-
PDF
- Split View
-
Views
-
Cite
Cite
Nobuyuki Nakajima, Akira Miyajima, Nobuo Shinohara, Wataru Obara, Tsunenori Kondo, Go Kimura, Haruki Kume, Hiroyuki Fujimoto, Takayuki Sugiyama, Norio Nonomura, Fumiya Hongo, Tomoharu Fukumori, Masayuki Takahashi, Hiro-omi Kanayama, Masatoshi Eto, Risk factors for recurrence after operation in patients with pT1a renal cell carcinoma: sub-analysis of the multi-institutional national database of the Japanese Urological Association, Japanese Journal of Clinical Oncology, Volume 52, Issue 3, March 2022, Pages 274–280, https://doi.org/10.1093/jjco/hyab201
Close - Share Icon Share
Abstract
More patients with renal cell carcinoma are now diagnosed with the disease in its early stages. Although patients with pT1a renal cell carcinoma have a good prognosis and low recurrence rate, a few patients still experience recurrence. Herein, we evaluated the clinicopathological risk factors for postoperative recurrence of pT1aN0M0 renal cell carcinoma.
An renal cell carcinoma survey was conducted by the Japanese Urological Association to register newly diagnosed cases of renal cell carcinoma. A total of 1418 patients diagnosed with pT1aN0M0 renal cell carcinoma who underwent surgery as the primary surgical treatment were included. We analyzed the recurrence-free survival using the Kaplan–Meier method and clinicopathological factors for recurrence using Cox proportional hazards models.
Among 1418 patients, 58 (4.1%) had recurrences after a median follow-up of 62.8 months. The median time to recurrence was 31.0 months. Metastases to the lungs and the bone were observed in 20 and 10 cases, respectively. Significant differences in sex, tumor size, Eastern Cooperative Oncology Group performance status, and dialysis history, preoperative hemoglobin levels, C-reactive protein levels and creatinine levels were observed between the recurrence and non-recurrence groups. Multivariate analysis identified male sex, high C-reactive protein level and tumor size ≥3 cm as independent risk factors. The 5-year recurrence-free survival of patients with 0, 1, 2 and 3 risk factors was 99.0, 97.2, 93.1 and 80.7%, respectively.
Male sex, tumor diameter and a high C-reactive protein level were independent recurrence risk factors for pT1a renal cell carcinoma; special attention should be paid to patients with these risk factors during postoperative follow-up.
Introduction
Currently, most renal cell carcinomas (RCCs) are identified in the early stage incidentally during health evaluation using ultrasound or computed tomography (T1a) (1,2). Generally, T1aN0M0 RCC has favorable pathological characteristics and prognosis. It has been reported that the 5-year recurrence-free survival rates of pT1a RCC are ~88.1–97.2% (3–7). The American Urological Association guidelines recommend active surveillance as one of the options for the management of localized renal masses (8).
On the other hand, distant metastases have been observed in some aggressive types of small renal cell carcinomas. Kume et al. reported that microvascular invasion (MVI) was an independent risk factor for recurrence in RCCs ≤3 cm in size (1). In addition, Takayama et al. reported that symptomatic cancer, a sarcomatoid component and a C-reactive protein (CRP) level ≥ 0.4 mg/dl were independent risk factors for cT1a RCC recurrence (9).
Identifying the risk factors for recurrence of RCC after nephrectomy is essential for patient counseling and the development of evidence-based surveillance programs. In the present study, we evaluated the clinicopathological risk factors for postoperative recurrence of pT1aN0M0 RCC using a subgroup of the RCC registry of The Cancer Registration Committee of the Japanese Urological Association. To the best of our knowledge, our study uses the largest sample size, compared with other such studies, to investigate the risk factors for pT1aN0M0 RCC recurrence in the Japanese population.
Patients and methods
The Cancer Registration Committee of the Japanese Urological Association conducted an RCC registry program in 2012. A total of 3663 cases of RCC diagnosed at 340 accredited training institutions in Japan in 2007 were included in this program (10).
A total of 1418 patients diagnosed with pT1aN0M0 RCC who received surgery as the primary treatment were included. Patients with <3 months of follow-up, those diagnosed with a recurrence within 1 month of the nephrectomy, those with bilateral tumors, those with multiple tumors in the kidney and those with oncocytoma were excluded from this study.
The clinicopathological characteristics of the patients we evaluated included age, sex, tumor laterality, tumor size, body mass index (BMI), reason for diagnosis, performance status (PS), medical history (diabetes mellitus, hypertension and hemodialysis), smoking history, preoperative blood test results [hemoglobin (Hb), lactate dehydrogenase (LDH), creatinine (Cr) and C-reactive protein (CRP)], surgical approach, type of nephrectomy and RCC histology. In this study, facility standard values for each facility were used to determine the lower or upper limit of normal for the blood tests.
Differences between patients with and without recurrence were evaluated using the Mann–Whitney U and chi-square tests. The Kaplan–Meier method was used to evaluate the incidence of recurrence-free survival (RFS), and the differences between survival curves were examined using the log-rank test. Univariate and multivariate Cox regression analyses were performed to identify the independent risk factors for recurrence. All statistical analyses were performed using JMP version 16 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at P < 0.05.
Results
The clinicopathological characteristics of the patients are summarized in Table 1. The median follow-up period was 62.8 months. Among the 1418 patients, 58 (4.1%) had recurrences. The 5-year recurrence-free rate was 95.8%. The median time to recurrence was 31.0 (1.1–67.6) months (Fig. 1). Metastases to the lungs and bone were observed in 20 and 10 cases, respectively, whereas local recurrence was seen in seven patients (Table 2). Patients were divided into a recurrence group and a non-recurrence group, and the prognosis between the two groups was compared. Male sex (recurrence vs. non-recurrence: 86.2% vs. 71.9%, P = 0.028), tumor size (2.80 ± 0.79 vs. 2.57 ± 0.77 cm, P = 0.018), Eastern Cooperative Oncology Group (ECOG) PS ≥ 1 (20.0% vs. 10.6%, P = 0.028), history of hemodialysis (10.5% vs. 4.5%, P = 0.036), a low Hb (32.8% vs. 14.9%, P < 0.001), and high levels of CRP (25.9% vs.11.7%, P < 0.001) and Cr (20.7% vs. 11.3%, P = 0.030) were significantly different between the two groups (Table 1).
