.21 | 1.01–1.44 | 0.034 |
| ALP | ≧300 (vs. <300) | 1.46 | 1.27–1.66 | <0.001 | 1.2 | 1.00–1.44 | 0.048 |
| AST | >30 (vs. ≤30) | 1.42 | 1.23–1.63 | <0.001 | 1.09 | 0.90–1.32 | 0.4 |
| Cre | ≧1.0 (vs. <1.0) | 1.43 | 1.18–1.72 | <0.001 | 1.48 | 1.20–1.81 | <0.001 |
| LDH | ≧250 (vs. <250) | 1.46 | 1.27–1.67 | <0.001 | 1.06 | 0.87–1.23 | 0.56 |
| Hb | <11 (vs. ≥11) | 1.27 | 1.11–1.44 | 0.004 | 1.05 | 0.90–1.22 | 0.52 |
| NLR | >3.0 (vs. ≤ 3.0) | 1.69 | 1.47–1.93 | <0.001 | 1.33 | 1.13–1.56 | <0.001 |
UM, upper/middle third of the stomach; L, lower third of the stomach; HER2, human epidermal growth factor receptor 2; Alb, albumin; ALP, alkaline phosphatase; AST, aspartate transaminase; Cre, creatinine; Hb, hemoglobin; LDH, lactate dehydrogenase; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; 95% CI, 95% confidence interval.
aGastrectomy, gastrectomy before and after first-line chemotherapy.
Figure 1(a)–(h) showed that each of the eight factors was significantly associated with shorter survival using the Kaplan–Meier method. The median OS with age < 40 was significantly poorer compared with age ≥ 40 (11.9 months vs. 7.2 months; P = 0.002). Additionally, patients with age < 40 showed poorer survival than patients with age ≥ 75 (7.2 months vs. 10.8 months; P = 0.01) (Supplemental Fig. 1). The median OS with PS ≥2 also had poorer survival compared with PS 0-1 (3.8 months vs. 12.4 months; P < 0.001). Moreover, the median OS was 15.6 months in patients with PS 0, 9.1 months in PS1, 4.1 months in PS2, 1.6 months in PS3, 3.5 months in PS4 (Supplemental Fig. 2). Significant differences were observed between PS0 and PS1 (P < 0.001), PS1 and PS2 (P < 0.001) and PS2 and PS3 (P = 0.047).
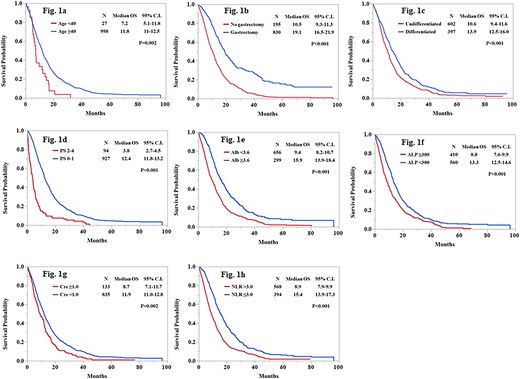
Kaplan–Meier estimates of overall survival according to each prognostic factors. (a) age (b) gastrectomy (c) histological type (d) PS (e) Alb (f) ALP (g) creatinine (h) NLR. OS, overall survival; CI, confidence interval; PS, performance status; Alb, albumin; ALP, alkaline phosphatase; NLR, neutrophil-to-lymphocyte ratio.
Number of prognostic factors
Based on eight prognostic factors, we categorized patients into three groups: the low-risk group (0–2), the moderate-risk group (3,4) and the high-risk group (5–8). Significant differences were observed among them (P < 0.001) (Supplemental Fig. 3). The median OS for the low risk, the moderate risk and the high-risk groups were 18.1, 11.7 and 5.9 months, respectively. The C-index for this classification was 0.64.
HER2 status and the use of trastuzumab
Patients using trastuzumab showed better survival than patients without (16.1 months vs. 11.1 months; P < 0.001). However, HER2-positive status was not associated with survival (positive, 13.1 months; negative, 11.8 months; P = 0.17) [Fig. 2(a) and (b)]. On the contrary, among HER2 positive patients (n = 170), those without trastuzumab treatment had poorer survival than those with (6.1 months vs. 16.0 months; P < 0.001) (Fig. 2(c)).
