-
PDF
- Split View
-
Views
-
Cite
Cite
Kenta Ishii, Yukihiro Yokoyama, Yoshihiro Nishida, Hiroshi Koike, Suguru Yamada, Yasuhiro Kodera, Naoto Sassa, Momokazu Gotoh, Masato Nagino, Characteristics of primary and repeated recurrent retroperitoneal liposarcoma: outcomes after aggressive surgeries at a single institution, Japanese Journal of Clinical Oncology, Volume 50, Issue 12, December 2020, Pages 1412–1418, https://doi.org/10.1093/jjco/hyaa126
Close - Share Icon Share
Abstract
This study sought to investigate the characteristics of primary and repeated recurrent retroperitoneal liposarcoma.
Patients treated with primary or recurrent retroperitoneal liposarcoma between 2005 and 2018 were retrospectively reviewed. Survival time analysis of recurrence-free survival and overall survival was conducted using Kaplan–Meier analysis and log-rank test.
Fifty-two patients with primary retroperitoneal liposarcoma were analysed. Amongst them, 46 patients (88%) had undergone surgery. Histologic grades included well-differentiated (n = 21), dedifferentiated (n = 21), myxoid (n = 3) and pleomorphic (n = 1) subtypes. The patients undergoing R0 resection in the first surgery had significantly higher recurrence-free survival rates compared with the patients undergoing non-R0 resection (3-year recurrence-free survival: 80 versus 38%; 5-year recurrence-free survival: 49 versus 29%, P = 0.033). Although overall survival rates tended to be higher in the patients undergoing R0 resection compared with the non-R0 resection, it did not reach to a statistical significant difference (5-year overall survival: 93 versus 75%; 10-year overall survival: 93 versus 59%, P = 0.124). The recurrence rates were 65, 67, 73 and 100%, and the median recurrence-free survival times were 46, 20, 9 and 3 months after the first, second, third and fourth surgeries, respectively. The 5-year overall survival rates were 82, 69, 40 and 0% after the first, second, third and fourth surgeries, respectively.
With repeated recurrence and surgeries, the time to recurrence decreased and the recurrence rate increased. R0 resection in the first surgery was considered the most important for longer recurrence-free survival and radical cure.
Introduction
Retroperitoneal sarcomas account for ~15% of soft tissue sarcomas in adults (1). Although complete surgical resection remains the most effective treatment for retroperitoneal sarcomas, the large size of these tumours, their inaccessible location and their proximity to vital structures often make complete resection difficult (2). As a result, local recurrence after surgery with curative intent is commonly observed in these tumours, and recurrence finally leads to tumour-related deaths (3,4).
Retroperitoneal sarcomas include various histologic subtypes, amongst which liposarcoma is the most commonly observed (5). Retroperitoneal liposarcoma (RLS) is usually found as a large tumour because it does not cause symptoms and develops silently in the retroperitoneal compartment. Unlike liposarcoma in the extremities, a large RLS is difficult to resect with a sufficient surgical margin. Moreover, it is sometimes difficult to macroscopically determine the borders of these tumours, particularly in well-differentiated liposarcoma. As a result, pathological evaluations for resected RLS specimens frequently demonstrate a positive surgical margin. For these reasons, local recurrence more commonly occurs in RLS than in other histologic subtype of retroperitoneal sarcoma (6). Because the cause of death in most patients with RLS is local recurrence without accompanying distant metastasis (5,7,8), understanding the characteristics of locally recurrent RLS is essential to improve the survival outcome of RLS.
The treatment option for locally recurrent RLS is surgical re-resection; however, the recurrence rate after the re-resection is also high (9). Therefore, surgery for locally recurrent RLS often results in repeated local recurrences and subsequent repeated surgeries (10). However, data regarding the clinicopathological characteristics of repeated recurrences of RLS and outcomes of subsequent repeated surgeries are limited.
Accordingly, the purpose of this study was to investigate the clinicopathological characteristics and outcomes of primary and recurrent RLS, with a particular focus on repeated recurrent RLS and subsequent repeated surgeries.
Methods
Participants
The medical records of patients who were admitted to Nagoya University Hospital between January 2005 and December 2018 and diagnosed as with primary or recurrent RLS were retrospectively reviewed. This study was approved by the ethics committee of Nagoya University Hospital (approval number 2019-0021) and registered in the University Hospital Medical Information Network (http://www.umin.ac.jp; UMIN-CTR ID: UMIN 000037502). Informed consent was obtained from all patients included in this study.
Data collection and definitions
The following demographic data were recorded: age at initial diagnosis, sex, chief complaint, tumour size and location, examination performed (biopsy, computed tomography [CT], magnetic resonance imaging [MRI] and positron emission tomography [PET]), preoperative diagnosis, adjuvant therapy, dates of first visit and surgery, pathological diagnosis and prognosis (recurrence and death). In patients with primary RLS, overall survival (OS) was defined as the time from the first visit to the time of death. In patients with recurrent RLS, OS was defined as the time from the radiographic detection of recurrence to the time of death. Recurrence-free survival (RFS) was defined as the time from surgery for primary or recurrent RLS to the time of recurrence or death. The surgery-to-recurrence interval was defined as the time of the previous surgery to the time of the next recurrence. The recurrence-to-surgery interval was defined as the time of recurrence to the time of surgery for recurrent RLS.
To describe locoregional disease patterns, ‘multifocal’ was defined as the presence of two or more non-contiguous tumours (11). The size of each tumour was evaluated by the maximum diameter of the tumour. Growth rate of the recurrence was defined as the tumour size (the maximum diameter in the case of a solitary tumour and the sum of all maximum diameters in the case of multifocal tumours) divided by the surgery-to-recurrence interval (9). Data associated with surgery (the operative procedure and post-operative complications) were also recorded. The severity of post-operative complications was classified using the Clavien–Dindo classification system (12). A significant complication was defined as a complication observed within 30 days after surgery with a Clavien–Dindo Grade ≥2.
