-
PDF
- Split View
-
Views
-
Cite
Cite
Kozaburo Tanuma, Koji Kawai, Satoshi Nitta, Masanobu Shiga, Takashi Kawahara, Hiromitsu Negoro, Mizuki Onozawa, Takamitsu Inoue, Hiroyuki Nishiyama, Jun Miyazaki, Improved survival of poor-risk non-seminomatous germ cell tumor patients: real-world data from a single institute in Japan, Japanese Journal of Clinical Oncology, Volume 53, Issue 1, January 2023, Pages 74–79, https://doi.org/10.1093/jjco/hyac151
Close - Share Icon Share
Abstract
The International Germ Cell Cancer Collaborative Group Update Consortium showed the improved survival of patients with a non-seminomatous germ cell tumor. We updated the survival data of the non-seminomatous germ cell tumor patients treated at our hospital.
We analyzed the outcomes of 138 patients treated in 1981–2018. We compared the survival of the patients treated in the early (1981–99) and later (2000–18) periods and determined the groups’ progression-free survival and overall survival using the Kaplan–Meier method. We used a web-based application of the International Germ Cell Cancer Collaborative Group Update model to calculate each patient’s predicted 3-year progression-free survival.
The 5-year progression-free survival rates of the good, intermediate and poor prognosis groups were 91, 83 and 64%, and their 5-year overall survival rates were 97, 89 and 82%, respectively. There were no significant differences in the progression-free survival or overall survival of the good and intermediate prognosis groups by treatment year. The 5-year progression-free survival of the poor prognosis group was almost identical in both treatment year (60 and 65%, respectively). By contrast, the 5-year overall survival in the later period (85%) was higher than that in the early period (70%). The median-predicted 3-year progression-free survival rates of the good, intermediate and poor prognosis groups were 92, 83 and 51% (P < 0.01), respectively. The concordance index for the good, intermediate and poor prognosis groups were 0.56, 0.79 and 0.67, respectively.
The survival of our poor prognosis non-seminomatous germ cell tumor patients improved over time. The 5-year overall survival of patients treated in 2000–18 reached 85%.
Introduction
Testicular germ cell tumor (GCT) is common in young men and is highly curable when appropriately treated by intensive chemotherapy and surgery. The prognosis of patients with an advanced testicular tumor has improved in recent decades and several factors are thought to be responsible for this progress, including the widespread use of first-line therapy with bleomycin, etoposide and cisplatin (BEP), the introduction of effective salvage chemotherapy and appropriate surgical intervention (1–3). Multiple investigations have also demonstrated that clinicians’ centralized cumulative experience was related to better treatment outcomes, especially in patients with poor prognoses (4–6).
The International Germ Cell Cancer Collaborative Group (IGCCCG) classification has been widely used to tailor the treatment of patients with advanced GCT since 1997 (7). According to the original IGCCCG data, the 5-year overall survival (OS) rates of good, intermediate and poor prognosis patients were 92, 80 and 48%, respectively (7). That classification was based on the data of GCT patients treated between 1975 and 1990, and thus a substantial number of patients were treated with older regimens that did not contain etoposide (7). With the widespread use of BEP since 1987, the treatment outcomes of GCT have improved, especially among poor prognosis patients (1). Based on this background, the IGCCCG Update Consortium collected data on 9728 men with metastatic non-seminomatous germ cell tumors (NSGCTs) treated between 1990 and 2013 (8), and the Consortium reported that the survival of NSGCT patients has indeed improved; in particular, the 5-year OS of NSGCT patients with a poor prognosis increased to 67% from 48% (8). The Consortium also developed the IGCCCG Update model to improve the individual prognostication for NSGCT patients. Using this model, an individual patient’s predicted 3-year progression-free survival (PFS) can be easily determined with an online calculator (9).
In 2006, we analyzed the outcome of 74 patients with advanced GCT who were treated at the Tsukuba University Hospital (TUH), and we reported that the 5-year PFS among the patients with a good, intermediate or poor prognosis was 90, 70 and 64%, respectively (10). In the present study, we collected the data of 138 patients with metastatic NSGCT who were treated at TUH during the period from 1981 and 2018 to determine whether the patient OS and PFS improved over time. In addition, using the IGCCCG Update model, we analyzed the distribution of the predicted 3-year PFS rates of the patients who were in each prognosis group based on the original IGCCCG classification.