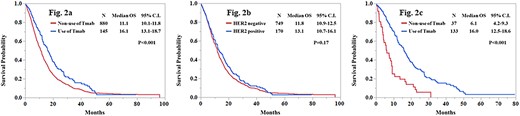
Kaplan–Meier estimates of overall survival according to the use of Tmab (a), HER2 status (b) and the use of Tmab among HER2 positive patients (c). OS, overall survival; CI, confidence interval; Tmab, trastuzumab; HER2, human epidermal growth factor receptor 2.
Discussion
In this retrospective 12 multicenter study, we identified eight significant prognostic factors in patients with AGC receiving chemotherapy: age < 40 years, PS ≥2, no gastrectomy, diffuse histological type, Alb <3.6, ALP ≥30, Cre ≥1.0 and NLR >3.0. This study showed the latest prognostic factors in AGC patients receiving chemotherapy under the current Japanese insurance system, which includes trastuzumab, ramucirumab and nivolumab. To the best of our knowledge, this is the first report to identify prognosticators using real-world data from multiple centers, including specialized cancer hospitals and community hospitals. Before initiating chemotherapy, predicting the prognosis of each patient using these eight reliable factors is necessary to create personalized chemotherapeutic strategies for patients with AGC in the current clinical practice.
We identified age < 40 years as one of the most significant independent prognostic factors. In addition, the prognosis of patients with age < 40 was poorer than that of patients with age ≥ 75 (7.2 months vs. 10.8 months) in appendix analysis. Although the prognosis of young adult patients with gastric cancer is controversial (25,26), our previous study and recent studies have shown that young patients with AGC at an advanced stage have a poor prognosis (22–24). However, very few reports have statistically analyzed the prognosis of young adult patients with AGC eligible for chemotherapy because of the low morbidity of these patients. Multivariate analysis revealed that age < 40 years was an independent prognostic factor for AGC patients before chemotherapy. Although the cause of poor prognosis in young adults has been considered to be poor PS at the time of diagnosis or their diffuse histology (23,24), our results indicate that young adult patients with AGC had poorer prognosis by age, not by the condition or histology. This shows the ineffectiveness of the present chemotherapy regimen for this age group. To ensure effective treatment, early intervention using genome analysis may help prolong the survival of these patients.
Although the efficacy of trastuzumab for HER2-positive AGC patients was demonstrated after the ToGA study (7), it is still controversial as to whether HER2 positivity in AGC is a poor prognostic factor (28,29). This is in contrast to breast cancer, which showed a negative prognostic effect. Thus, our study analyzed the prognostic role of HER2 status as tumor factor of AGC recognized before treatment. In addition, we evaluated the efficacy of trastuzumab for HER2 status. Our analysis showed that trastuzumab is highly effective in patients with HER2-positive AGC, even in the Japanese clinical setting. Using trastuzumab was significantly associated with longer survival than that of patients not using it (16.1 months vs. 11.1 months). In HER2-positive patients, not using trastuzumab led significantly shorter overall survival (16.0 months vs. 6.1 months, P < 0.001). On the other hand, HER2 status was not a prognostic factor in the univariate analysis. These results may be biased by the patient’s condition where trastuzumab could not be used despite being HER2-positive. However, this result also highlighted the importance of using trastuzumab for HER2-positive patients. Further research adjusting for each background factor is required.
The importance of PS as a prognostic factor has been repeatedly proven (13–17,27,30). Here, we affirmed its significance using our real-world practical data. In addition, our appendix analysis showed that the median OS was significantly associated with worsening PS. This highlighted the importance of PS for chemotherapy (the median OS; 15.6 months in PS0, 9.1 months in PS1, 4.1 months in PS2, 1.6 months in PS3). To devise better chemotherapeutic strategies for patients with AGC, we should be aware that even a difference between 0 and 1 PS can greatly affect prognosis. Moreover, patients with PS ≥ 2 have a very poor prognosis, even after receiving chemotherapy. Fundamentally, many of the clinical trials had been set at only PS 0 and 1, and the effectiveness of chemotherapy was not proven at PS ≥2. We should be aware of these when considering treatment plans for patients with poor PS.