Pathology and treatment
In this study, RLS was diagnosed by experienced pathologists and classified based on recent World Health Organization criteria into four histologic subtypes such as well-differentiated, dedifferentiated, myxoid and pleomorphic RLS. Microscopic margin status (R0 or R1) was also evaluated by the pathologists. A multidisciplinary sarcoma team composed of specialists in the Departments of Surgical Oncology, Medical Oncology and Radiation Oncology determined the treatment strategies for primary and recurrent RLS. In brief, an aggressive surgical resection, including adjacent organ resection, was challenged when the patient fulfilled the following criteria: (i) there was a possibility of R0 resection and radical cure, (ii) the adjacent organ resection was necessary for R0 resection, (iii) RLS was the major prognostic determinant of the patients and (iv) the patient has a tolerance for extended surgical resection. Chemotherapy, radiation therapy or both were performed when radical surgery was considered impossible. Preoperative or post-operative adjuvant therapy, including chemotherapy, radiation therapy or both, was also performed in some patients after discussion by the multidisciplinary team. In particular, neoadjuvant therapy was aggressively considered when the tumour size was huge, and the tumour invaded multiple organs. Adjuvant therapy (especially radiation therapy) was also aggressively considered when the surgery resulted in R1 resection or the tumour was close to the surgical margin. After the surgical resection of primary or recurrent RLS, follow-up examination of all patients with CT of the chest, abdomen and pelvis was carried out every 3–6 months for at least 5 years. Additional radiographic evaluation with MRI and PET was conducted when CT revealed a suspected recurrent lesion of suspected recurrence.
| . | Total n = 52 . | Surgery n = 46 . | No surgery n = 6 . | P value . |
|---|---|---|---|---|
| Age (years) | 61 (53–68) | 61 (54–68) | 55 (42–70) | 0.475 |
| Male sex, n (%) | 29 (56) | 25 (54) | 4 (67) | 0.568 |
| Chief complaint, n (%) | 0.249 | |||
| Pain | 24 (46) | 19 (41) | 5 (83) | |
| Palpable mass | 14 (27) | 14 (30) | 1 (17) | |
| Incidental (from imaging) | 8 (17) | 8 (17) | 0 (0) | |
| Unknown | 6 (12) | 6 (13) | 0 (0) | |
| Tumour size (cm) | 15 (10–23) | 15 (10–23) | 15 (9–23) | 0.891 |
| Tumour laterality (right side), n (%) | 28 (54) | 26 (56) | 2 (33) | 0.397 |
| Examination before treatment, n (%) | ||||
| Biopsy, n (%) | 21 (40) | 16 (35) | 5 (83) | 0.034 |
| CT, n (%) | 40 (77) | 34 (74) | 6 (100) | 0.316 |
| MRI, n (%) | 24 (46) | 20 (43) | 4 (67) | 0.397 |
| PET, n (%) | 13 (25) | 11 (24) | 2 (33) | 0.632 |
| Diagnosis of liposarcoma before treatment, n (%) | 40 (77) | 35 (76) | 5 (83) | 0.692 |
| Multifocal disease, n (%) | 15 (29) | 14 (30) | 1 (17) | 0.659 |
| Lymph node metastasis, n (%) | 4 (8) | 2 (4) | 2 (33) | 0.061 |
| Distant metastasis, n (%) | 3 (6) | 0 (0) | 3 (50) | 0.001 |
| Pathological subtype, n (%) | 0.497 | |||
| Well-differentiated | 22 (42) | 21 (46) | 1 (17) | |
| Dedifferentiated | 25 (48) | 21 (46) | 4 (67) | |
| Myxoid | 4 (8) | 3 (7) | 1 (17) | |
| Pleomorphic | 1 (2) | 1 (2) | 0 (0) |
| . | Total n = 52 . | Surgery n = 46 . | No surgery n = 6 . | P value . |
|---|---|---|---|---|
| Age (years) | 61 (53–68) | 61 (54–68) | 55 (42–70) | 0.475 |
| Male sex, n (%) | 29 (56) | 25 (54) | 4 (67) | 0.568 |
| Chief complaint, n (%) | 0.249 | |||
| Pain | 24 (46) | 19 (41) | 5 (83) | |
| Palpable mass | 14 (27) | 14 (30) | 1 (17) | |
| Incidental (from imaging) | 8 (17) | 8 (17) | 0 (0) | |
| Unknown | 6 (12) | 6 (13) | 0 (0) | |
| Tumour size (cm) | 15 (10–23) | 15 (10–23) | 15 (9–23) | 0.891 |
| Tumour laterality (right side), n (%) | 28 (54) | 26 (56) | 2 (33) | 0.397 |
| Examination before treatment, n (%) | ||||
| Biopsy, n (%) | 21 (40) | 16 (35) | 5 (83) | 0.034 |
| CT, n (%) | 40 (77) | 34 (74) | 6 (100) | 0.316 |
| MRI, n (%) | 24 (46) | 20 (43) | 4 (67) | 0.397 |
| PET, n (%) | 13 (25) | 11 (24) | 2 (33) | 0.632 |
| Diagnosis of liposarcoma before treatment, n (%) | 40 (77) | 35 (76) | 5 (83) | 0.692 |
| Multifocal disease, n (%) | 15 (29) | 14 (30) | 1 (17) | 0.659 |
| Lymph node metastasis, n (%) | 4 (8) | 2 (4) | 2 (33) | 0.061 |
| Distant metastasis, n (%) | 3 (6) | 0 (0) | 3 (50) | 0.001 |
| Pathological subtype, n (%) | 0.497 | |||
| Well-differentiated | 22 (42) | 21 (46) | 1 (17) | |
| Dedifferentiated | 25 (48) | 21 (46) | 4 (67) | |
| Myxoid | 4 (8) | 3 (7) | 1 (17) | |
| Pleomorphic | 1 (2) | 1 (2) | 0 (0) |
Data are presented as medians with IQRs or numbers with percentages (%).
RLS, retroperitoneal liposarcoma; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; IQR, interquartile range.