Patients and methods
Patients
Between January 1981 and August 2018, 138 patients with metastatic NSGCT began treatment at TUH. Of them, 38 patients were treated during the early period (i.e. 1981–99), and the remaining 100 patients were treated during the later period (2000–18). The characteristics of the patients and treatment procedures are summarized in Table 1: 134 patients had metastatic testicular cancer, and the remaining 4 patients (2.9%) had mediastinal germ cell cancer. The median age at diagnosis was 31 years (range: 17–66 years). The patients had been classified into the relevant prognostic category according to the original IGCCCG classification (7): 36 patients (26.1%) were classified as having good prognosis disease, 42 patients (30.4%) as intermediate and 60 patients (43.5%) as were classified as having poor prognosis disease. As shown in Fig. 1, the proportion of patients with a poor prognosis increased from 26% in the early period to 50% in the later period.
The characteristics and treatment procedures of 138 patients treated for metastatic non-seminomatous germ cell tumor between 1981 and 2018 at Tsukuba University Hospital
| Baseline characteristics . | n (%) . | Treatment profile . | n (%) . |
|---|---|---|---|
| Age | First-line chemotherapy | 138 (100.0) | |
| Median (range) | 31 (17–66) | PVB | 19 (13.8) |
| Original IGCCCG groups | BEP | 103 (74.6) | |
| Good | 36 (26.1) | VIP | 10 (7.2) |
| Intermediate | 42 (30.4) | EP | 6 (4.3) |
| Poor | 60 (43.5) | Second-line chemotherapy | 63 (45.7) |
| Presence or absence of progression | TIP | 50 (36.2) | |
| No progression | 103 (74.6) | HDCT | 7 (5.1) |
| Progression | 35 (25.4) | VIP | 2 (1.4) |
| Overall survival | Others | 4 (2.9) | |
| Alive | 121 (87.7) | Third-line chemotherapy | 23 (16.7) |
| Death | 17 (12.3) | GEMOX | 11 (8.0) |
| Primary sites | HDCT | 4 (2.9) | |
| Testis | 134 (97.1) | CPT11 + NDP/CDDP | 3 (2.2) |
| Mediastinum | 4 (2.9) | Others | 5 (3.6) |
| Visceral metastases | Fourth-line chemotherapy | 12 (8.7) | |
| Lung metastases | 76 (55.1) | CPT11 + NDP/CDDP | 9 (6.5) |
| NPVM | 36 (26.1) | Others | 3 (2.2) |
| Pretreatment AFP levels | Post-chemotheraphy surgery | 91 (66.0) | |
| <1000 ng/ml | 103 (74.6) | RPLND | 64 (46.4) |
| 1000–10 000 ng/ml | 25 (18.1) | Thoracotomy | 28(20.3) |
| >10 000 ng/ml | 10 (7.3) | Others | 13 (9.4) |
| Pretreatment hCG levels | |||
| <5000 IU/l | 90 (65.2) | ||
| 5000–50 000 IU/l | 17 (12.3) | ||
| >50 000 IU/l | 31 (22.5) | ||
| Pretreatment LDH levels | |||
| ≦ 2.5 × ULN | 82 (59.4) | ||
| >2.5 × ULN | 56 (40.6) | ||
| Baseline characteristics . | n (%) . | Treatment profile . | n (%) . |
|---|---|---|---|
| Age | First-line chemotherapy | 138 (100.0) | |
| Median (range) | 31 (17–66) | PVB | 19 (13.8) |
| Original IGCCCG groups | BEP | 103 (74.6) | |
