-
PDF
- Split View
-
Views
-
Cite
Cite
Kazuhiro Suzuki, Nobuaki Matsubara, Hirotaka Kazama, Takeshi Seto, Shoko Tsukube, Hideyasu Matsuyama, Safety and efficacy of cabazitaxel in 660 patients with metastatic castration-resistant prostate cancer in real-world settings: results of a Japanese post-marketing surveillance study, Japanese Journal of Clinical Oncology, Volume 49, Issue 12, December 2019, Pages 1157–1163, https://doi.org/10.1093/jjco/hyz108
Close - Share Icon Share
Abstract
To evaluate the real-world safety and efficacy of cabazitaxel in Japanese patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with a docetaxel-containing regimen.
This prospective multicenter observational study registered all patients with mCRPC treated with cabazitaxel following its launch in Japan in September 2014. Patient enrollment continued until at least 500 patients were enrolled. Adverse drug reactions (ADRs) were evaluated according to CTCAE ver. 4.0. Efficacy endpoints were assessed for up to 1 year, and included prostate specific antigen (PSA) response rates (defined as a decrease of ≥30% or ≥50% from baseline), overall survival (OS), and time to treatment failure (TTF).
A total of 660 mCRPC patients were enrolled across 316 centers by June 2016. Frequent ADRs (any grade) were neutropenia (49.1%), febrile neutropenia (18.0%) and anemia (15.0%). Most ADRs occurred in cycle 1. Neutropenia and febrile neutropenia were significantly less frequent in patients who received prophylactic granulocyte colony-stimulating factor. The PSA response rates for decrease of ≥30% or ≥50% from baseline were 28.1% and 17.5%, respectively, in patients with baseline PSA of ≥5 ng/ml. Median OS and TTF were 319 days (95% confidence interval: 293.0–361.0) and 116 days (95% confidence interval: 108.0–135.0), respectively.
This study of cabazitaxel in 660 Japanese patients treated in real-world settings, the largest study of cabazitaxel to date, demonstrated a safety profile that was generally consistent with those of pivotal clinical studies. Cabazitaxel was also effective in terms of the PSA response, OS, and TTF.
Introduction
Prostate cancer (PC) is a relatively common cancer in developed countries, and is usually associated with a high survival rate. In the GLOBOCAN surveillance program, the age-standardized incidence rate was 30.4 per 100,000 person-years and the mortality rate was just 5.0 per 100,000 person-years in Japan (1). Meanwhile, the age-standardized 5-year survival rate was reported to increase from 85.9% in 2000–04 to 93.0% in 2010–14 (2), which may reflect continued improvements in treatment strategies.
Androgen deprivation therapy alone or in combination with an antiandrogen is widely performed for patients with advanced or metastatic PC, but almost all patients develop castration-resistant prostate cancer (CRPC) (3).
In the last 10 years, docetaxel-based chemotherapy has become the mainstay option for the treatment of patients with CRPC. More recently, several new treatment options have been introduced, including the androgen signaling inhibitors enzalutamide and abiraterone, the radionuclide radium-223, and the new taxane cabazitaxel (4,5). These drugs are now recommended for the treatment of CRPC in western guidelines for PC (6) and in the Japanese Urological Association evidence-based clinical practice guidelines for PC (7).
Cabazitaxel is a second-generation taxane that exhibited anti-tumor activity in docetaxel-sensitive and -resistant cell lines with stronger suppression of microtubule dynamics and better intracellular retention than docetaxel (8–11). Cabazitaxel was approved in 2010 in the USA as a second-line chemotherapy in combination with prednisone for the treatment of patients with hormone-refractory metastatic CRPC (mCRPC) previously treated with a docetaxel based on the results of the Phase III TROPIC study (12). Cabazitaxel was then approved in 2014 in Japan following a Phase I study that confirmed the pharmacokinetics and safety of cabazitaxel in Japanese patients were consistent with those reported in the prior global studies (13,14).
Several studies have also demonstrated that the safety profile of cabazitaxel, including the rates of hematologic and gastrointestinal adverse events (AEs), is generally consistent with those of the first-generation taxanes, docetaxel and paclitaxel (15–17). The results of clinical studies and post-marketing surveillance in real-world settings also suggest that the AEs of cabazitaxel can be managed by careful monitoring and dose reduction (18,19).