| Clinical/pathological characteristic . | Total n = 1418 . | With recurrence n = 58 (%) . | Without recurrence n = 1360 (%) . | P value . |
|---|---|---|---|---|
| Follow-up period (day, mean ± SD) | 1709.8 ± 548.4 | 1713.1 ± 487.2 | 1709.7 ± 551.1 | 0.96 |
| Age(years, mean ± SD) | 62.9 ± 12.1 | 65.9 ± 11.0 | 62.7 ± 12.1 | 0.066 |
| Sex | 0.062 | |||
| Male | 1031 (72.7) | 50 (86.2) | 981 (72.1) | |
| Female | 386 (27.2) | 8 (13.8) | 378 (27.8) | |
| Unknown | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Side | 0.97 | |||
| Right | 771 (54.4) | 31 (53.4) | 740 (54.4) | |
| Left | 647 (45.6) | 27 (46.6) | 620 (45.6) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.80 ± 0.79 | 2.57 ± 0.77 | 0.018 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 22.85 ± 4.11 | 23.48 ± 3.53 | 0.14 |
| Reason for diagnosis | 0.59 | |||
| Symptomatic | 110 (7.8) | 7 (12.1) | 103 (7.6) | |
| Incidental | 1295 (91.3) | 50 (86.2) | 1245 (91.5) | |
| Other | 13 (1.0) | 1 (1.7) | 12 (1.0) | |
| PS | 0.037 | |||
| 0 | 1232 (86.9) | 44 (75.9) | 1188 (87.4) | |
| ≧1 | 151 (10.6) | 11 (19.0) | 140 (10.3) | |
| Unknown | 35 (2.5) | 3 (5.2) | 32 (2.4) | |
| Hypertension | 490 (34.6) | 24 (41.4) | 466 (34.3) | 0.33 |
| Diabetes mellitus | 192 (13.5) | 12 (20.7) | 180 (13.2) | 0.11 |
| Smoking history | 334 (23.6) | 18 (31.0) | 316 (23.2) | 0.12 |
| Hemodialysis | 67 (4.7) | 6 (10.3) | 61 (4.5) | 0.036 |
| Low hemoglobin level | 214 (15.1) | 19 (32.8) | 195 (14.4) | < 0.001 |
| High LDH level | 37 (2.6) | 2 (3.5) | 35 (2.6) | 0.75 |
| High creatinine level | 160 (11.3) | 12 (20.7) | 148 (10.9) | 0.030 |
| High CRP level | 132 (9.3) | 14 (24.1) | 118 (8.7) | <0.001 |
| Surgical approach | 0.43 | |||
| Open | 713 (50.3) | 27 (46.6) | 686 (50.4) | |
| Laparoscopic | 658 (46.4) | 31 (53.4) | 627 (46.1) | |
| Portless endoscopic surgery | 44 (3.1) | 0 (0) | 44 (3.2) | |
| Type of nephrectomy | 0.27 | |||
| Total | 933 (65.8) | 35 (60.3) | 898 (66.0) | |
| Partial | 483 (34.1) | 22 (37.9) | 461 (33.9) | |
| RCC histology | 0.94 | |||
| Clear | 1236 (87.2) | 50 (86.2) | 1186 (87.2) | |
| Papillary | 65 (4.6) | 4 (6.9) | 61 (4.5) | |
| Chromophobe | 49 (3.5) | 1 (1.7) | 48 (3.5) | |
| Other | 68 (4.8) | 3 (5.2) | 65 (4.8) |
| Clinical/pathological characteristic . | Total n = 1418 . | With recurrence n = 58 (%) . | Without recurrence n = 1360 (%) . | P value . |
|---|---|---|---|---|
| Follow-up period (day, mean ± SD) | 1709.8 ± 548.4 | 1713.1 ± 487.2 | 1709.7 ± 551.1 | 0.96 |
| Age(years, mean ± SD) | 62.9 ± 12.1 | 65.9 ± 11.0 | 62.7 ± 12.1 | 0.066 |
| Sex | 0.062 | |||
| Male | 1031 (72.7) | 50 (86.2) | 981 (72.1) | |
| Female | 386 (27.2) | 8 (13.8) | 378 (27.8) | |
| Unknown | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Side | 0.97 | |||
| Right | 771 (54.4) | 31 (53.4) | 740 (54.4) | |
| Left | 647 (45.6) | 27 (46.6) | 620 (45.6) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.80 ± 0.79 | 2.57 ± 0.77 | 0.018 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 22.85 ± 4.11 | 23.48 ± 3.53 | 0.14 |
| Reason for diagnosis | 0.59 | |||
| Symptomatic | 110 (7.8) | 7 (12.1) | 103 (7.6) | |
| Incidental | 1295 (91.3) | 50 (86.2) | 1245 (91.5) | |
| Other | 13 (1.0) | 1 (1.7) | 12 (1.0) | |
| PS | 0.037 | |||
| 0 | 1232 (86.9) | 44 (75.9) | 1188 (87.4) | |
| ≧1 | 151 (10.6) | 11 (19.0) | 140 (10.3) | |
| Unknown | 35 (2.5) | 3 (5.2) | 32 (2.4) | |
| Hypertension | 490 (34.6) | 24 (41.4) | 466 (34.3) | 0.33 |
| Diabetes mellitus | 192 (13.5) | 12 (20.7) | 180 (13.2) | 0.11 |
| Smoking history | 334 (23.6) | 18 (31.0) | 316 (23.2) | 0.12 |
| Hemodialysis | 67 (4.7) | 6 (10.3) | 61 (4.5) | 0.036 |
| Low hemoglobin level | 214 (15.1) | 19 (32.8) | 195 (14.4) | < 0.001 |
| High LDH level | 37 (2.6) | 2 (3.5) | 35 (2.6) | 0.75 |
| High creatinine level | 160 (11.3) | 12 (20.7) | 148 (10.9) | 0.030 |
| High CRP level | 132 (9.3) | 14 (24.1) | 118 (8.7) | <0.001 |
| Surgical approach | 0.43 | |||
| Open | 713 (50.3) | 27 (46.6) | 686 (50.4) | |
| Laparoscopic | 658 (46.4) | 31 (53.4) | 627 (46.1) | |
| Portless endoscopic surgery | 44 (3.1) | 0 (0) | 44 (3.2) | |
| Type of nephrectomy | 0.27 | |||
| Total | 933 (65.8) | 35 (60.3) | 898 (66.0) | |
| Partial | 483 (34.1) | 22 (37.9) | 461 (33.9) | |
| RCC histology | 0.94 | |||
| Clear | 1236 (87.2) | 50 (86.2) | 1186 (87.2) | |
| Papillary | 65 (4.6) | 4 (6.9) | 61 (4.5) | |
| Chromophobe | 49 (3.5) | 1 (1.7) | 48 (3.5) | |
| Other | 68 (4.8) | 3 (5.2) | 65 (4.8) |
BMI, body mass index; PS, performance status; LDH, lactate dehydrogenase; CRP, c-reactive protein; RCC, renal cell carcinoma.