| Good | 36 (26.1) | VIP | 10 (7.2) |
| Intermediate | 42 (30.4) | EP | 6 (4.3) |
| Poor | 60 (43.5) | Second-line chemotherapy | 63 (45.7) |
| Presence or absence of progression | TIP | 50 (36.2) | |
| No progression | 103 (74.6) | HDCT | 7 (5.1) |
| Progression | 35 (25.4) | VIP | 2 (1.4) |
| Overall survival | Others | 4 (2.9) | |
| Alive | 121 (87.7) | Third-line chemotherapy | 23 (16.7) |
| Death | 17 (12.3) | GEMOX | 11 (8.0) |
| Primary sites | HDCT | 4 (2.9) | |
| Testis | 134 (97.1) | CPT11 + NDP/CDDP | 3 (2.2) |
| Mediastinum | 4 (2.9) | Others | 5 (3.6) |
| Visceral metastases | Fourth-line chemotherapy | 12 (8.7) | |
| Lung metastases | 76 (55.1) | CPT11 + NDP/CDDP | 9 (6.5) |
| NPVM | 36 (26.1) | Others | 3 (2.2) |
| Pretreatment AFP levels | Post-chemotheraphy surgery | 91 (66.0) | |
| <1000 ng/ml | 103 (74.6) | RPLND | 64 (46.4) |
| 1000–10 000 ng/ml | 25 (18.1) | Thoracotomy | 28(20.3) |
| >10 000 ng/ml | 10 (7.3) | Others | 13 (9.4) |
| Pretreatment hCG levels | |||
| <5000 IU/l | 90 (65.2) | ||
| 5000–50 000 IU/l | 17 (12.3) | ||
| >50 000 IU/l | 31 (22.5) | ||
| Pretreatment LDH levels | |||
| ≦ 2.5 × ULN | 82 (59.4) | ||
| >2.5 × ULN | 56 (40.6) | ||
IGCCCG, International Germ Cell Cancer Collaborative Group; PVB, bleomycin, vinblastine and cisplatin; BEP, bleomycin, etoposide, and cisplatin; VIP, etoposide, ifosfamide, and cisplatin; EP, etoposide and cisplatin; TIP, paclitaxel, ifosfamide, and cisplatin; HDCT, high-dose chemotherapy; GEMOX, gemcitabine and oxaliplatin; CPT11, irinotecan; NDP, nedaplatin; CDDP, cisplatin; NPVM, non-pulmonary visceral metastases; AFP, alpha-fetoprotein; hCG, human chorionic gonadotropin; LDH, lactate dehydrogenase; ULN, upper limit of normal; RPLND, retroperitoneal lymph node dissection.
The characteristics and treatment procedures of 138 patients treated for metastatic non-seminomatous germ cell tumor between 1981 and 2018 at Tsukuba University Hospital
| Baseline characteristics . | n (%) . | Treatment profile . | n (%) . |
|---|---|---|---|
| Age | First-line chemotherapy | 138 (100.0) | |
| Median (range) | 31 (17–66) | PVB | 19 (13.8) |
| Original IGCCCG groups | BEP | 103 (74.6) | |
| Good | 36 (26.1) | VIP | 10 (7.2) |
| Intermediate | 42 (30.4) | EP | 6 (4.3) |
| Poor | 60 (43.5) | Second-line chemotherapy | 63 (45.7) |
| Presence or absence of progression | TIP | 50 (36.2) | |
| No progression | 103 (74.6) | HDCT | 7 (5.1) |
| Progression | 35 (25.4) | VIP | 2 (1.4) |
| Overall survival | Others | 4 (2.9) | |
| Alive | 121 (87.7) | Third-line chemotherapy | 23 (16.7) |
| Death | 17 (12.3) | GEMOX | 11 (8.0) |
| Primary sites | HDCT | 4 (2.9) | |
| Testis | 134 (97.1) | CPT11 + NDP/CDDP | 3 (2.2) |
| Mediastinum | 4 (2.9) | Others | 5 (3.6) |
| Visceral metastases | Fourth-line chemotherapy | 12 (8.7) | |