| . | Total n = 52 . | Surgery n = 46 . | No surgery n = 6 . | P value . |
|---|---|---|---|---|
| Age (years) | 61 (53–68) | 61 (54–68) | 55 (42–70) | 0.475 |
| Male sex, n (%) | 29 (56) | 25 (54) | 4 (67) | 0.568 |
| Chief complaint, n (%) | 0.249 | |||
| Pain | 24 (46) | 19 (41) | 5 (83) | |
| Palpable mass | 14 (27) | 14 (30) | 1 (17) | |
| Incidental (from imaging) | 8 (17) | 8 (17) | 0 (0) | |
| Unknown | 6 (12) | 6 (13) | 0 (0) | |
| Tumour size (cm) | 15 (10–23) | 15 (10–23) | 15 (9–23) | 0.891 |
| Tumour laterality (right side), n (%) | 28 (54) | 26 (56) | 2 (33) | 0.397 |
| Examination before treatment, n (%) | ||||
| Biopsy, n (%) | 21 (40) | 16 (35) | 5 (83) | 0.034 |
| CT, n (%) | 40 (77) | 34 (74) | 6 (100) | 0.316 |
| MRI, n (%) | 24 (46) | 20 (43) | 4 (67) | 0.397 |
| PET, n (%) | 13 (25) | 11 (24) | 2 (33) | 0.632 |
| Diagnosis of liposarcoma before treatment, n (%) | 40 (77) | 35 (76) | 5 (83) | 0.692 |
| Multifocal disease, n (%) | 15 (29) | 14 (30) | 1 (17) | 0.659 |
| Lymph node metastasis, n (%) | 4 (8) | 2 (4) | 2 (33) | 0.061 |
| Distant metastasis, n (%) | 3 (6) | 0 (0) | 3 (50) | 0.001 |
| Pathological subtype, n (%) | 0.497 | |||
| Well-differentiated | 22 (42) | 21 (46) | 1 (17) | |
| Dedifferentiated | 25 (48) | 21 (46) | 4 (67) | |
| Myxoid | 4 (8) | 3 (7) | 1 (17) | |
| Pleomorphic | 1 (2) | 1 (2) | 0 (0) |
| . | Total n = 52 . | Surgery n = 46 . | No surgery n = 6 . | P value . |
|---|---|---|---|---|
| Age (years) | 61 (53–68) | 61 (54–68) | 55 (42–70) | 0.475 |
| Male sex, n (%) | 29 (56) | 25 (54) | 4 (67) | 0.568 |
| Chief complaint, n (%) | 0.249 | |||
| Pain | 24 (46) | 19 (41) | 5 (83) | |
| Palpable mass | 14 (27) | 14 (30) | 1 (17) | |
| Incidental (from imaging) | 8 (17) | 8 (17) | 0 (0) | |
| Unknown | 6 (12) | 6 (13) | 0 (0) | |
| Tumour size (cm) | 15 (10–23) | 15 (10–23) | 15 (9–23) | 0.891 |
| Tumour laterality (right side), n (%) | 28 (54) | 26 (56) | 2 (33) | 0.397 |
| Examination before treatment, n (%) | ||||
| Biopsy, n (%) | 21 (40) | 16 (35) | 5 (83) | 0.034 |
| CT, n (%) | 40 (77) | 34 (74) | 6 (100) | 0.316 |
| MRI, n (%) | 24 (46) | 20 (43) | 4 (67) | 0.397 |
| PET, n (%) | 13 (25) | 11 (24) | 2 (33) | 0.632 |
| Diagnosis of liposarcoma before treatment, n (%) | 40 (77) | 35 (76) | 5 (83) | 0.692 |
| Multifocal disease, n (%) | 15 (29) | 14 (30) | 1 (17) | 0.659 |
| Lymph node metastasis, n (%) | 4 (8) | 2 (4) | 2 (33) | 0.061 |
| Distant metastasis, n (%) | 3 (6) | 0 (0) | 3 (50) | 0.001 |
| Pathological subtype, n (%) | 0.497 | |||
| Well-differentiated | 22 (42) | 21 (46) | 1 (17) | |
| Dedifferentiated | 25 (48) | 21 (46) | 4 (67) | |
| Myxoid | 4 (8) | 3 (7) | 1 (17) | |
| Pleomorphic | 1 (2) | 1 (2) | 0 (0) |
Data are presented as medians with IQRs or numbers with percentages (%).
RLS, retroperitoneal liposarcoma; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; IQR, interquartile range.
Statistical analysis
Data for continuous variables are expressed as medians with interquartile ranges (IQRs) because many continuous variables were not normally distributed. Comparisons between the two groups were conducted using the non-parametric Wilcoxon rank-sum test. Categorical variables are described as numbers with percentages, and differences between the two groups were analysed using Pearson’s χ2 test unless the expected score in any of the cells was ≤5; Fisher’s exact test was used in such situations.
For survival time analysis, OS and RFS curves, 3- and 5-year RFS rates, 5- and 10-year OS rates and median survival times (MSTs) with 95% confidence intervals (CIs) were estimated using the Kaplan–Meier method. The log-rank test was applied to assess differences in OS and RFS between groups. Univariate Cox regression analyses were conducted to identify significant prognostic factors associated with OS and RFS in the patients who had undergone surgery. In addition, the significant prognostic factors were separately assessed in well-differentiated RLS and dedifferentiated RLS as a subgroup analysis.
All statistical analyses were conducted using IBM SPSS Statistics version 24.0 for Windows (SPSS, Inc., Chicago, IL, USA). A two-tailed P value of <0.05 indicated statistical significance.
Results
Participants and baseline characteristics
A total of 52 patients were analysed. The median age was 61 years (IQR 53–68), and 29 patients (56%) were male. Amongst the 52 patients, 22 patients (42%) had the well-differentiated subtype, 25 patients (48%) had the dedifferentiated subtype, 4 patients (8%) had the myxoid subtype and 1 patient (2%) had the pleomorphic subtype. During the study period, 13 patients (25%) died of liposarcoma and 2 patients died of other causes. The median follow-up time for the 37 surviving patients was 64 months (IQR 35–101). Forty-six patients (88%) underwent surgery with curative intent for primary RLS. The remaining 6 patients underwent chemotherapy and/or radiation therapy because of far locally advancement tumour (n = 3) or distant metastasis (n = 3). Age, sex, chief complaint, tumour size and location and pathological subtype were not significantly different between patients that did or did not undergo surgery (Table 1).
Surgery for primary RLS
In 36 (78%) of the 46 patients who underwent surgery for primary RLS, combined resection of other organs was required. These concomitantly resected organs were the kidney and/or adrenal gland (n = 24), digestive tract (n = 9), muscle and/or bone (n = 6), pancreas and/or spleen (n = 5), gonads (n = 4) and major vessels (n = 2). Pathologic evidence of direct organ invasion of the tumour was observed in 9 out of the 36 patients (25%). Significant post-operative complications occurred in 6 patients (13%). These complications were pancreatic fistula (n = 2), duodenal stenosis (n = 1), pneumonia (n = 1), portal vein thrombosis (n = 1) and lymphorrhoea (n = 1). As shown by survival time analysis, the OS in patients who underwent surgery for primary RLS was significantly longer than that in patients who did not undergo surgery (Fig. 1).
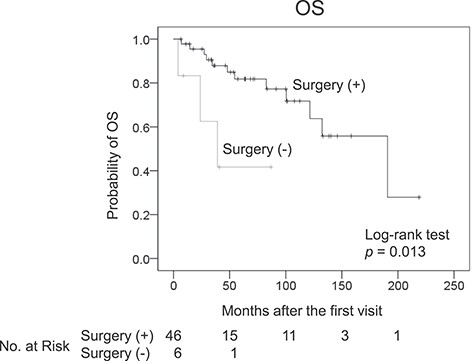
Kaplan–Meier plot for the overall survival (OS) of patients with and without surgery for primary retroperitoneal liposarcoma.