| Clinical/pathological characteristic . | Total n = 1418 . | With recurrence n = 58 (%) . | Without recurrence n = 1360 (%) . | P value . |
|---|---|---|---|---|
| Follow-up period (day, mean ± SD) | 1709.8 ± 548.4 | 1713.1 ± 487.2 | 1709.7 ± 551.1 | 0.96 |
| Age(years, mean ± SD) | 62.9 ± 12.1 | 65.9 ± 11.0 | 62.7 ± 12.1 | 0.066 |
| Sex | 0.062 | |||
| Male | 1031 (72.7) | 50 (86.2) | 981 (72.1) | |
| Female | 386 (27.2) | 8 (13.8) | 378 (27.8) | |
| Unknown | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Side | 0.97 | |||
| Right | 771 (54.4) | 31 (53.4) | 740 (54.4) | |
| Left | 647 (45.6) | 27 (46.6) | 620 (45.6) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.80 ± 0.79 | 2.57 ± 0.77 | 0.018 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 22.85 ± 4.11 | 23.48 ± 3.53 | 0.14 |
| Reason for diagnosis | 0.59 | |||
| Symptomatic | 110 (7.8) | 7 (12.1) | 103 (7.6) | |
| Incidental | 1295 (91.3) | 50 (86.2) | 1245 (91.5) | |
| Other | 13 (1.0) | 1 (1.7) | 12 (1.0) | |
| PS | 0.037 | |||
| 0 | 1232 (86.9) | 44 (75.9) | 1188 (87.4) | |
| ≧1 | 151 (10.6) | 11 (19.0) | 140 (10.3) | |
| Unknown | 35 (2.5) | 3 (5.2) | 32 (2.4) | |
| Hypertension | 490 (34.6) | 24 (41.4) | 466 (34.3) | 0.33 |
| Diabetes mellitus | 192 (13.5) | 12 (20.7) | 180 (13.2) | 0.11 |
| Smoking history | 334 (23.6) | 18 (31.0) | 316 (23.2) | 0.12 |
| Hemodialysis | 67 (4.7) | 6 (10.3) | 61 (4.5) | 0.036 |
| Low hemoglobin level | 214 (15.1) | 19 (32.8) | 195 (14.4) | < 0.001 |
| High LDH level | 37 (2.6) | 2 (3.5) | 35 (2.6) | 0.75 |
| High creatinine level | 160 (11.3) | 12 (20.7) | 148 (10.9) | 0.030 |
| High CRP level | 132 (9.3) | 14 (24.1) | 118 (8.7) | <0.001 |
| Surgical approach | 0.43 | |||
| Open | 713 (50.3) | 27 (46.6) | 686 (50.4) | |
| Laparoscopic | 658 (46.4) | 31 (53.4) | 627 (46.1) | |
| Portless endoscopic surgery | 44 (3.1) | 0 (0) | 44 (3.2) | |
| Type of nephrectomy | 0.27 | |||
| Total | 933 (65.8) | 35 (60.3) | 898 (66.0) | |
| Partial | 483 (34.1) | 22 (37.9) | 461 (33.9) | |
| RCC histology | 0.94 | |||
| Clear | 1236 (87.2) | 50 (86.2) | 1186 (87.2) | |
| Papillary | 65 (4.6) | 4 (6.9) | 61 (4.5) | |
| Chromophobe | 49 (3.5) | 1 (1.7) | 48 (3.5) | |
| Other | 68 (4.8) | 3 (5.2) | 65 (4.8) |
| Clinical/pathological characteristic . | Total n = 1418 . | With recurrence n = 58 (%) . | Without recurrence n = 1360 (%) . | P value . |
|---|---|---|---|---|
| Follow-up period (day, mean ± SD) | 1709.8 ± 548.4 | 1713.1 ± 487.2 | 1709.7 ± 551.1 | 0.96 |
| Age(years, mean ± SD) | 62.9 ± 12.1 | 65.9 ± 11.0 | 62.7 ± 12.1 | 0.066 |
| Sex | 0.062 | |||
| Male | 1031 (72.7) | 50 (86.2) | 981 (72.1) | |
| Female | 386 (27.2) | 8 (13.8) | 378 (27.8) | |
| Unknown | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Side | 0.97 | |||
| Right | 771 (54.4) | 31 (53.4) | 740 (54.4) | |
| Left | 647 (45.6) | 27 (46.6) | 620 (45.6) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.80 ± 0.79 | 2.57 ± 0.77 | 0.018 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 22.85 ± 4.11 | 23.48 ± 3.53 | 0.14 |
| Reason for diagnosis | 0.59 | |||
| Symptomatic | 110 (7.8) | 7 (12.1) | 103 (7.6) | |
| Incidental | 1295 (91.3) | 50 (86.2) | 1245 (91.5) | |
| Other | 13 (1.0) | 1 (1.7) | 12 (1.0) | |
| PS | 0.037 | |||
| 0 | 1232 (86.9) | 44 (75.9) | 1188 (87.4) | |
| ≧1 | 151 (10.6) | 11 (19.0) | 140 (10.3) | |
| Unknown | 35 (2.5) | 3 (5.2) | 32 (2.4) | |
| Hypertension | 490 (34.6) | 24 (41.4) | 466 (34.3) | 0.33 |
| Diabetes mellitus | 192 (13.5) | 12 (20.7) | 180 (13.2) | 0.11 |
| Smoking history | 334 (23.6) | 18 (31.0) | 316 (23.2) | 0.12 |
| Hemodialysis | 67 (4.7) | 6 (10.3) | 61 (4.5) | 0.036 |
| Low hemoglobin level | 214 (15.1) | 19 (32.8) | 195 (14.4) | < 0.001 |
| High LDH level | 37 (2.6) | 2 (3.5) | 35 (2.6) | 0.75 |
| High creatinine level | 160 (11.3) | 12 (20.7) | 148 (10.9) | 0.030 |
| High CRP level | 132 (9.3) | 14 (24.1) | 118 (8.7) | <0.001 |
| Surgical approach | 0.43 | |||
| Open | 713 (50.3) | 27 (46.6) | 686 (50.4) | |
| Laparoscopic | 658 (46.4) | 31 (53.4) | 627 (46.1) | |
| Portless endoscopic surgery | 44 (3.1) | 0 (0) | 44 (3.2) | |
| Type of nephrectomy | 0.27 | |||
| Total | 933 (65.8) | 35 (60.3) | 898 (66.0) | |
| Partial | 483 (34.1) | 22 (37.9) | 461 (33.9) | |
| RCC histology | 0.94 | |||
| Clear | 1236 (87.2) | 50 (86.2) | 1186 (87.2) | |
| Papillary | 65 (4.6) | 4 (6.9) | 61 (4.5) | |
| Chromophobe | 49 (3.5) | 1 (1.7) | 48 (3.5) | |
| Other | 68 (4.8) | 3 (5.2) | 65 (4.8) |
BMI, body mass index; PS, performance status; LDH, lactate dehydrogenase; CRP, c-reactive protein; RCC, renal cell carcinoma.