| Lung metastases | 76 (55.1) | CPT11 + NDP/CDDP | 9 (6.5) |
| NPVM | 36 (26.1) | Others | 3 (2.2) |
| Pretreatment AFP levels | Post-chemotheraphy surgery | 91 (66.0) | |
| <1000 ng/ml | 103 (74.6) | RPLND | 64 (46.4) |
| 1000–10 000 ng/ml | 25 (18.1) | Thoracotomy | 28(20.3) |
| >10 000 ng/ml | 10 (7.3) | Others | 13 (9.4) |
| Pretreatment hCG levels | |||
| <5000 IU/l | 90 (65.2) | ||
| 5000–50 000 IU/l | 17 (12.3) | ||
| >50 000 IU/l | 31 (22.5) | ||
| Pretreatment LDH levels | |||
| ≦ 2.5 × ULN | 82 (59.4) | ||
| >2.5 × ULN | 56 (40.6) | ||
| Baseline characteristics . | n (%) . | Treatment profile . | n (%) . |
|---|---|---|---|
| Age | First-line chemotherapy | 138 (100.0) | |
| Median (range) | 31 (17–66) | PVB | 19 (13.8) |
| Original IGCCCG groups | BEP | 103 (74.6) | |
| Good | 36 (26.1) | VIP | 10 (7.2) |
| Intermediate | 42 (30.4) | EP | 6 (4.3) |
| Poor | 60 (43.5) | Second-line chemotherapy | 63 (45.7) |
| Presence or absence of progression | TIP | 50 (36.2) | |
| No progression | 103 (74.6) | HDCT | 7 (5.1) |
| Progression | 35 (25.4) | VIP | 2 (1.4) |
| Overall survival | Others | 4 (2.9) | |
| Alive | 121 (87.7) | Third-line chemotherapy | 23 (16.7) |
| Death | 17 (12.3) | GEMOX | 11 (8.0) |
| Primary sites | HDCT | 4 (2.9) | |
| Testis | 134 (97.1) | CPT11 + NDP/CDDP | 3 (2.2) |
| Mediastinum | 4 (2.9) | Others | 5 (3.6) |
| Visceral metastases | Fourth-line chemotherapy | 12 (8.7) | |
| Lung metastases | 76 (55.1) | CPT11 + NDP/CDDP | 9 (6.5) |
| NPVM | 36 (26.1) | Others | 3 (2.2) |
| Pretreatment AFP levels | Post-chemotheraphy surgery | 91 (66.0) | |
| <1000 ng/ml | 103 (74.6) | RPLND | 64 (46.4) |
| 1000–10 000 ng/ml | 25 (18.1) | Thoracotomy | 28(20.3) |
| >10 000 ng/ml | 10 (7.3) | Others | 13 (9.4) |
| Pretreatment hCG levels | |||
| <5000 IU/l | 90 (65.2) | ||
| 5000–50 000 IU/l | 17 (12.3) | ||
| >50 000 IU/l | 31 (22.5) | ||
| Pretreatment LDH levels | |||
| ≦ 2.5 × ULN | 82 (59.4) | ||
| >2.5 × ULN | 56 (40.6) | ||
IGCCCG, International Germ Cell Cancer Collaborative Group; PVB, bleomycin, vinblastine and cisplatin; BEP, bleomycin, etoposide, and cisplatin; VIP, etoposide, ifosfamide, and cisplatin; EP, etoposide and cisplatin; TIP, paclitaxel, ifosfamide, and cisplatin; HDCT, high-dose chemotherapy; GEMOX, gemcitabine and oxaliplatin; CPT11, irinotecan; NDP, nedaplatin; CDDP, cisplatin; NPVM, non-pulmonary visceral metastases; AFP, alpha-fetoprotein; hCG, human chorionic gonadotropin; LDH, lactate dehydrogenase; ULN, upper limit of normal; RPLND, retroperitoneal lymph node dissection.
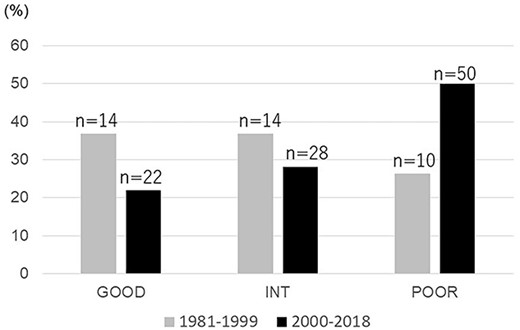
Distribution of the IGCCCG prognostic groups among the present patients according to the period during which they were treated at Tsukuba University Hospital for a non-seminomatous germ cell tumor (NSGCT) (1981–99 vs. 2000–18).