In the TROPIC study, cabazitaxel significantly increased the prostate specific antigen (PSA) response rate and improved overall survival (OS) by 2.4 months (15.1 vs 12.7 months; P < 0.0001) compared with mitoxantrone (12). In CAPRISTANA, an international multicenter, observational, prospective cohort study involving 189 patients treated with cabaxitazel in clinical settings, the median OS, progression-free survival, and time to treatment failure (TTF) were 13.2, 5.6 and 4.4 months, respectively (20). These efficacy results were consistent with those of the TROPIC, pre-registration Phase III study.
In the Phase I study in Japan, in which 44 patients received the maximum tolerated dose of cabazitaxel, the PSA response rate was 29.3% (n = 12/41) (14). The most frequent AEs (any grade) were neutropenia (100%), febrile neutropenia (54.5%), fatigue (54.5%), nausea (52.3%) and diarrhea (50.0%). Most patients received granulocyte colony-stimulating factor (G-CSF) in cycle 1 (86.4%) or in subsequent cycles (81.8%).
A retrospective, observational study of 47 patients (median age 70 years, range 46–85 years) treated with cabazitaxel in Japan provided further evidence that cabazitaxel was tolerable in Japanese patients with mCRPC, and the median OS was 16.1 months (21). However, these efficacy data from real-world daily practice are based on relatively small numbers of patients who were treated at a single hospital. Therefore, in order to confirm the efficacy and safety in real-world settings, observational data from a larger number of patients treated at multiple centers are needed.
Following the approval of cabazitaxel in Japan, a post-marketing surveillance study (PMS) was implemented with the objectives of monitoring its safety and tolerability in real-world clinical practice. Here, we report the results of this PMS, including those on its safety and efficacy.
Methods
Ethics
This PMS was designed by Sanofi K.K. in collaboration with and with the approval of the Japanese Pharmaceutical and Medical Devices Agency (PMDA). It was conducted in compliance with the Ministerial Ordinance on Good Postmarketing Study Practice for Drugs (GPSP) in Japan. In accordance with Japanese regulations and because data were collected using anonymized forms that could not be linked to individual patients, it was not necessary to obtain informed consent from the patients.
Survey objectives
The objectives of this PMS were to collect information on the safety and efficacy of cabazitaxel in real-world clinical settings in Japan for the treatment of mCRPC. This included recording unexpected adverse drug reactions (ADRs) and ADRs occurring in clinical practice. We also assessed factors that might influence the safety or efficacy of cabazitaxel in clinical use. In this paper, we describe the results observed in the full cohort of patients.
Patients and PMS design
This PMS registered all patients who were scheduled to start treatment with cabazitaxel for docetaxel-refractory mCRPC starting September 2014. It was planned to continue registration until the end of the 4-year registration period or once ~500 patients had been registered, whichever came first. The study was conducted under contracts with each participating site.
The investigators completed case-report forms to record follow-up data for patients who started and discontinued treatment or who completed treatment < 1 year after the start of cabazitaxel treatment and in patients who continued treatment for ≥1 year from the start of cabazitaxel treatment. The observation period (1 year) was set by the PMDA in consideration of a Phase I study in which only 10.4% of patients (5/48) received cabazitaxel for ≥1 year and because most AEs occurred within 1 year of the first dose without a marked increase in the incidences of AEs beyond this time.
As this was a non-interventional, observational study, all treatment decisions, including the dose and schedule of cabazitaxel, prophylaxis and concomitant therapies, were made by the patient’s physician in accordance with routine clinical practice and local treatment recommendations. Prophylaxis could include G-CSF at the physician’s discretion.
The approved dose and administration of cabazitaxel as described in the package insert for cabazitaxel (Jevtana® 25 mg/m2) and involves infusion over 1 hour every 3 weeks in combination with oral prednisolone administered daily throughout treatment. In December 2014, about 6 months after the approval of cabazitaxel, its package insert was amended to recommend prophylactic G-CSF especially for patients suseptible to febrile neutropenia.