Prognostic factors associated with RFS and OS
Of the 46 patients who underwent surgery for primary RLS, 30 patients (65%) experienced recurrence and 10 patients (22%) died of recurrence after surgery. The recurrence site was local in 30 patients and distant in 2 patients (lung). The 3- and 5-year RFS rates and MST after the first surgery were 53% (95% CI 38–68), 36% (95% CI 21–51) and 46 months (95% CI 16–76), respectively. The 5- and 10-year OS rates and MST in the 46 patients were 82% (95% CI 70–94), 72% (95% CI 55–89) and 191 months (95% CI 106–275), respectively. Univariate Cox regression analysis demonstrated that R0 resection was associated with a longer RFS-first and the well-differentiated subtype was associated with both a longer RFS-first and a longer OS (Table 2). The patients undergoing R0 resection in the first surgery had significantly higher RFS rates compared with the patients undergoing non-R0 resection (3-year RFS: 80 versus 38%; 5-year RFS: 49 versus 29%, P = 0.033). When the patients with well-differentiated RLS and dedifferentiated RLS were separately analysed, the superiority of R0 resection for a longer RFS-first did not reach to a statistical significance (P = 0.066 in well-differentiated RLS and P = 0.866 in dedifferentiated RLS). In addition, subgroup analysis limited to those with dedifferentiated RLS showed that combined resection of other organs was associated with a longer RFS-first, although pathological evidence of direct tumour invasion to other organs was observed in only 7 out of 17 patients (Supplementary Fig. 1). Although OS rates tended to be higher in the patients undergoing R0 resection compared with the non-R0 resection, it did not reach to a statistical significant difference (5-year OS: 93 versus 75%; 10-year OS: 93 versus 59%, P = 0.124).
Univariate Cox regression analysis of RFS after the first surgery (RFS-first) and OS
| . | RFS-first . | OS . | ||||
|---|---|---|---|---|---|---|
| . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age at the first surgery (years) | 0.987 | 0.962–1.013 | 0.315 | 1.018 | 0.968–1.071 | 0.484 |
| Male sex | 1.987 | 0.929–4.251 | 0.077 | 2.692 | 0.789–9.184 | 0.114 |
| Tumour size >15 cm | 0.584 | 0.274–2.244 | 0.163 | 0.402 | 0.120–1.135 | 0.140 |
| Tumour laterality (right side) | 0.678 | 0.316–1.454 | 0.318 | 0.895 | 0.259–3.100 | 0.861 |
| Multifocal disease | 1.858 | 0.814–4.245 | 0.141 | 1.855 | 0.532–6.472 | 0.332 |
| Preoperative biopsy | 1.132 | 0.576–2.226 | 0.719 | 1.298 | 0.423–3.983 | 0.684 |
| Diagnosis of liposarcoma before surgery | 0.508 | 0.231–1.121 | 0.094 | 0.467 | 0.135–1.619 | 0.230 |
| Lymph node metastasis | 1.124 | 0.151–8.371 | 0.909 | 0.046 | 0.000–137600 | 0.685 |
| Other organ resection | 1.590 | 0.619–4.079 | 0.335 | 1.864 | 0.392–8.849 | 0.434 |
| Significant post-operative complications | 0.709 | 0.414–1.216 | 0.211 | 0.839 | 0.295–2.387 | 0.742 |
| R0 resection | 0.423 | 0.187–0.956 | 0.039 | 0.319 | 0.068–1.483 | 0.145 |
| Well-differentiated subtype | 0.289 | 0.127–0.656 | 0.003 | 0.190 | 0.048–0.749 | 0.018 |
| Adjuvant therapy | 0.571 | 0.198–1.642 | 0.298 | 0.408 | 0.052–3.208 | 0.394 |
| . | RFS-first . | OS . | ||||
|---|---|---|---|---|---|---|
| . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age at the first surgery (years) | 0.987 | 0.962–1.013 | 0.315 | 1.018 | 0.968–1.071 | 0.484 |
| Male sex | 1.987 | 0.929–4.251 | 0.077 | 2.692 | 0.789–9.184 | 0.114 |
| Tumour size >15 cm | 0.584 | 0.274–2.244 | 0.163 | 0.402 | 0.120–1.135 | 0.140 |
| Tumour laterality (right side) | 0.678 | 0.316–1.454 | 0.318 | 0.895 | 0.259–3.100 | 0.861 |
| Multifocal disease | 1.858 | 0.814–4.245 | 0.141 | 1.855 | 0.532–6.472 | 0.332 |
| Preoperative biopsy | 1.132 | 0.576–2.226 | 0.719 | 1.298 | 0.423–3.983 | 0.684 |
| Diagnosis of liposarcoma before surgery | 0.508 | 0.231–1.121 | 0.094 | 0.467 | 0.135–1.619 | 0.230 |
| Lymph node metastasis | 1.124 | 0.151–8.371 | 0.909 | 0.046 | 0.000–137600 | 0.685 |
| Other organ resection | 1.590 | 0.619–4.079 | 0.335 | 1.864 | 0.392–8.849 | 0.434 |
| Significant post-operative complications | 0.709 | 0.414–1.216 | 0.211 | 0.839 | 0.295–2.387 | 0.742 |
| R0 resection | 0.423 | 0.187–0.956 | 0.039 | 0.319 | 0.068–1.483 | 0.145 |
| Well-differentiated subtype | 0.289 | 0.127–0.656 | 0.003 | 0.190 | 0.048–0.749 | 0.018 |
| Adjuvant therapy | 0.571 | 0.198–1.642 | 0.298 | 0.408 | 0.052–3.208 | 0.394 |
RFS, recurrence-free survival; OS, overall survival; CI, confidence interval.
A significant post-operative complication was defined as a complication observed within 30 days after surgery with Clavien–Dindo Grade ≥2. Adjuvant therapy includes chemotherapy, radiation therapy or both performed before or after surgery.