Four laboratory tests, such as NLR, ALP, Alb and Cre, taken before initiating chemotherapy are also useful for predicting the prognosis for patients with AGC. The serum levels of ALP and Alb have been identified as prognostic factors in previous studies (13,14,16,31). Meanwhile, NLR seems to be a newly established prognostic factor for various cancer (19–21). In this study using real-world data, these three factors were also identified as significant and useful prognostic factors. In addition, we are the first to show that Cre level is an independent prognostic factor. Few reports have focused on evaluating Cre levels for prognostication. Although this result may be derived from the close association between renal function and the selection of treatment strategy for AGC, clinicians need to consider this when starting and managing chemotherapy for patients with poor renal function.
We also demonstrated that a number of prognostic factors could be utilized to categorize patients into three groups, in a manner similar to some previous studies (13–18,27,30). Using the subjects of our study, we compared the ability of prognostic classification between our index and modified JCOG index (17), which was latest index before our study (Supplemental Fig. 4). Similar to the result of our index, it also showed a good stratification that the median OS of the low, moderate and high-risk group were 5.7, 9.6 and 18.5 months, respectively. However, the survival curve for the moderate risk group was not centrally positioned between that of low- and high-risk group. There was a difference of 4 months in the median OS between the low- and moderate-risk groups, and a difference of 9 months between the moderate and high-risk groups. On the other hand, according to our index, the difference in median OS among the three groups is ⁓6 months each. Furthermore, our index was able to extract a larger number of high-risk patients who required attention during chemotherapy, compared with the modified JCOG index; 199 patients vs 166 patients. We analyzed the C-index using the modified JCOG index and our classification method to assess their statistical validity. Both yielded results of 0.65 and 0.64, respectively, which were not particularly good. This highlights the difficulty of accurately predicting prognosis solely based on the prognostic factors identified at present, indicating the need for further investigation to achieve accurate prognostic predictions.
Our study has some strengths and novelties. First, there is no report to identify the prognosis of AGC using clinical data from multiple centers, including specialized cancer hospitals and community hospitals. Previous reports had been limited to sub-analysis of clinical trials or retrospective studies in a single specialized cancer hospital. We investigated and reviewed over 1000 patient’s detailed clinical course from medical records and collected many clinical parameters in a database dedicated to this study. These data are closest to that of actual clinical practice compared with previous reports. In fact, the median age of patients started chemotherapy was 68 years, and PS2 or higher was included in ⁓10%. Second, this is the first study to analyze the prognostic role of age by dividing it by 40 years or younger, not the elderly, in patients with AGC. As a result, age < 40 years was an independent prognostic factor for AGC patients. Recently, adolescents and young adults (15–39 years) with cancer is important issue in recent years (32). Our sub-analysis also indicated the prognosis of patients with age < 40 was poorer than that of patients with age ≥ 75. Third, this study reflects the current reality of chemotherapy for AGC. Chemotherapy for the AGC patients is changing year after year. Nevertheless, well-known prognostic factors, such as PS, histological type and no gastrectomy, were unchanging with previous studies, in spite of the differences in the patients’ condition and environment or era. Thus, our study proved the importance of these factors, again.
The limitations of this study must be addressed. First, it is retrospective. Second, it is impossible to eliminate the effects of therapeutic factors associated with the overall survival of AGC patients. Our study included various treatment regimens for first-line chemotherapy to reveal the real-world data of patients with AGC. Third, the available drugs for AGC patients were changed during the analysis period. Under the Japanese insurance system, trastuzumab, ramucirumab and nivolumab were used in 2011, 2015 and 2017, respectively. Fourth, our data cannot include the most recent topic in which nivolumab was added as a first-line treatment. This may alter the effectiveness of chemotherapy in the future.
In conclusion, we showed the current reality of chemotherapy for patients with AGC under the Japanese insurance system at 12 centers, including specialized cancer hospitals and community hospitals. We identified eight prognostic factors for patients with AGC receiving chemotherapy in clinical practice. In particular, young adults with AGC were proven to be a newly important prognostic factor. The high efficacy of trastuzumab has also been demonstrated in our setting. We believe that our analysis using real-world data is very meaningful in confirming the true efficacy of chemotherapy for AGC in practice and in helping clinicians set a treatment strategy for each patient.
Conflict of interest
The authors declare that they have no conflicts of interest with respect to this study.
References
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines, Vol. 2010, 3rd edition,
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines, Vol. 2017, 4th edition
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines Vol. 2018, 5th edition
Japanese Gastric Cancer Association.