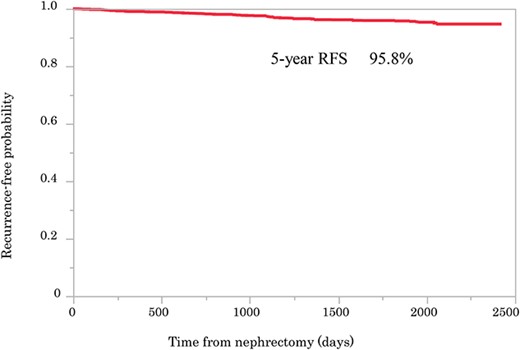
Kaplan–Meier curve showing recurrence-free probability after surgery.
| Recurrence lesionLesion . | Number of cases (%) . |
|---|---|
| Lung | 20 (34.5) |
| Bone | 10 (17.2) |
| Ipsilateral kidney (preserved*) | 8 (13.8) |
| Local | 7 (12.1) |
| Contralateral kidney | 6 (10.3) |
| Pancreas | 3 (5.2) |
| Lympho node | 2 (3.4) |
| Liver | 2 (3.4) |
| Adrenal gland | 2 (3.4) |
| Recurrence lesionLesion . | Number of cases (%) . |
|---|---|
| Lung | 20 (34.5) |
| Bone | 10 (17.2) |
| Ipsilateral kidney (preserved*) | 8 (13.8) |
| Local | 7 (12.1) |
| Contralateral kidney | 6 (10.3) |
| Pancreas | 3 (5.2) |
| Lympho node | 2 (3.4) |
| Liver | 2 (3.4) |
| Adrenal gland | 2 (3.4) |
*Remaining kidney after partial nephrectomy.
| Recurrence lesionLesion . | Number of cases (%) . |
|---|---|
| Lung | 20 (34.5) |
| Bone | 10 (17.2) |
| Ipsilateral kidney (preserved*) | 8 (13.8) |
| Local | 7 (12.1) |
| Contralateral kidney | 6 (10.3) |
| Pancreas | 3 (5.2) |
| Lympho node | 2 (3.4) |
| Liver | 2 (3.4) |
| Adrenal gland | 2 (3.4) |
| Recurrence lesionLesion . | Number of cases (%) . |
|---|---|
| Lung | 20 (34.5) |
| Bone | 10 (17.2) |
| Ipsilateral kidney (preserved*) | 8 (13.8) |
| Local | 7 (12.1) |
| Contralateral kidney | 6 (10.3) |
| Pancreas | 3 (5.2) |
| Lympho node | 2 (3.4) |
| Liver | 2 (3.4) |
| Adrenal gland | 2 (3.4) |
*Remaining kidney after partial nephrectomy.
Univariate analysis identified age (≥60; hazard ratio, 1.79; 95% CI, 1.03–3.27; P = 0.040), sex (Male; hazard ratio, 2.32; 95% CI, 1.17–5.30; P = 0.015), tumor size (≥3 cm; hazard ratio, 2.00; 95% CI, 1.19–3.39; P = 0.009), PS (≥1; hazard ratio, 2.45; 95% CI, 1.20–4.56; P = 0.016), hemoglobin level (low; hazard ratio, 2.90; 95% CI, 1.64–4.95; P < 0.001), and creatinine (high; hazard ratio, 2.11; 95% CI, 1.07–3.84; P = 0.033) and CRP (high; hazard ratio, 3.41; 95% CI, 1.79–6.11; P < 0.001) levels as significant risk factors for recurrence. Sex (Male; hazard ratio, 3.06; 95% CI, 1.41–8.02; P = 0.003), tumor size (≥3 cm; hazard ratio, 1.74; 95% CI, 1.01–3.03; P = 0.0472) and a high CRP level (high; hazard ratio, 2.27; 95% CI, 1.10–4.35; P = 0.0279) were also identified as significant risk factors for tumor recurrence in the multivariate analysis (Table 3); RFS curves for these risk factors are presented in Fig. 2. There were significant differences in the RFS between the patients with 0, 1, 2 or 3 risk factors, and the 5-year RFS rates were 99., 97.2, 93.1 and 80.7%, respectively.
| Variables . | Categories . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| . | . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . |
| Age | |||||||
| < 60 | |||||||
| ≧ 60 | 1.79 | 1.026–3.273 | 0.040 | 1.43 | 0.796–2.668 | 0.24 | |
| Sex | |||||||
| Female | |||||||
| Male | 2.32 | 1.168–5.302 | 0.015 | 3.06 | 1.409–8/021 | 0.003 | |
| Side | |||||||
| Right | |||||||
| Left | 1.03 | 0.610–1.721 | 0.92 | ||||
| Tumor size (cm) | |||||||
| <3 | |||||||
| ≧3 | 2.00 | 1.192–3.391 | 0.009 | 1.74 | 1.007–3.035 | 0.046 | |
| BMI(kg/m2) | |||||||
| <25 | |||||||
| ≧25 | 0.92 | 0.510–1.625 | 0.79 | ||||
| Reason for diagnosis | |||||||
| Incidental | |||||||
| Symptomatic | 1.76 | 0.798–3.882 | 0.16 | ||||
| PS | |||||||
| 0 | |||||||
| ≧1 | 2.45 | 1.199–4.563 | 0.016 | 1.59 | 0.731–3.169 | 0.23 | |
| Hypertension | |||||||
| − | |||||||
| + | 1.33 | 0.779–2.245 | 0.29 | ||||
| Diabetes mellitus | |||||||
| − | |||||||
| + | 1.70 | 0.858–3.114 | 0.12 | ||||
| Smoking history | |||||||
| − | |||||||
| + | 1.66 | 0.885–3.033 | 0.11 | ||||
| Hemodialysis | |||||||
| − | |||||||
| + | 2.48 | 0.951–5.324 | 0.062 | ||||
| Low hemoglobin level | |||||||
| − | |||||||
| + | 2.90 | 1.642—4.954 | <0.001 | 1.91 | 0.951–3.670 | 0.080 | |
| High LDH level | |||||||
| − | |||||||
| + | 1.42 | 0.233–4.558 | 0.64 | ||||
| High Creatinine level | |||||||
| − | |||||||
| + | 2.11 | 1.066–3.844 | 0.033 | 1.07 | 0.475–2.234 | 0.97 | |
| High CRP level | |||||||
| − | |||||||
| + | 3.41 | 1.789–6.105 | <0.001 | 2.27 | 1.099–4.354 | 0.027 | |
| Type of nephrectomy | |||||||
| Partial | |||||||
| Total | 0.79 | 0.465–1.360 | 0.38 | ||||
| RCC histology | |||||||
| Clear | |||||||
| Non clear | 1.92 | 0.969–3.510 | 0.061 | ||||
| Variables . | Categories . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| . | . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . |
| Age | |||||||
| < 60 | |||||||
| ≧ 60 | 1.79 | 1.026–3.273 | 0.040 | 1.43 | 0.796–2.668 | 0.24 | |
| Sex | |||||||
| Female | |||||||
| Male | 2.32 | 1.168–5.302 | 0.015 | 3.06 | 1.409–8/021 | 0.003 | |
| Side | |||||||
| Right | |||||||
| Left | 1.03 | 0.610–1.721 | 0.92 | ||||
| Tumor size (cm) | |||||||
| <3 | |||||||
| ≧3 | 2.00 | 1.192–3.391 | 0.009 | 1.74 | 1.007–3.035 | 0.046 | |
| BMI(kg/m2) | |||||||
| <25 | |||||||
| ≧25 | 0.92 | 0.510–1.625 | 0.79 | ||||