Univariate Cox regression analysis of RFS after the first surgery (RFS-first) and OS
| . | RFS-first . | OS . | ||||
|---|---|---|---|---|---|---|
| . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age at the first surgery (years) | 0.987 | 0.962–1.013 | 0.315 | 1.018 | 0.968–1.071 | 0.484 |
| Male sex | 1.987 | 0.929–4.251 | 0.077 | 2.692 | 0.789–9.184 | 0.114 |
| Tumour size >15 cm | 0.584 | 0.274–2.244 | 0.163 | 0.402 | 0.120–1.135 | 0.140 |
| Tumour laterality (right side) | 0.678 | 0.316–1.454 | 0.318 | 0.895 | 0.259–3.100 | 0.861 |
| Multifocal disease | 1.858 | 0.814–4.245 | 0.141 | 1.855 | 0.532–6.472 | 0.332 |
| Preoperative biopsy | 1.132 | 0.576–2.226 | 0.719 | 1.298 | 0.423–3.983 | 0.684 |
| Diagnosis of liposarcoma before surgery | 0.508 | 0.231–1.121 | 0.094 | 0.467 | 0.135–1.619 | 0.230 |
| Lymph node metastasis | 1.124 | 0.151–8.371 | 0.909 | 0.046 | 0.000–137600 | 0.685 |
| Other organ resection | 1.590 | 0.619–4.079 | 0.335 | 1.864 | 0.392–8.849 | 0.434 |
| Significant post-operative complications | 0.709 | 0.414–1.216 | 0.211 | 0.839 | 0.295–2.387 | 0.742 |
| R0 resection | 0.423 | 0.187–0.956 | 0.039 | 0.319 | 0.068–1.483 | 0.145 |
| Well-differentiated subtype | 0.289 | 0.127–0.656 | 0.003 | 0.190 | 0.048–0.749 | 0.018 |
| Adjuvant therapy | 0.571 | 0.198–1.642 | 0.298 | 0.408 | 0.052–3.208 | 0.394 |
| . | RFS-first . | OS . | ||||
|---|---|---|---|---|---|---|
| . | Hazard ratio . | 95% CI . | P value . | Hazard ratio . | 95% CI . | P value . |
| Age at the first surgery (years) | 0.987 | 0.962–1.013 | 0.315 | 1.018 | 0.968–1.071 | 0.484 |
| Male sex | 1.987 | 0.929–4.251 | 0.077 | 2.692 | 0.789–9.184 | 0.114 |
| Tumour size >15 cm | 0.584 | 0.274–2.244 | 0.163 | 0.402 | 0.120–1.135 | 0.140 |
| Tumour laterality (right side) | 0.678 | 0.316–1.454 | 0.318 | 0.895 | 0.259–3.100 | 0.861 |
| Multifocal disease | 1.858 | 0.814–4.245 | 0.141 | 1.855 | 0.532–6.472 | 0.332 |
| Preoperative biopsy | 1.132 | 0.576–2.226 | 0.719 | 1.298 | 0.423–3.983 | 0.684 |
| Diagnosis of liposarcoma before surgery | 0.508 | 0.231–1.121 | 0.094 | 0.467 | 0.135–1.619 | 0.230 |
| Lymph node metastasis | 1.124 | 0.151–8.371 | 0.909 | 0.046 | 0.000–137600 | 0.685 |
| Other organ resection | 1.590 | 0.619–4.079 | 0.335 | 1.864 | 0.392–8.849 | 0.434 |
| Significant post-operative complications | 0.709 | 0.414–1.216 | 0.211 | 0.839 | 0.295–2.387 | 0.742 |
| R0 resection | 0.423 | 0.187–0.956 | 0.039 | 0.319 | 0.068–1.483 | 0.145 |
| Well-differentiated subtype | 0.289 | 0.127–0.656 | 0.003 | 0.190 | 0.048–0.749 | 0.018 |
| Adjuvant therapy | 0.571 | 0.198–1.642 | 0.298 | 0.408 | 0.052–3.208 | 0.394 |
RFS, recurrence-free survival; OS, overall survival; CI, confidence interval.
A significant post-operative complication was defined as a complication observed within 30 days after surgery with Clavien–Dindo Grade ≥2. Adjuvant therapy includes chemotherapy, radiation therapy or both performed before or after surgery.
Characteristics of recurrent RLS
Of the 30 patients who experienced a first recurrence, 24 patients underwent a second surgery. Amongst these 24 patients, 16 patients (67%) experienced a second recurrence. The second recurrence site was local in 14 patients and distant in 2 patients (liver and lung). Amongst these 16 patients, 11 patients underwent a third surgery for the second recurrence and 8 patients (73%) experienced a third recurrence. Amongst the 8 patients with a third recurrence, 4 patients underwent a fourth surgery and all the 4 patients (100%) experienced a fourth recurrence. Amongst the 4 patients with a fourth recurrence, 2 patients underwent subsequent surgeries (up to fifth and sixth surgeries). All surgeries were performed with curative intent. Clinicopathological characteristics at the time of the first–fourth recurrences are summarized in Table 3. With an increasing number of recurrences, the surgery-to-recurrence interval and the proportion of the well-differentiated subtype decreased and the proportion of the multifocal local recurrence, dedifferentiated subtype and growth rate increased. Of the 22 patients initially diagnosed with well-differentiated liposarcoma, 6 patients (27%) developed recurrence with a dedifferentiated component through repeated recurrences.
| . | First recurrence n = 30 . | Second recurrence n = 16 . | Third recurrence n = 8 . | Fourth recurrence n = 4 . |
|---|---|---|---|---|
| Age at recurrence (years) | 64 (55–70) | 64 (49–70) | 59 (49–70) | 67 (55–74) |
| Male gender, n (%) | 18 (60) | 5 (31) | 1 (13) | 1 (25) |
| Surgery-to-recurrence interval (months) | 18 (10–49) | 21 (8–41) | 9 (5–14) | 4 (3–8) |
| Multifocal disease, n (%) | 14 (47) | 11 (69) | 7 (88) | 3 (75) |
| Growth rate (cm/month) | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 1.1 (0.1–1.8) | 2.3 (1.6–2.9) |
| Local recurrence, n (%) | 30 (100) | 14 (88) | 8 (100) | 4 (100) |
| Distant metastasis, n (%) | 2 (7) | 2 (13) | 0 (0) | 0(0) |
| Pathological subtype, n (%) | ||||
| Well-differentiated | 11 (37) | 6 (38) | 1 (13) | 0 (0) |
| Dedifferentiated | 11 (37) | 5 (31) | 3 (38) | 2 (50) |
| Myxoid | 2 (7) | 0 (0) | 0 (0) | 0 (0) |
| Pleomorphic | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 6 (20) | 5 (31) | 4 (50) | 2 (50) |
| . | First recurrence n = 30 . | Second recurrence n = 16 . | Third recurrence n = 8 . | Fourth recurrence n = 4 . |
|---|---|---|---|---|
| Age at recurrence (years) | 64 (55–70) | 64 (49–70) | 59 (49–70) | 67 (55–74) |
| Male gender, n (%) | 18 (60) | 5 (31) | 1 (13) | 1 (25) |
| Surgery-to-recurrence interval (months) | 18 (10–49) | 21 (8–41) | 9 (5–14) | 4 (3–8) |
| Multifocal disease, n (%) | 14 (47) | 11 (69) | 7 (88) | 3 (75) |
| Growth rate (cm/month) | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 1.1 (0.1–1.8) | 2.3 (1.6–2.9) |
| Local recurrence, n (%) | 30 (100) | 14 (88) | 8 (100) | 4 (100) |
| Distant metastasis, n (%) | 2 (7) | 2 (13) | 0 (0) | 0(0) |
| Pathological subtype, n (%) | ||||
| Well-differentiated | 11 (37) | 6 (38) | 1 (13) | 0 (0) |
| Dedifferentiated | 11 (37) | 5 (31) | 3 (38) | 2 (50) |
| Myxoid | 2 (7) | 0 (0) | 0 (0) | 0 (0) |
| Pleomorphic | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 6 (20) | 5 (31) | 4 (50) | 2 (50) |
Data are presented as medians with IQRs or numbers with percentages.