Data collection
The case-report forms completed before starting cabazitaxel recorded the following information: patient demographics, Eastern Cooperative Oncology Group performance status, disease characteristics, treatment history and concomitant therapies for PC, and PSA levels. The case-report forms were also completed during each treatment cycle through to the end of the observation period/treatment discontinuation/death: exposure to cabazitaxel and prednisolone in each cycle, premedications, use of concomitant drugs, prophylactic use of G-CSF, PSA and AEs/ADRS.
Case-report forms for AE/ADR reporting recorded the following information: date of onset, AE term, grade (Common Terminology Criteria for Adverse Events version 4.0), seriousness, intervention, outcome, date of outcome/date outcome confirmed, causal relationship with cabazitaxel, possible causative factors other than cabazitaxel and abnormal laboratory values related to AEs. The following AEs were defined as priority survey items: neutropenia, febrile neutropenia, renal failure (including acute renal failure), sepsis and other serious infections, anemia, diarrhea, and peripheral neuropathy.
The patient’s survival status was followed up to 1 year. AEs/ADRs were recorded in the safety observation period, which was defined as whichever of the following was shortest: from first administration of cabazitaxel to the first administration of cabazitaxel over 1 year, to 30 days after the last administration of cabazitaxel, or during the survival period within 1 year.
Efficacy assessment
The efficacy of cabazitaxel was evaluated in terms of PSA response rates, OS and TTF. For PSA response rates, we assessed the proportions of patients with ≥50% or ≥30% decreases in PSA from baseline levels ≥20 ng/ml and ≥5 ng/ml. OS and TTF were determined as the times from the initiation of cabazitaxel to death (any cause) or discontinuation of cabazitaxel (any reason), respectively.
Statistical analysis
The target sample size was planned to be 500 patients for comprehensive evaluation of the safety profile of cabazitaxel. This sample size was deemed sufficient based on the incidence rates of the priority survey items that exceeded 1.0% in the Japanese Phase I study (14) and in the international Phase III studies (12,22).
Data from all patients who received at least one dose of cabazitaxel were included in the safety evaluation.
Baseline patient characteristics were summarized descriptively in terms of the mean ± standard deviation, median (range), or number (percent) of patients. The frequencies of ADRs were also analyzed descriptively in terms of the number (percent) of patients. For efficacy, the PSA response rate was summarized as the number (percent) of patients showing a PSA response of ≥50% or ≥30% from each of the two baseline levels (≥20 and ≥5 ng/ml). Median OS and TTF with 95% confidence intervals (CI) were estimated using the Kaplan–Meier method.
The proportions of patients who experienced neutropenia or febrile neutropenia were compared between those who received prophylactic G-CSF and those who did not using Fisher’s exact test. No other statistical comparisons were made.
Results
Patients
We initially planned to stop patient registration once the landmark of ~500 patients had been reached (or reaching the end of the 4-year registration period), but a total of 662 patients had been registered as of June 2015. Therefore, further registration was stopped at this time and all registered patients were to be included in the analyses. Of these 662 patients, 2 patients were excluded from the full analysis population because the case-report form was unavailable for the first patient and was completed by unauthorized personnel (and deemed ineligible) for the second patient. Therefore, we analyzed data for 660 patients. Patients were followed up through to June 2016.
The baseline characteristics of patients are shown in Table 1. The mean ± SD age was 70.0 ± 7.0 years, 526 patients (79.7%) were ≥65 years old and 180 patients (27.3%) were ≥75 years old. ECOG-PS was ≥1 and Gleason score was 8–10 in 37.4% and 78.2% of the patients, respectively. Bone metastases were found prior to start of cabazitaxel in 88.0% of the patients. The median (range) PSA level at baseline was 164.9 ng/ml (0.01−16 697.2 ng/ml). Nearly all of the patients had previously received endocrine therapy and/or chemotherapy; and 97.9%, 79.9% and 55.0% of patients had previously received docetaxel, enzalutamide,and abiraterone, respectively. Nearly half (48.3%) of patients were previously treated with both enzalutamide and abiraterone.