| Reason for diagnosis | |||||||
| Incidental | |||||||
| Symptomatic | 1.76 | 0.798–3.882 | 0.16 | ||||
| PS | |||||||
| 0 | |||||||
| ≧1 | 2.45 | 1.199–4.563 | 0.016 | 1.59 | 0.731–3.169 | 0.23 | |
| Hypertension | |||||||
| − | |||||||
| + | 1.33 | 0.779–2.245 | 0.29 | ||||
| Diabetes mellitus | |||||||
| − | |||||||
| + | 1.70 | 0.858–3.114 | 0.12 | ||||
| Smoking history | |||||||
| − | |||||||
| + | 1.66 | 0.885–3.033 | 0.11 | ||||
| Hemodialysis | |||||||
| − | |||||||
| + | 2.48 | 0.951–5.324 | 0.062 | ||||
| Low hemoglobin level | |||||||
| − | |||||||
| + | 2.90 | 1.642—4.954 | <0.001 | 1.91 | 0.951–3.670 | 0.080 | |
| High LDH level | |||||||
| − | |||||||
| + | 1.42 | 0.233–4.558 | 0.64 | ||||
| High Creatinine level | |||||||
| − | |||||||
| + | 2.11 | 1.066–3.844 | 0.033 | 1.07 | 0.475–2.234 | 0.97 | |
| High CRP level | |||||||
| − | |||||||
| + | 3.41 | 1.789–6.105 | <0.001 | 2.27 | 1.099–4.354 | 0.027 | |
| Type of nephrectomy | |||||||
| Partial | |||||||
| Total | 0.79 | 0.465–1.360 | 0.38 | ||||
| RCC histology | |||||||
| Clear | |||||||
| Non clear | 1.92 | 0.969–3.510 | 0.061 | ||||
BMI, body mass index; PS, performance status; LDH, lactate dehydrogenase; CRP, c-reactive protein; RCC, renal cell carcinoma.
| Variables . | Categories . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| . | . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . |
| Age | |||||||
| < 60 | |||||||
| ≧ 60 | 1.79 | 1.026–3.273 | 0.040 | 1.43 | 0.796–2.668 | 0.24 | |
| Sex | |||||||
| Female | |||||||
| Male | 2.32 | 1.168–5.302 | 0.015 | 3.06 | 1.409–8/021 | 0.003 | |
| Side | |||||||
| Right | |||||||
| Left | 1.03 | 0.610–1.721 | 0.92 | ||||
| Tumor size (cm) | |||||||
| <3 | |||||||
| ≧3 | 2.00 | 1.192–3.391 | 0.009 | 1.74 | 1.007–3.035 | 0.046 | |
| BMI(kg/m2) | |||||||
| <25 | |||||||
| ≧25 | 0.92 | 0.510–1.625 | 0.79 | ||||
| Reason for diagnosis | |||||||
| Incidental | |||||||
| Symptomatic | 1.76 | 0.798–3.882 | 0.16 | ||||
| PS | |||||||
| 0 | |||||||
| ≧1 | 2.45 | 1.199–4.563 | 0.016 | 1.59 | 0.731–3.169 | 0.23 | |
| Hypertension | |||||||
| − | |||||||
| + | 1.33 | 0.779–2.245 | 0.29 | ||||
| Diabetes mellitus | |||||||
| − | |||||||
| + | 1.70 | 0.858–3.114 | 0.12 | ||||
| Smoking history | |||||||
| − | |||||||
| + | 1.66 | 0.885–3.033 | 0.11 | ||||
| Hemodialysis | |||||||
| − | |||||||
| + | 2.48 | 0.951–5.324 | 0.062 | ||||
| Low hemoglobin level | |||||||
| − | |||||||
| + | 2.90 | 1.642—4.954 | <0.001 | 1.91 | 0.951–3.670 | 0.080 | |
| High LDH level | |||||||
| − | |||||||
| + | 1.42 | 0.233–4.558 | 0.64 | ||||
| High Creatinine level | |||||||
| − | |||||||
| + | 2.11 | 1.066–3.844 | 0.033 | 1.07 | 0.475–2.234 | 0.97 | |
| High CRP level | |||||||
| − | |||||||
| + | 3.41 | 1.789–6.105 | <0.001 | 2.27 | 1.099–4.354 | 0.027 | |
| Type of nephrectomy | |||||||
| Partial | |||||||
| Total | 0.79 | 0.465–1.360 | 0.38 | ||||
| RCC histology | |||||||
| Clear | |||||||
| Non clear | 1.92 | 0.969–3.510 | 0.061 | ||||
| Variables . | Categories . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|
| . | . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . |
| Age | |||||||
| < 60 | |||||||
| ≧ 60 | 1.79 | 1.026–3.273 | 0.040 | 1.43 | 0.796–2.668 | 0.24 | |
| Sex | |||||||
| Female | |||||||
| Male | 2.32 | 1.168–5.302 | 0.015 | 3.06 | 1.409–8/021 | 0.003 | |
| Side | |||||||
| Right | |||||||
| Left | 1.03 | 0.610–1.721 | 0.92 | ||||
| Tumor size (cm) | |||||||
| <3 | |||||||
| ≧3 | 2.00 | 1.192–3.391 | 0.009 | 1.74 | 1.007–3.035 | 0.046 | |
| BMI(kg/m2) | |||||||
| <25 | |||||||
| ≧25 | 0.92 | 0.510–1.625 | 0.79 | ||||
| Reason for diagnosis | |||||||
| Incidental | |||||||
| Symptomatic | 1.76 | 0.798–3.882 | 0.16 | ||||
| PS | |||||||
| 0 | |||||||
| ≧1 | 2.45 | 1.199–4.563 | 0.016 | 1.59 | 0.731–3.169 | 0.23 | |
| Hypertension | |||||||
| − | |||||||
| + | 1.33 | 0.779–2.245 | 0.29 | ||||
| Diabetes mellitus | |||||||
| − | |||||||
| + | 1.70 | 0.858–3.114 | 0.12 | ||||
| Smoking history | |||||||
| − | |||||||
| + | 1.66 | 0.885–3.033 | 0.11 | ||||
| Hemodialysis | |||||||
| − | |||||||
| + | 2.48 | 0.951–5.324 | 0.062 | ||||
| Low hemoglobin level | |||||||
| − | |||||||
| + | 2.90 | 1.642—4.954 | <0.001 | 1.91 | 0.951–3.670 | 0.080 | |
| High LDH level | |||||||
| − | |||||||
| + | 1.42 | 0.233–4.558 | 0.64 | ||||
| High Creatinine level | |||||||
| − | |||||||
| + | 2.11 | 1.066–3.844 | 0.033 | 1.07 | 0.475–2.234 | 0.97 | |
| High CRP level | |||||||
| − | |||||||
| + | 3.41 | 1.789–6.105 | <0.001 | 2.27 | 1.099–4.354 | 0.027 | |
| Type of nephrectomy | |||||||
| Partial | |||||||
| Total | 0.79 | 0.465–1.360 | 0.38 | ||||
| RCC histology | |||||||
| Clear | |||||||
| Non clear | 1.92 | 0.969–3.510 | 0.061 | ||||
BMI, body mass index; PS, performance status; LDH, lactate dehydrogenase; CRP, c-reactive protein; RCC, renal cell carcinoma.