Treatment
As shown in Table 1, 103 (74.6%) patients received BEP as the induction chemotherapy (11). Nineteen patients received bleomycin, vinblastine and cisplatin (12). All of these patients were treated before 1993. The remaining 16 patients received etoposide, ifosfamide and cisplatin (VIP) (13) or etoposide and cisplatin (14) as an alternative induction chemotherapy. In principle, the chemotherapy program included three courses of BEP for good prognosis patients and four courses of BEP for intermediate and poor prognosis patients.
When a patient’s response to the initial induction chemotherapy was unfavorable, second-line chemotherapy was started with the subsequent treatment cycle as consolidation therapy, and this treatment was repeated until the normalization of tumor markers was observed. Disease progression was defined as increases in tumor markers or the development of new or enlarging metastases on radiological examinations. If disease progression with an increased tumor marker was observed during or after induction chemotherapy, second-line chemotherapy was performed as salvage therapy.
Consequently, 63 (45.7%) patients received second-line chemotherapy in which the most frequently used regimen was paclitaxel, ifosfamide and cisplatin (TIP) (15,16). The other second-line or salvage chemotherapy used was high-dose chemotherapy (17,18), VIP or others (Table 1). Twenty-three patients (16.7%) received third-line and 12 patients (8.7%) received fourth-line chemotherapy, which included gemcitabine and oxaliplatin (GEMOX) (19) or irinotecan and nedaplatin (20).
Patients underwent the surgical resection of residual operable masses when all tumor markers were normalized by chemotherapy, but surgery was not performed on patients with adequately responding retroperitoneal lymph node (RPLN) masses (<1 cm in dia.). As a result, 91 patients (66.0%) underwent post-chemotherapy surgery; the most frequently performed surgery was RPLN dissection, followed by thoracotomy.
Calculation of the predicted 3-year PFS
For the calculation of the predicted 3-year PFS of each patient, we used the online calculator (9) based on the results of the IGCCCG Update analysis. The valuables used for the calculation were the patient’s age at diagnosis, site of primary, the presence/absence of lung metastases or non-pulmonary visceral metastases, the alpha-fetoprotein and human chorionic gonadotropin levels and the presence/absence of a lactate dehydrogenase value >2.5× the upper limit of normal.
Statistical analyses
Survival curves were constructed by the Kaplan–Meier method, and the differences among groups were compared using the log-rank test. The distributions of the predicted 3-year PFS of patients in each paired group were compared by the Mann–Whitney U-test. The ability of the predicted 3-year PFS to predict clinical PFS of each prognosis group was assessed with calculation of Harrell’s concordance index (c-index). The levels of significance was set at P < 0.05. Statistical analyses were performed using Jump software (SAS, Cary, NC).
Ethical considerations
The study protocol and data processing were approved by the TUH Ethical Board (R04-097).
Results
Oncological outcomes according to original IGCCCG
Overall, 35 (25.4%) of the 138 patients suffered from disease progression. The original IGCCCG risk groups of these patients were poor prognosis in 23 patients (65.7%), intermediate prognosis in 9 patients and good prognosis in 3 patients. The disease state of 30 patients (85.7%) progressed with germ cell cancer, that of 4 patients progressed with a growing teratoma and that of 1 patient progressed with a primitive neuroectodermal tumor, which was considered a malignant somatic transformation arising from a teratoma. Among the 35 patients who experienced disease progression, 19 patients were successfully salvaged with chemotherapy and/or surgery. The remaining 16 patients (11 with poor prognosis disease and 5 with intermediate prognosis disease) died due to their cancer. One successfully salvaged patient died from another disease 25 years after his last GCT treatment. Another patient died from a treatment-related complication without disease progression. Over a median follow-up of 103 months (range: 24–405 months), 120 of the 138 patients survived.