| . | First recurrence n = 30 . | Second recurrence n = 16 . | Third recurrence n = 8 . | Fourth recurrence n = 4 . |
|---|---|---|---|---|
| Age at recurrence (years) | 64 (55–70) | 64 (49–70) | 59 (49–70) | 67 (55–74) |
| Male gender, n (%) | 18 (60) | 5 (31) | 1 (13) | 1 (25) |
| Surgery-to-recurrence interval (months) | 18 (10–49) | 21 (8–41) | 9 (5–14) | 4 (3–8) |
| Multifocal disease, n (%) | 14 (47) | 11 (69) | 7 (88) | 3 (75) |
| Growth rate (cm/month) | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 1.1 (0.1–1.8) | 2.3 (1.6–2.9) |
| Local recurrence, n (%) | 30 (100) | 14 (88) | 8 (100) | 4 (100) |
| Distant metastasis, n (%) | 2 (7) | 2 (13) | 0 (0) | 0(0) |
| Pathological subtype, n (%) | ||||
| Well-differentiated | 11 (37) | 6 (38) | 1 (13) | 0 (0) |
| Dedifferentiated | 11 (37) | 5 (31) | 3 (38) | 2 (50) |
| Myxoid | 2 (7) | 0 (0) | 0 (0) | 0 (0) |
| Pleomorphic | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 6 (20) | 5 (31) | 4 (50) | 2 (50) |
| . | First recurrence n = 30 . | Second recurrence n = 16 . | Third recurrence n = 8 . | Fourth recurrence n = 4 . |
|---|---|---|---|---|
| Age at recurrence (years) | 64 (55–70) | 64 (49–70) | 59 (49–70) | 67 (55–74) |
| Male gender, n (%) | 18 (60) | 5 (31) | 1 (13) | 1 (25) |
| Surgery-to-recurrence interval (months) | 18 (10–49) | 21 (8–41) | 9 (5–14) | 4 (3–8) |
| Multifocal disease, n (%) | 14 (47) | 11 (69) | 7 (88) | 3 (75) |
| Growth rate (cm/month) | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 1.1 (0.1–1.8) | 2.3 (1.6–2.9) |
| Local recurrence, n (%) | 30 (100) | 14 (88) | 8 (100) | 4 (100) |
| Distant metastasis, n (%) | 2 (7) | 2 (13) | 0 (0) | 0(0) |
| Pathological subtype, n (%) | ||||
| Well-differentiated | 11 (37) | 6 (38) | 1 (13) | 0 (0) |
| Dedifferentiated | 11 (37) | 5 (31) | 3 (38) | 2 (50) |
| Myxoid | 2 (7) | 0 (0) | 0 (0) | 0 (0) |
| Pleomorphic | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 6 (20) | 5 (31) | 4 (50) | 2 (50) |
Data are presented as medians with IQRs or numbers with percentages.
| . | First surgery n = 46 . | Second surgery n = 24 . | Third surgery n = 11 . | Fourth surgery n = 4 . |
|---|---|---|---|---|
| Age at surgery (years) | 61 (54–68) | 63 (48–69) | 56 (45–69) | 66 (54–73) |
| Male sex, n (%) | 25 (54) | 12 (50) | 2 (18) | 1 (25) |
| Recurrence-to-surgery interval (months) | NA | 3 (1–6) | 4 (1–7) | 10 (1–21) |
| Other organ resection, n (%) | 36 (78) | 17 (71) | 2 (18) | 1 (25) |
| Pathological tumour invasion to other organs, n (%) | 9/36 (25) | 5/17 (29) | 2/2 (100) | 1/1 (100) |
| R0 resection, n (%) | 16 (35) | 8 (33) | 1 (9) | 0 (0) |
| Significant post-operative complications, n (%) | 6 (13) | 7 (29) | 2 (18) | 0 (0) |
| Post-operative 90-day mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Recurrence after surgery, n (%) | 30 (65) | 16 (67) | 8 (73) | 4 (100) |
| Median survival time for RFS (months) | 46 (16–76) | 24 (18–30) | 9 (8–9) | 3 (1–5) |
| 3-year RFS (%) | 53 (38–68) | 34 (12–56) | 20 (0–45) | 0 (0–0) |
| 5-year RFS (%) | 36 (21–51) | 0 (0–0) | 10 (0–29) | 0 (0–0) |
| Median survival time for OS (months) | 191 (106–275) | 90 (54–125) | 56 (30–82) | 35 (24–45) |
| 5-year OS after surgery (%) | 82 (70–94) | 69 (47–90) | 40 (0–79) | 0 (0–0) |
| 10-year OS after surgery (%) | 72 (55–89) | 35 (6–65) | 0 (0–0) | 0 (0–0) |
| . | First surgery n = 46 . | Second surgery n = 24 . | Third surgery n = 11 . | Fourth surgery n = 4 . |
|---|---|---|---|---|
| Age at surgery (years) | 61 (54–68) | 63 (48–69) | 56 (45–69) | 66 (54–73) |
| Male sex, n (%) | 25 (54) | 12 (50) | 2 (18) | 1 (25) |
| Recurrence-to-surgery interval (months) | NA | 3 (1–6) | 4 (1–7) | 10 (1–21) |
| Other organ resection, n (%) | 36 (78) | 17 (71) | 2 (18) | 1 (25) |
| Pathological tumour invasion to other organs, n (%) | 9/36 (25) | 5/17 (29) | 2/2 (100) | 1/1 (100) |
| R0 resection, n (%) | 16 (35) | 8 (33) | 1 (9) | 0 (0) |
| Significant post-operative complications, n (%) | 6 (13) | 7 (29) | 2 (18) | 0 (0) |
| Post-operative 90-day mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Recurrence after surgery, n (%) | 30 (65) | 16 (67) | 8 (73) | 4 (100) |
| Median survival time for RFS (months) | 46 (16–76) | 24 (18–30) | 9 (8–9) | 3 (1–5) |
| 3-year RFS (%) | 53 (38–68) | 34 (12–56) | 20 (0–45) | 0 (0–0) |
| 5-year RFS (%) | 36 (21–51) | 0 (0–0) | 10 (0–29) | 0 (0–0) |
| Median survival time for OS (months) | 191 (106–275) | 90 (54–125) | 56 (30–82) | 35 (24–45) |
| 5-year OS after surgery (%) | 82 (70–94) | 69 (47–90) | 40 (0–79) | 0 (0–0) |
| 10-year OS after surgery (%) | 72 (55–89) | 35 (6–65) | 0 (0–0) | 0 (0–0) |
NA, not applied.