| Characteristics . | Value . |
|---|---|
| N | 660 |
| Age (years, mean ± SD) | 70.0 ± 7.0 |
| <65 years old, n (%) | 133 (20.2%) |
| ≥65 to <75 years old, n (%) | 346 (52.4%) |
| ≥75 years old, n (%) | 180 (27.3%) |
| BSA (m2) | |
| Mean ± SD | 1.65 ± 0.15 |
| Median (range) | 1.66 (1.26–2.20) |
| Time since initial diagnosis (years, mean ± SD) | 5.1 ± 3.3 |
| ECOG PS, n (%) | |
| 0 | 412 (62.4%) |
| 1 | 194 (29.4%) |
| ≥2 | 53 (8.0%) |
| Gleason score, n (%) | |
| 2–7 moderately differentiated | 104 (15.8%) |
| 8–10 poorly differentiated | 516 (78.2%) |
| Metastatic sites prior to cabazitaxel, n (%) | |
| Bone | 581 (88.0%) |
| Seminal vesicle | 78 (11.8%) |
| Bladder | 65 (9.9%) |
| Lung | 70 (10.6%) |
| Liver | 88 (13.3%) |
| PSA, ng/ml | |
| Available n | 654 |
| Mean ± SD | 501.1 ± 1199.9 |
| Median (range) | 164.9 (0.01–16 697.2) |
| Concomitant diseases, n (%) | |
| Presence | 275 (41.7%) |
| Liver disorder (presence) | 17 (2.6%) |
| Renal impairment (presence) | 14 (2.1%) |
| Allergic history | 19 (2.9%) |
| Bone marrow suppression | 41 (6.2%) |
| Infection | 19 (2.9%) |
| Prior treatments, n (%) | |
| Curative local excision | 212 (32.1%) |
| New-generation AR inhibitors | 571 (86.5%) |
| Enzalutamide | 527 (79.9%) |
| Abiraterone | 363 (55.0%) |
| Enzalutamide and abiraterone | 319 (48.3%) |
| Docetaxel chemotherapy | 646 (97.9%) |
| Initial dose of docetaxel | |
| 75 mg/m2 | 127 (19.2%) |
| 70 mg/m2 | 234 (35.5%) |
| 60 mg/m2 | 131 (19.8%) |
| Other | 137 (20.8%) |
| Number of cycles of docetaxel | |
| Mean ± SD | 12.6 ± 12.3 |
| Median (range) | 9.0 (1–143) |
| Palliative radiation therapy | 197 (29.9%) |
| Characteristics . | Value . |
|---|---|
| N | 660 |
| Age (years, mean ± SD) | 70.0 ± 7.0 |
| <65 years old, n (%) | 133 (20.2%) |
| ≥65 to <75 years old, n (%) | 346 (52.4%) |
| ≥75 years old, n (%) | 180 (27.3%) |
| BSA (m2) | |
| Mean ± SD | 1.65 ± 0.15 |
| Median (range) | 1.66 (1.26–2.20) |
| Time since initial diagnosis (years, mean ± SD) | 5.1 ± 3.3 |
| ECOG PS, n (%) | |
| 0 | 412 (62.4%) |
| 1 | 194 (29.4%) |
| ≥2 | 53 (8.0%) |
| Gleason score, n (%) | |
| 2–7 moderately differentiated | 104 (15.8%) |
| 8–10 poorly differentiated | 516 (78.2%) |
| Metastatic sites prior to cabazitaxel, n (%) | |
| Bone | 581 (88.0%) |
| Seminal vesicle | 78 (11.8%) |
| Bladder | 65 (9.9%) |
| Lung | 70 (10.6%) |
| Liver | 88 (13.3%) |
| PSA, ng/ml | |
| Available n | 654 |
| Mean ± SD | 501.1 ± 1199.9 |
| Median (range) | 164.9 (0.01–16 697.2) |
| Concomitant diseases, n (%) | |
| Presence | 275 (41.7%) |
| Liver disorder (presence) | 17 (2.6%) |
| Renal impairment (presence) | 14 (2.1%) |
| Allergic history | 19 (2.9%) |
| Bone marrow suppression | 41 (6.2%) |
| Infection | 19 (2.9%) |
| Prior treatments, n (%) | |
| Curative local excision | 212 (32.1%) |
| New-generation AR inhibitors | 571 (86.5%) |
| Enzalutamide | 527 (79.9%) |
| Abiraterone | 363 (55.0%) |
| Enzalutamide and abiraterone | 319 (48.3%) |
| Docetaxel chemotherapy | 646 (97.9%) |
| Initial dose of docetaxel | |
| 75 mg/m2 | 127 (19.2%) |
| 70 mg/m2 | 234 (35.5%) |
| 60 mg/m2 | 131 (19.8%) |
| Other | 137 (20.8%) |
| Number of cycles of docetaxel | |
| Mean ± SD | 12.6 ± 12.3 |
| Median (range) | 9.0 (1–143) |
| Palliative radiation therapy | 197 (29.9%) |
SD, standard deviation; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; PS, performance status; PSA, prostate specific antigen; AR, androgen receptor.