The Kaplan–Meier curves for the patients’ PFS and OS according to IGCCCG are presented in Fig. 2. The patients with good prognosis disease had a 91% 5-year PFS rate; the corresponding values of the intermediate and poor prognosis groups were 83 and 64%, respectively. The differences among the three prognosis groups were significant (P = 0.005). The respective 5-year OS rates of the good and intermediate prognosis groups were 97 and 89% and that of the poor prognosis group was 82%; there were no significant differences in the 5-year OS rates among the three groups.

(A) The progression-free survival (PFS) and (B) overall survival (OS) of the patients according to the IGCCCG classification.
Oncological outcomes according to treatment year
We compared the survival of the NSGCT patients treated during the early period (1981–99) with that of the patients treated during the later period (2000–18). The 5-year PFS and 5-year OS rates of the good prognosis group treated in the early period were 94 and 94%, and the corresponding rates of the patients treated in the later period were 89 and 100%, respectively. The 5-year PFS and 5-year OS rates of intermediate prognosis patients treated during the earlier period were 79 and 85% and those of the patients treated during the later period were 86 and 92%, respectively; there was no significant difference in the 5-year PFS or OS rates between the two treatment periods.
As shown in Fig. 3, the 5-year PFS of the poor prognosis group was almost identical in both treatment year (60% in the earlier period and 65% in the latter period, respectively). By contrast, the 5-year OS in the later period was 85%, which was higher than that in the early period (70%). But, the difference was not significant (P = 0.2).
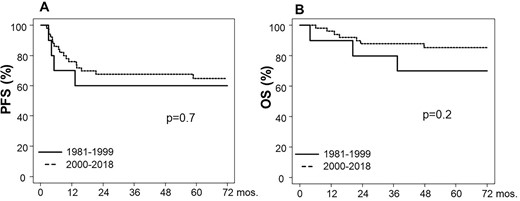
(A) PFS and (B) OS of the poor prognosis NSGCT patients according to treatment period.
Distribution of the predicted 3-year PFS rates according to the IGCCCG update
Figure 4A illustrates the distribution of the predicted 3-year PFS values among the original IGCCCG prognostic groups. In the good prognosis group, the median-predicted 3-year PFS was 92% and the distribution was relatively homogenous, ranging from 79 to 96%. The median-predicted 3-year PFS rates of the intermediate and poor prognosis groups were 83 and 51%, respectively, and there were significant differences in the predicted 3-year PFS of each original IGCCCG prognostic group (P < 0.001). In the poor prognosis group, the distribution of the predicted 3-year PFS values was particularly heterogenous, ranging from 7 to 85%.
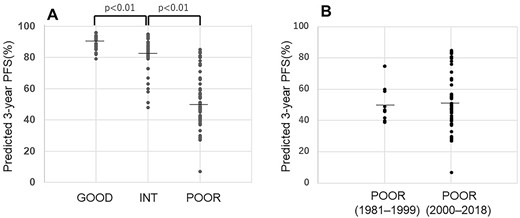
(A) The predicted 3-year PFS according to the IGCCCG prognostic groups. (B) The predicted 3-year PFS of the poor prognosis patients according to the treatment period.
We next compared the predicted the 3-year PFS between the earlier and later treatment periods. The median-predicted the 3-year PFS of the good prognosis patients in each treatment period were 92 and 91%, and those of the intermediate prognosis patients were 83 and 83%, respectively. Also, as shown in Fig. 4B, the median-predicted 3-year PFS of the poor prognosis patients was almost same for both treatment periods, at 48% and 51%.
Finally, the ability of the predicted 3-year PFS to predict the clinical PFS of each prognosis group was assessed with calculation of Harrell’s c-index. The c-index for the good, intermediate and poor prognosis groups were 0.56, 0.79 and 0.67, respectively.
Discussion
We analyzed the oncological outcomes of patients with advanced NSGCT who were treated at our institution over a 38-year period, and the results demonstrated that the 5-year OS rates of the patients with a good, intermediate or poor prognosis were 97, 89 and 82%, respectively. The 5-year OS rates of the good and intermediate prognosis groups are almost identical to the survival data of the IGCCCG Update Consortium (8). By contrast, the 5-year OS rate of the poor prognosis patients in our series was 82%, which is somewhat better than the IGCCCG update data in which the 5-year OS rate of poor prognosis patients is 69% (8). However, it is not appropriate to compare the outcomes of our present study and that of the IGCCCG Update Consortium (8) because of the different study scales and study periods. Rather, our results are consistent with a retrospective study of NSGCT patients treated at five university hospitals in Japan, which showed a 5-year OS rate of 83% for 67 poor prognosis testicular cancer patients treated between 2000 and 2010 (21).