Data are presented as medians with IQRs or numbers with percentages (%), except for RFS and OS, which are presented as median survival times, and 3- and 5-year RFS and 5- and 10-year OS, which are presented as percentages. Median survival times for RFS and OS and percentages for the 3- and 5-year RFS and 5- and 10-year OS are presented with 95% CIs. A significant post-operative complication was defined as a complication observed within 30 days after surgery with Clavien–Dindo Grade ≥2.
| . | First surgery n = 46 . | Second surgery n = 24 . | Third surgery n = 11 . | Fourth surgery n = 4 . |
|---|---|---|---|---|
| Age at surgery (years) | 61 (54–68) | 63 (48–69) | 56 (45–69) | 66 (54–73) |
| Male sex, n (%) | 25 (54) | 12 (50) | 2 (18) | 1 (25) |
| Recurrence-to-surgery interval (months) | NA | 3 (1–6) | 4 (1–7) | 10 (1–21) |
| Other organ resection, n (%) | 36 (78) | 17 (71) | 2 (18) | 1 (25) |
| Pathological tumour invasion to other organs, n (%) | 9/36 (25) | 5/17 (29) | 2/2 (100) | 1/1 (100) |
| R0 resection, n (%) | 16 (35) | 8 (33) | 1 (9) | 0 (0) |
| Significant post-operative complications, n (%) | 6 (13) | 7 (29) | 2 (18) | 0 (0) |
| Post-operative 90-day mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Recurrence after surgery, n (%) | 30 (65) | 16 (67) | 8 (73) | 4 (100) |
| Median survival time for RFS (months) | 46 (16–76) | 24 (18–30) | 9 (8–9) | 3 (1–5) |
| 3-year RFS (%) | 53 (38–68) | 34 (12–56) | 20 (0–45) | 0 (0–0) |
| 5-year RFS (%) | 36 (21–51) | 0 (0–0) | 10 (0–29) | 0 (0–0) |
| Median survival time for OS (months) | 191 (106–275) | 90 (54–125) | 56 (30–82) | 35 (24–45) |
| 5-year OS after surgery (%) | 82 (70–94) | 69 (47–90) | 40 (0–79) | 0 (0–0) |
| 10-year OS after surgery (%) | 72 (55–89) | 35 (6–65) | 0 (0–0) | 0 (0–0) |
| . | First surgery n = 46 . | Second surgery n = 24 . | Third surgery n = 11 . | Fourth surgery n = 4 . |
|---|---|---|---|---|
| Age at surgery (years) | 61 (54–68) | 63 (48–69) | 56 (45–69) | 66 (54–73) |
| Male sex, n (%) | 25 (54) | 12 (50) | 2 (18) | 1 (25) |
| Recurrence-to-surgery interval (months) | NA | 3 (1–6) | 4 (1–7) | 10 (1–21) |
| Other organ resection, n (%) | 36 (78) | 17 (71) | 2 (18) | 1 (25) |
| Pathological tumour invasion to other organs, n (%) | 9/36 (25) | 5/17 (29) | 2/2 (100) | 1/1 (100) |
| R0 resection, n (%) | 16 (35) | 8 (33) | 1 (9) | 0 (0) |
| Significant post-operative complications, n (%) | 6 (13) | 7 (29) | 2 (18) | 0 (0) |
| Post-operative 90-day mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Recurrence after surgery, n (%) | 30 (65) | 16 (67) | 8 (73) | 4 (100) |
| Median survival time for RFS (months) | 46 (16–76) | 24 (18–30) | 9 (8–9) | 3 (1–5) |
| 3-year RFS (%) | 53 (38–68) | 34 (12–56) | 20 (0–45) | 0 (0–0) |
| 5-year RFS (%) | 36 (21–51) | 0 (0–0) | 10 (0–29) | 0 (0–0) |
| Median survival time for OS (months) | 191 (106–275) | 90 (54–125) | 56 (30–82) | 35 (24–45) |
| 5-year OS after surgery (%) | 82 (70–94) | 69 (47–90) | 40 (0–79) | 0 (0–0) |
| 10-year OS after surgery (%) | 72 (55–89) | 35 (6–65) | 0 (0–0) | 0 (0–0) |
NA, not applied.
Data are presented as medians with IQRs or numbers with percentages (%), except for RFS and OS, which are presented as median survival times, and 3- and 5-year RFS and 5- and 10-year OS, which are presented as percentages. Median survival times for RFS and OS and percentages for the 3- and 5-year RFS and 5- and 10-year OS are presented with 95% CIs. A significant post-operative complication was defined as a complication observed within 30 days after surgery with Clavien–Dindo Grade ≥2.
Surgery for recurrent RLS
Table 4 summarizes the characteristics and outcomes of the first–fourth surgeries. The post-operative morbidities of the second–fourth surgeries were not different from that of the first surgery, and no patients died post-operatively (within 90 days after surgery). Combined other organ resection was performed in 17 out of 24 patients (71%) in the second surgery, and the concomitantly resected organs were the digestive tract (n = 11), pancreas and/or spleen (n = 4), kidney (n = 2), muscle and/or bone (n = 2), major vessels (n = 2) and liver (n = 1). Pathological tumour invasion was observed in 5 out of these 17 patients (29%). Achieving R0 resection was rare in the third and fourth surgeries, and the MST for RFS became shorter with an increasing number of recurrences (Fig. 2). Amongst 30 patients with first recurrence, surgery was performed in 24 patients and the remaining 6 patients received chemotherapy (n = 4) and radiation therapy (n = 2). The MST for OS after the second surgery was 90 months (95% CI 54–125), whereas that of the patients without the second surgery was 21 months (95% CI 3–39, P = 0.018, Fig. 3A). Amongst 16 patients with a second recurrence, surgery was performed in 11 patients and the remaining 5 patients received chemotherapy (n = 2), chemoradiotherapy (n = 1) or best supportive care (n = 2). The MST for OS after the third surgery was 56 months (95% CI 30–82) and that of the patients without the third surgery was 16 months (95% CI 0–35, P = 0.100, Fig. 3B).
Discussion
In this study, we investigated the clinicopathological characteristics of primary and recurrent RLS and the outcomes of surgery for primary and recurrent RLS. Repeated local recurrences were common in RLS, and the malignant potential of the tumours seemed to increase with repeated recurrences. R0 resection in the first surgery for primary RLS and well-differentiated subtype were associated with longer RFS. The RFS after the third and subsequent surgeries for repeated recurrent RLS were poor, probably because achieving R0 resection was quite difficult in these surgeries. Although the third surgery did not yield a significantly longer OS compared with that without the third surgery, the OS after the third surgery was acceptable (MST 56 months; 5-year OS rate 40%).