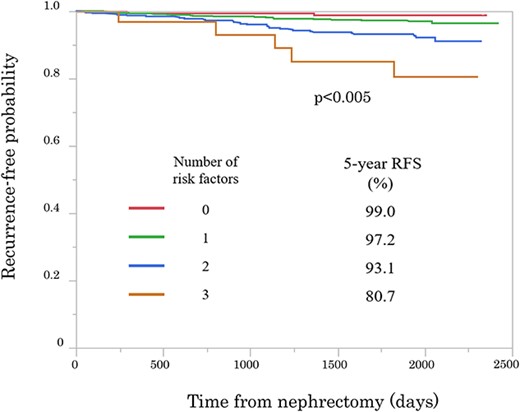
Kaplan–Meier curves showing recurrence-free probability after nephrectomy according to number of risk factors (Male sex; tumor size and high CRP level).
Discussion
To the best of our knowledge, the present study uses the largest sample size to investigate the risk factors for pT1aN0M0 RCC recurrence in the Japanese population compared with that of the previous literature. In the current study, among 1418 patients with RCC, 58 patients (4.1%) experienced tumor recurrence. The 5-year recurrence-free rate was 95.8%. This result was compatible with previous reports (3–6). Tumor recurrence to the lungs and the bone was observed in 20 (34.5%) and 10 (17.2%) cases, respectively. It is well known that the most common site of RCC recurrence is the lung. Regarding bone recurrence in patients with pT1aN0M0 RCC, Nishikimi et al. reported that the site of recurrence in 11 of 25 (44%) patients was the bone (5). However, in the studies by Kim et al. and Ito et al., bone recurrence was seen in one of nine (11.1%) and none of the five (0%) patients with tumor recurrence (3,6), respectively. It remains unclear whether the bone is a common site of recurrence in pT1a renal cancer.
Patients with recurrence were more likely to be men and have an ECOG PS ≥ 1, along with a history of hemodialysis, low Hb level, high CRP and Cr levels and increased tumor size. Univariate analysis revealed that age, sex, tumor size, PS, low Hb level and high Cr and CRP levels were significant risk factors for recurrence. Sex, tumor size and a high CRP level were also independent risk factors for recurrence on multivariate analysis.
Several reports from Western countries have described sex differences in RCC histology and prognosis (11,12). Aron et al. analyzed 35 336 cases of RCC at all stages and reported that women had a greater incidence of the clear cell subtype of RCC and a decreased incidence of the papillary subtype. Despite the unfavorable prognosis of the clear cell RCC subtype, women had a better prognosis (overall survival) than men (11). In contrast, Zaitsu et al. analyzed 5265 case of RCC at all stages and reported that there were no significant sex differences in the prevalence of clear cell subtypes in Japan, and sex differences in RCC histology may not affect RCC survival (13). Although the present study included only cases of pT1a, men had a greater incidence of clear cell carcinoma (Table 4), which was not consistent with previous reports. Men also presented with a higher BMI and incidence of smoking history. The recurrence rate by sex was determined using a propensity score matching method (Males and Females were matched 1:1 using nearest-neighbor matching without replacement, with a caliper of 0.2) with BMI and smoking history as covariates, thus showing that the recurrence rate was 3.9% (12/310 cases) in males and 1.9% (6/310 cases) in females. The RFS was calculated using the Kaplan–Meier method, and the difference between the two groups was compared with the log-rank test, which showed that there was no significant difference (P = 0.183; data not shown). Therefore, the clinicopathological sex differences may have led to an unfavorable prognosis in men.
| Clinical/pathological characteristic . | Total n = 1418 . | Malen = 1031 (%) . | Female n = 386b(%) . | P value . |
|---|---|---|---|---|
| Age(years, mean ± SD) | 62.9 ± 12.1 | 62.0 ± 12.0 | 65.2 ± 12.0 | <0.001 |
| Side | 0.70 | |||
| Right | 771 (54.4) | 557 (54.0) | 213 (55.2) | |
| Left | 647 (45.6) | 474 (46.0) | 173 (47.8) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.57 ± 0.76 | 2.60 ± 0.80 | 0.50 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 23.69 ± 3.45 | 22.84 ± 3.76 | <0.001 |
| Reason for diagnosis | 0.12 | |||
| Symptomatic | 110 (7.8) | 74 (7.2) | 36 (9.3) | |
| Incidental | 1295 (91.3) | 945 (91.7) | 349 (90.4) | |
| Other | 13 (1.0) | 12 (1.2) | 1 (0.3) | |
| Performance status | 0.78 | |||
| 0 | 1231 (86.8) | 902 (87.5) | 329 (85.2) | |
| ≧1 | 151 (10.6) | 109 (10.6) | 42 (10.9) | |
| Unknown | 36 (2.5) | 20 (1.9) | 15 (3.9) | |
| Hypertension | 490 (34.6) | 350 (34.0) | 139 (36.0) | 0.69 |
| Diabetes mellitus | 192 (13.5) | 141 (13.7) | 51 (13.2) | 0.70 |
| Smoking history | 334 (23.6) | 309 (30.0) | 25 (6.5) | <0.001 |
| Hemodialysis | 67 (4.7) | 50 (4.9) | 17 (4.4) | 0.72 |
| Low hemoglobin level | 214 (15.6) | 156 (15.1) | 58 (15.0) | 0.81 |
| High LDH level | 37 (2.1) | 26 (2.5) | 11 (2.8) | 0.84 |
| High creatinine level | 160 (11.3) | 114 (11.1) | 46 (11.9) | 0.75 |
| High CRP level | 132 (9.3) | 94 (9.1) | 38 (9.8) | 0.81 |
| Surgical approach | 0.73 | |||
| Open | 713 (50.3) | 523 (50.7) | 189 (49.0) | |
| Laparo | 658 (46.4) | 477 (46.3) | 181 (46.9) | |
| Portless endoscopic surgery | 44 (3.1) | 29 (2.8) | 15 (3.9) | |
| Type of nephrectomy | 0.015 | |||
| Total | 933 (65.8) | 657 (63.7) | 276 (71.5) | |
| Partial | 483 (34.1) | 373 (36.2) | 110 (18.5) | |
| RCC histology | <0.001 | |||
| Clear | 1236 (87.2) | 920 (89.2) | 315 (81.6) | |