| Characteristics . | Value . |
|---|---|
| N | 660 |
| Age (years, mean ± SD) | 70.0 ± 7.0 |
| <65 years old, n (%) | 133 (20.2%) |
| ≥65 to <75 years old, n (%) | 346 (52.4%) |
| ≥75 years old, n (%) | 180 (27.3%) |
| BSA (m2) | |
| Mean ± SD | 1.65 ± 0.15 |
| Median (range) | 1.66 (1.26–2.20) |
| Time since initial diagnosis (years, mean ± SD) | 5.1 ± 3.3 |
| ECOG PS, n (%) | |
| 0 | 412 (62.4%) |
| 1 | 194 (29.4%) |
| ≥2 | 53 (8.0%) |
| Gleason score, n (%) | |
| 2–7 moderately differentiated | 104 (15.8%) |
| 8–10 poorly differentiated | 516 (78.2%) |
| Metastatic sites prior to cabazitaxel, n (%) | |
| Bone | 581 (88.0%) |
| Seminal vesicle | 78 (11.8%) |
| Bladder | 65 (9.9%) |
| Lung | 70 (10.6%) |
| Liver | 88 (13.3%) |
| PSA, ng/ml | |
| Available n | 654 |
| Mean ± SD | 501.1 ± 1199.9 |
| Median (range) | 164.9 (0.01–16 697.2) |
| Concomitant diseases, n (%) | |
| Presence | 275 (41.7%) |
| Liver disorder (presence) | 17 (2.6%) |
| Renal impairment (presence) | 14 (2.1%) |
| Allergic history | 19 (2.9%) |
| Bone marrow suppression | 41 (6.2%) |
| Infection | 19 (2.9%) |
| Prior treatments, n (%) | |
| Curative local excision | 212 (32.1%) |
| New-generation AR inhibitors | 571 (86.5%) |
| Enzalutamide | 527 (79.9%) |
| Abiraterone | 363 (55.0%) |
| Enzalutamide and abiraterone | 319 (48.3%) |
| Docetaxel chemotherapy | 646 (97.9%) |
| Initial dose of docetaxel | |
| 75 mg/m2 | 127 (19.2%) |
| 70 mg/m2 | 234 (35.5%) |
| 60 mg/m2 | 131 (19.8%) |
| Other | 137 (20.8%) |
| Number of cycles of docetaxel | |
| Mean ± SD | 12.6 ± 12.3 |
| Median (range) | 9.0 (1–143) |
| Palliative radiation therapy | 197 (29.9%) |
| Characteristics . | Value . |
|---|---|
| N | 660 |
| Age (years, mean ± SD) | 70.0 ± 7.0 |
| <65 years old, n (%) | 133 (20.2%) |
| ≥65 to <75 years old, n (%) | 346 (52.4%) |
| ≥75 years old, n (%) | 180 (27.3%) |
| BSA (m2) | |
| Mean ± SD | 1.65 ± 0.15 |
| Median (range) | 1.66 (1.26–2.20) |
| Time since initial diagnosis (years, mean ± SD) | 5.1 ± 3.3 |
| ECOG PS, n (%) | |
| 0 | 412 (62.4%) |
| 1 | 194 (29.4%) |
| ≥2 | 53 (8.0%) |
| Gleason score, n (%) | |
| 2–7 moderately differentiated | 104 (15.8%) |
| 8–10 poorly differentiated | 516 (78.2%) |
| Metastatic sites prior to cabazitaxel, n (%) | |
| Bone | 581 (88.0%) |
| Seminal vesicle | 78 (11.8%) |
| Bladder | 65 (9.9%) |
| Lung | 70 (10.6%) |
| Liver | 88 (13.3%) |
| PSA, ng/ml | |
| Available n | 654 |
| Mean ± SD | 501.1 ± 1199.9 |
| Median (range) | 164.9 (0.01–16 697.2) |
| Concomitant diseases, n (%) | |
| Presence | 275 (41.7%) |
| Liver disorder (presence) | 17 (2.6%) |
| Renal impairment (presence) | 14 (2.1%) |
| Allergic history | 19 (2.9%) |