Complete surgical resection of primary RLS remains the mainstay of curative treatment for RLS. In this study, the well-differentiated subtype and R0 resection in the first surgery were associated with better outcomes, whereas combined resection of adjacent organs did not contribute to prolong RFS or OS (Table 2). These results correspond with those of previous studies (8,13,14). However, several other authors have reported that aggressive primary surgery with combined resection of other organs led to better local control (15,16). In this study, when patients were limited to those with primary dedifferentiated RLS that is expected to have shorter RFS than well-differentiated RLS, combined resection of other organs was associated with a longer RFS. Therefore, for primary dedifferentiated RLS, an extended surgery including combined resection of other organs is currently recommended to secure a negative surgical margin and achieve prolonged local control. However, whether extended surgery should be performed and whether an adjacent organ should be preserved as much as possible in case of primary well-differentiated RLS remain uncertain. These issues need to be clarified in a future prospective study.
Repeated local recurrences following surgical resection of primary and locally recurrent RLS are frequently observed; as a result, surgeons often wonder if and how many times surgical resection should be repeated. In the present study, although R0 resection was usually not achieved in surgery for locally recurrent RLS, a second surgery for the first local recurrence of RLS improved survival compared with that with no surgery, as shown in previous studies (5,17). This study also intended to evaluate the benefits of third and subsequent surgeries for repeated local recurrences of RLS. The third surgery could not yield either enough RFS or significantly longer OS compared with that without the third surgery. The OS after the third surgery was not poor; however, this may be because the RLS usually grows slowly especially in well-differentiated subtype, and the local recurrence is not immediately life-threatening. In addition, insufficient sample size and difference of patient and tumour backgrounds made it difficult to assess the effects of the third and subsequent surgeries. An interventional study or large cohort study enough to adjust the background differences is needed to determine the benefits of the third and subsequent surgeries.
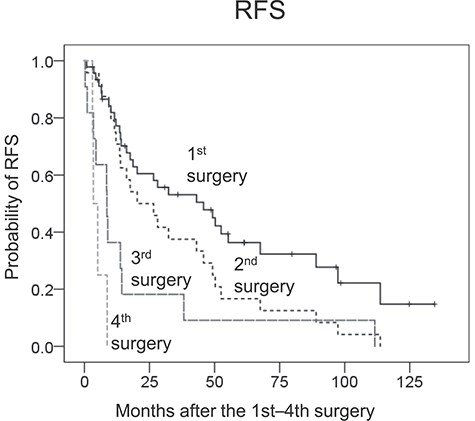
Kaplan–Meier plots for the recurrence-free survival (RFS) of patients after the first, second, third and fourth surgeries.
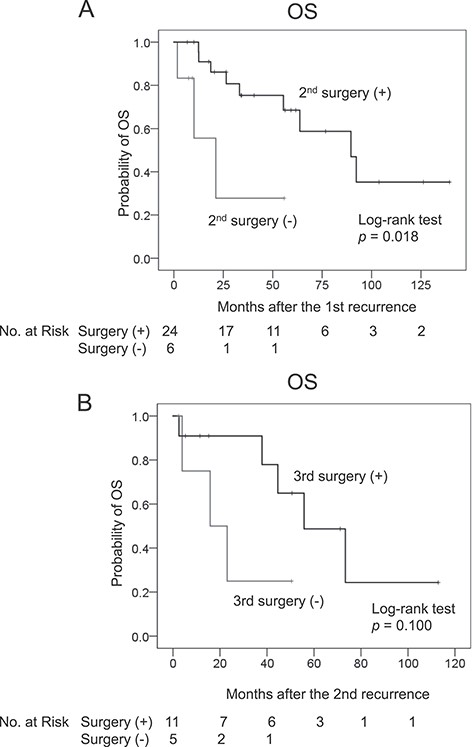
Kaplan–Meier plots for the OS of patients after (A) the second and (B) third surgeries.
In this study, the RLS growth rate often increased, and some patients with initially well-differentiated RLS developed recurrence with a dedifferentiated component through repeating recurrences. Park et al. (9) have demonstrated that the local recurrence growth rate was a strong prognostic factor after complete resection of the first local recurrences; however, they did not refer to the growth rate in the second and subsequent recurrences. This study first provided the data regarding the growth rate in the second and subsequent recurrences. Several authors reported that 7–17% of patients who initially had well-differentiated RLS and developed a first local recurrence exhibited recurrence as dedifferentiated RLS (8,18). Singer et al. (8) also reported that the rate of this transformation was increased by up to 44% for the second local recurrence. These characteristics of RLS may be one of the reasons why the third and subsequent surgeries did not yield an enough RFS in the current study. The pathological mechanism of this transformation has not been well investigated. Possible explanations are that mutations causing this transformation sometimes occur through repeating recurrences or that tumour cells with potential malignancy as dedifferentiated liposarcoma have already diffusely invaded the area around the tumour. Further study is necessary to elucidate the pathological mechanism of this transformation in RLS.
The present study has several limitations. First, this study was conducted at a single institution, and the sample size was not large. Ideally, the survival benefit of surgery for repeated recurrent RLS should be evaluated by multivariate analysis, but this was difficult because of the small sample size in this study. Second, the data in this study were retrospectively collected and analysed. Some prognostic factors, such as the French Federation Nationale des Centres de Lutte Contre le Cancer grade, were missing and could not be included in the analysis. A multicentre prospective cohort study in which a large number of patients with multiple recurrent RLS are enrolled and predetermined variables are analysed is warranted. Third, although treatment strategies were discussed by the multidisciplinary sarcoma team at our institution, chemotherapy regimens and the indication of adjuvant therapy were not completely consistent during the study period. These variations may have affected the OS and RFS of the patients and biased the results of comparisons of the group that underwent surgery and the group that did not undergo surgery. A consistent strategy regarding non-surgical treatment needs to be established at our institution. Finally, adjuvant therapy (radiation therapy and/or chemotherapy) was not performed in the patients that underwent third and subsequent surgeries for repeated local recurrences. Further studies may demonstrate that the third and subsequent surgeries with adjuvant therapy provide better outcomes.
In conclusion, repeated local recurrences are commonly observed even after aggressive surgery for primary and recurrent RLS. The first surgery for primary RLS and the second surgery for the first local recurrences were potentially curative treatment, and R0 resection in the first surgery was considered the most important. Although the third and subsequent surgeries could not provide enough RFS or significantly longer OS compared with that of patients that did not receive surgery, the survival outcome after the third surgery for the second recurrences was not poor. Further study to investigate the effect of adjuvant therapies, including radiation and chemotherapy, for not only the primary tumour but also repeated recurrent RLS is warranted.
Conflict of interest statement
There is no conflict of interest.