| Papillary | 65 (4.6) | 45 (4.4) | 20 (5.2) | |
| Chromophobe | 49 (3.5) | 26 (2.5) | 23 (6.0) | |
| Other | 68 (4.8) | 40 (3.9) | 28 |
| Clinical/pathological characteristic . | Total n = 1418 . | Malen = 1031 (%) . | Female n = 386b(%) . | P value . |
|---|---|---|---|---|
| Age(years, mean ± SD) | 62.9 ± 12.1 | 62.0 ± 12.0 | 65.2 ± 12.0 | <0.001 |
| Side | 0.70 | |||
| Right | 771 (54.4) | 557 (54.0) | 213 (55.2) | |
| Left | 647 (45.6) | 474 (46.0) | 173 (47.8) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.57 ± 0.76 | 2.60 ± 0.80 | 0.50 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 23.69 ± 3.45 | 22.84 ± 3.76 | <0.001 |
| Reason for diagnosis | 0.12 | |||
| Symptomatic | 110 (7.8) | 74 (7.2) | 36 (9.3) | |
| Incidental | 1295 (91.3) | 945 (91.7) | 349 (90.4) | |
| Other | 13 (1.0) | 12 (1.2) | 1 (0.3) | |
| Performance status | 0.78 | |||
| 0 | 1231 (86.8) | 902 (87.5) | 329 (85.2) | |
| ≧1 | 151 (10.6) | 109 (10.6) | 42 (10.9) | |
| Unknown | 36 (2.5) | 20 (1.9) | 15 (3.9) | |
| Hypertension | 490 (34.6) | 350 (34.0) | 139 (36.0) | 0.69 |
| Diabetes mellitus | 192 (13.5) | 141 (13.7) | 51 (13.2) | 0.70 |
| Smoking history | 334 (23.6) | 309 (30.0) | 25 (6.5) | <0.001 |
| Hemodialysis | 67 (4.7) | 50 (4.9) | 17 (4.4) | 0.72 |
| Low hemoglobin level | 214 (15.6) | 156 (15.1) | 58 (15.0) | 0.81 |
| High LDH level | 37 (2.1) | 26 (2.5) | 11 (2.8) | 0.84 |
| High creatinine level | 160 (11.3) | 114 (11.1) | 46 (11.9) | 0.75 |
| High CRP level | 132 (9.3) | 94 (9.1) | 38 (9.8) | 0.81 |
| Surgical approach | 0.73 | |||
| Open | 713 (50.3) | 523 (50.7) | 189 (49.0) | |
| Laparo | 658 (46.4) | 477 (46.3) | 181 (46.9) | |
| Portless endoscopic surgery | 44 (3.1) | 29 (2.8) | 15 (3.9) | |
| Type of nephrectomy | 0.015 | |||
| Total | 933 (65.8) | 657 (63.7) | 276 (71.5) | |
| Partial | 483 (34.1) | 373 (36.2) | 110 (18.5) | |
| RCC histology | <0.001 | |||
| Clear | 1236 (87.2) | 920 (89.2) | 315 (81.6) | |
| Papillary | 65 (4.6) | 45 (4.4) | 20 (5.2) | |
| Chromophobe | 49 (3.5) | 26 (2.5) | 23 (6.0) | |
| Other | 68 (4.8) | 40 (3.9) | 28 |
Mann–Whitney U and chi-square tests
BMI, body mass index; PS, performance status; LDH, lactate dehydrogenase; CRP, C-reactive protein; RCC, renal cell carcinoma.
| Clinical/pathological characteristic . | Total n = 1418 . | Malen = 1031 (%) . | Female n = 386b(%) . | P value . |
|---|---|---|---|---|
| Age(years, mean ± SD) | 62.9 ± 12.1 | 62.0 ± 12.0 | 65.2 ± 12.0 | <0.001 |
| Side | 0.70 | |||
| Right | 771 (54.4) | 557 (54.0) | 213 (55.2) | |
| Left | 647 (45.6) | 474 (46.0) | 173 (47.8) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.57 ± 0.76 | 2.60 ± 0.80 | 0.50 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 23.69 ± 3.45 | 22.84 ± 3.76 | <0.001 |
| Reason for diagnosis | 0.12 | |||
| Symptomatic | 110 (7.8) | 74 (7.2) | 36 (9.3) | |
| Incidental | 1295 (91.3) | 945 (91.7) | 349 (90.4) | |
| Other | 13 (1.0) | 12 (1.2) | 1 (0.3) | |
| Performance status | 0.78 | |||
| 0 | 1231 (86.8) | 902 (87.5) | 329 (85.2) | |
| ≧1 | 151 (10.6) | 109 (10.6) | 42 (10.9) | |
| Unknown | 36 (2.5) | 20 (1.9) | 15 (3.9) | |
| Hypertension | 490 (34.6) | 350 (34.0) | 139 (36.0) | 0.69 |
| Diabetes mellitus | 192 (13.5) | 141 (13.7) | 51 (13.2) | 0.70 |
| Smoking history | 334 (23.6) | 309 (30.0) | 25 (6.5) | <0.001 |
| Hemodialysis | 67 (4.7) | 50 (4.9) | 17 (4.4) | 0.72 |
| Low hemoglobin level | 214 (15.6) | 156 (15.1) | 58 (15.0) | 0.81 |
| High LDH level | 37 (2.1) | 26 (2.5) | 11 (2.8) | 0.84 |
| High creatinine level | 160 (11.3) | 114 (11.1) | 46 (11.9) | 0.75 |
| High CRP level | 132 (9.3) | 94 (9.1) | 38 (9.8) | 0.81 |
| Surgical approach | 0.73 | |||
| Open | 713 (50.3) | 523 (50.7) | 189 (49.0) | |
| Laparo | 658 (46.4) | 477 (46.3) | 181 (46.9) | |
| Portless endoscopic surgery | 44 (3.1) | 29 (2.8) | 15 (3.9) | |
| Type of nephrectomy | 0.015 | |||
| Total | 933 (65.8) | 657 (63.7) | 276 (71.5) | |
| Partial | 483 (34.1) | 373 (36.2) | 110 (18.5) | |
| RCC histology | <0.001 | |||
| Clear | 1236 (87.2) | 920 (89.2) | 315 (81.6) | |
| Papillary | 65 (4.6) | 45 (4.4) | 20 (5.2) | |
| Chromophobe | 49 (3.5) | 26 (2.5) | 23 (6.0) | |
| Other | 68 (4.8) | 40 (3.9) | 28 |
| Clinical/pathological characteristic . | Total n = 1418 . | Malen = 1031 (%) . | Female n = 386b(%) . | P value . |
|---|---|---|---|---|
| Age(years, mean ± SD) | 62.9 ± 12.1 | 62.0 ± 12.0 | 65.2 ± 12.0 | <0.001 |
| Side | 0.70 | |||
| Right | 771 (54.4) | 557 (54.0) | 213 (55.2) | |
| Left | 647 (45.6) | 474 (46.0) | 173 (47.8) | |
| Tumor size (cm, mean ± SD) | 2.58 ± 0.77 | 2.57 ± 0.76 | 2.60 ± 0.80 | 0.50 |
| BMI (kg/m2, mean ± SD) | 23.46 ± 3.56 | 23.69 ± 3.45 | 22.84 ± 3.76 | <0.001 |
| Reason for diagnosis | 0.12 | |||
| Symptomatic | 110 (7.8) | 74 (7.2) | 36 (9.3) | |
| Incidental | 1295 (91.3) | 945 (91.7) | 349 (90.4) | |
| Other | 13 (1.0) | 12 (1.2) | 1 (0.3) | |
| Performance status | 0.78 | |||
| 0 | 1231 (86.8) | 902 (87.5) | 329 (85.2) | |
| ≧1 | 151 (10.6) | 109 (10.6) | 42 (10.9) | |
| Unknown | 36 (2.5) | 20 (1.9) | 15 (3.9) | |