We next compared the survival of the patients treated at our hospital in the early period and those treated in a later period, and our analysis revealed no significant difference in the survival of the good and intermediate groups between the two treatment periods. However, the 5-year OS of the patients with a poor prognosis rose from 70% in the early period to 85% in the later period. We consider multiple factors responsible for this improvement among poor prognosis patients.
First, as shown in Fig. 1, the proportion of patients with a poor prognosis increased from 26% in the early period to 50% in the later period. This is due to our risk-adapted management through a regional medical network involving the coordination of TUH and four key branch hospitals in Ibaraki Prefecture (22). In this system, patients with poor prognosis disease and patients who have risk factors for chemotherapy are principally referred to TUH at the start of induction chemotherapy. Such centralized cumulative experience is known to be related to better treatment outcome, especially in patients with poor prognosis (4–6).
Second, we introduced TIP as salvage chemotherapy in 2000, when the efficacy of TIP as a second-line chemotherapy was reported (15,16). TIP has remained our hospital’s standard second-line chemotherapy for NSGCT. During this study’s earlier period, 10 patients suffered from disease progression. Of them, no patient received TIP as a second-line chemotherapy. By contrast, 21 (84%) of the 25 progressed patients in the later period received TIP as a second-line chemotherapy. As a third-line or fourth-line chemotherapy, 12 (48%) of the progressed patients in the later period were treated with GEMOX or irinotecan and nedaplatin; by contrast, in the early period, only 1 patient (10%) received chemotherapy with those modern regimens. It is thus possible that the accumulation of experience with the use of modern second-line or fourth-line chemotherapy and post-chemotherapy surgery has contributed to the improvement of treatment outcomes revealed herein. Yamashita et al. also reported that the OS of poor prognosis testicular cancer patients between 2000 and 2019 improved compared with that between 1980 and 1999 in a single Japanese institute (23).
The IGCCCG Update Consortium suggests that the original IGCCCG classification can remain a reference standard for treatment decisions in daily practice because the classification still distinguishes three prognostic groups among patients with metastatic NSGCT (8). The Consortium also developed the IGCCCG Update model, which allows more granular individual prognostications. The model added two new variables: the presence of lung metastases and the patient’s age (8). As shown in Fig. 4A, the distribution of the predicted 3-year PFS rates calculated by a web-based application was significantly different among the three original IGCCCG prognostic groups. It is also notable that the distribution of the predicted 3-year PFS rates among patients with poor prognosis disease was extremely heterogenous, ranging from 7 to 85%. The c-index of the predicted 3-year PFS for intermediate prognosis group was high as 0.79, and this was followed by poor prognosis groups (0.67). Therefore, the predicted 3-year PFS showed well discrimination in both groups.
We next compared the predicted 3-year PFS between two treatment periods with the speculation that patients who were treated during the later period might be a more favorable risk group due to earlier diagnoses and better diagnostic tools. However, we observed no significant difference in the 3-year PFS rate between the earlier and later periods in the good and intermediate prognosis groups. In addition, the median-predicted 3-year PFS rate of the poor prognosis patients was almost the same in each treatment period, i.e. 49 and 51% (Fig. 4B). These findings support the possibility that the improved survival (especially among the patients with a poor prognosis) is largely due to advances in disease management rather than to a migration of the disease stage from the earlier diagnoses.
In conclusion, the survival of the poor prognosis NSGCT patients treated at our hospital has improved over time since 1981. The 5-year OS of the patients with a poor prognosis treated during the years from 2000 to 2018 reached 85%. It is possible that the accumulation of experience with the use of modern salvage chemotherapy and post-chemotherapy surgery contributed to the improvement of treatment outcomes.
Conflict of interest statement
None declared.