| Hypertension | 490 (34.6) | 350 (34.0) | 139 (36.0) | 0.69 |
| Diabetes mellitus | 192 (13.5) | 141 (13.7) | 51 (13.2) | 0.70 |
| Smoking history | 334 (23.6) | 309 (30.0) | 25 (6.5) | <0.001 |
| Hemodialysis | 67 (4.7) | 50 (4.9) | 17 (4.4) | 0.72 |
| Low hemoglobin level | 214 (15.6) | 156 (15.1) | 58 (15.0) | 0.81 |
| High LDH level | 37 (2.1) | 26 (2.5) | 11 (2.8) | 0.84 |
| High creatinine level | 160 (11.3) | 114 (11.1) | 46 (11.9) | 0.75 |
| High CRP level | 132 (9.3) | 94 (9.1) | 38 (9.8) | 0.81 |
| Surgical approach | 0.73 | |||
| Open | 713 (50.3) | 523 (50.7) | 189 (49.0) | |
| Laparo | 658 (46.4) | 477 (46.3) | 181 (46.9) | |
| Portless endoscopic surgery | 44 (3.1) | 29 (2.8) | 15 (3.9) | |
| Type of nephrectomy | 0.015 | |||
| Total | 933 (65.8) | 657 (63.7) | 276 (71.5) | |
| Partial | 483 (34.1) | 373 (36.2) | 110 (18.5) | |
| RCC histology | <0.001 | |||
| Clear | 1236 (87.2) | 920 (89.2) | 315 (81.6) | |
| Papillary | 65 (4.6) | 45 (4.4) | 20 (5.2) | |
| Chromophobe | 49 (3.5) | 26 (2.5) | 23 (6.0) | |
| Other | 68 (4.8) | 40 (3.9) | 28 |
Mann–Whitney U and chi-square tests
BMI, body mass index; PS, performance status; LDH, lactate dehydrogenase; CRP, C-reactive protein; RCC, renal cell carcinoma.
For many years, tumor size has been considered one of the most important prognostic factors of RCC. The T1a, T1b, T2a and T2b stages are defined by tumor size (14). Several studies have reported that tumor size is a significant risk factor for synchronous and asynchronous metastases (15–17). Houston et al. reported that the metastasis-free survival rate was significantly associated with tumor size, and the incidence of metastatic disease was negligible in patients with tumors <3 cm in size (16). On the other hand, Klatte et al. reported that tumor size alone was not an independent prognostic risk factor in patients with RCCs < 4 cm in size (18).
In the current study, no recurrence was observed among patients with a tumor diameter < 1 cm (Fig. 3). On the other hand, the recurrence rate among patients with tumors > 3 cm in diameter was 5.8%, which is consistent with previous reports (3,17). Similar to those of previous reports, the results of our study suggest that patients with a tumor size of >3 cm should be given particular attention.
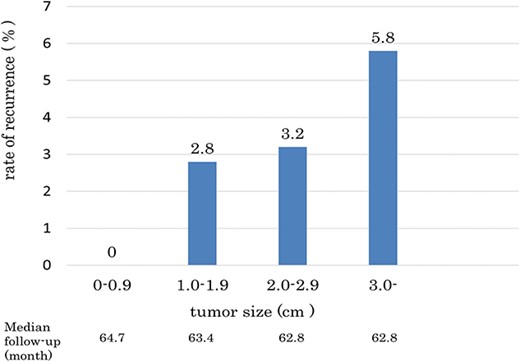
Regarding CRP level, several studies have reported that an increased pre- or postoperative CRP level is an independent prognostic factor in localized RCC (19). Takayama et al. reported that a CRP level of ≥0.4 mg/dl or more was a significant risk factor for metastasis [including synchronous and metachronous metastases; (9)]. Ito et al. also reported that a CRP level ≥ 1.0 mg/dl is an independent predictor of recurrence and prognosis in patients with N0M0 RCC (19). Since the exact CRP values were not evaluated in the present study, further studies are required to establish the role of the CRP level in relation to recurrence.
Identifying the risk factors for recurrence of RCC after nephrectomy is essential for patient counseling and the development of evidence-based surveillance programs. To date, several postoperative recurrence-predicting nomograms have been reported (20–22). The RFS was analyzed according to the number of risk factors identified in the multivariate analysis (Male sex, tumor size and a high CRP level). The 5-year RFS of patients with 0, 1, 2 and 3 risk factors was 99.0, 97.2, 93.1 and 80.7%, respectively. Patients with these three risk factors have especially poor RFS (5-year RFS was ~80%) and require special attention during follow-up.
Our study has several limitations: the data were collected retrospectively, and the exact values for blood test results were not obtained; we only obtained information on whether the test result values were higher or lower than the standard values of each facility. Furthermore, the detailed histological data were not obtained; MVI, a sarcomatoid component and the Fuhrman grade, tumor necrosis, growth pattern and CT findings have been reported as pathological prognostic factors of T1a or T1 RCC (1,2,4,5,9 and 23). Although detailed pathological and imaging analysis are necessary for more accurate prognosis estimation, the results of the present study are meaningful in that the prognosis could easily be estimated based on the preoperative findings.
Conclusions
Sex (male), tumor diameter (particularly ≥3 cm) and a high CRP level are independent recurrence risk factors for pT1a renal cell carcinoma, and extensive care is required during the postoperative follow-up for patients with these risk factors.
Acknowledgement
This abstract was presented at the 55th Annual Meeting of the Japan Society of Clinical Oncology, 20 October–22 October 2017, Yokohama, Japan. The authors would like to thank all Japanese Urological Association members for collecting the clinicopathological data.
Funding
No funding to declare.
Conflicts of interest
The authors have no conflicts of interest.
Availability of data and materials
None.