| Bone marrow suppression | 41 (6.2%) |
| Infection | 19 (2.9%) |
| Prior treatments, n (%) | |
| Curative local excision | 212 (32.1%) |
| New-generation AR inhibitors | 571 (86.5%) |
| Enzalutamide | 527 (79.9%) |
| Abiraterone | 363 (55.0%) |
| Enzalutamide and abiraterone | 319 (48.3%) |
| Docetaxel chemotherapy | 646 (97.9%) |
| Initial dose of docetaxel | |
| 75 mg/m2 | 127 (19.2%) |
| 70 mg/m2 | 234 (35.5%) |
| 60 mg/m2 | 131 (19.8%) |
| Other | 137 (20.8%) |
| Number of cycles of docetaxel | |
| Mean ± SD | 12.6 ± 12.3 |
| Median (range) | 9.0 (1–143) |
| Palliative radiation therapy | 197 (29.9%) |
SD, standard deviation; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; PS, performance status; PSA, prostate specific antigen; AR, androgen receptor.
Overall, 377 patients (57.1%) died during the study period owing to primary disease (349, 92.6%), AEs (24, 6.4%), ADRs (15, 4.0%) or other reasons (12, 3.2%).
Cabazitaxel exposure
The exposure of cabazitaxel treatment is shown in Table 2. The median initial dose and median dose per cycle were 20.0 mg/m2 and 20.0 mg/m2/cycle, respectively. More patients received an initial dose or dose per cycle of 20 to <25 mg/m2 than other dose levels (<15, 15 to <20, and ≥25 mg/m2). The median actual dose intensity was 5.6 mg/m2/week, and the median relative dose intensity of cabazitaxel was 67.2%. The median number of cycles of cabazitaxel was 4.0, and the median duration of each cycle was 28 days.
| Doses and treatment duration . | Value . | |
|---|---|---|
| N | 660 | |
| Initial dose (mg/m2) | ||
| Median (range) | 20.0 | (10.0–26.3) |
| <15 | 17 | (2.6%) |
| 15 to <20 | 117 | (17.7%) |
| 20 to <25 | 327 | (49.6%) |
| ≥25 | 199 | (30.2%) |
| Dose per cycle (mg/m2/cycle) | ||
| Median (range) | 20.0 | (10.0–25.5) |
| <15 | 15 | (2.3%) |
| 15 to <20 | 151 | (22.9%) |
| 20 to <25 | 376 | (57.0%) |
| ≥25 | 118 | (17.9%) |
| Cumulative dose (mg/m2), median (range) | 84.4 | (10.0–445.0) |
| Actual dose intensity (mg/m2/week), median (range) | 5.6 | (1.5–8.4) |
| Relative dose intensity* (%), median (range) | 67.2 | (17.8–101.0) |
| Number of treatment cycles, median (range) | 4.0 | (1–18) |
| Duration of each treatment cycle (days), median (range) | 28 | (10–202) |
| Doses and treatment duration . | Value . | |
|---|---|---|
| N | 660 | |
| Initial dose (mg/m2) | ||
| Median (range) | 20.0 | (10.0–26.3) |
| <15 | 17 | (2.6%) |
| 15 to <20 | 117 | (17.7%) |
| 20 to <25 | 327 | (49.6%) |
| ≥25 | 199 | (30.2%) |
| Dose per cycle (mg/m2/cycle) | ||
| Median (range) | 20.0 | (10.0–25.5) |
| <15 | 15 | (2.3%) |
| 15 to <20 | 151 | (22.9%) |
| 20 to <25 | 376 | (57.0%) |
| ≥25 | 118 | (17.9%) |
| Cumulative dose (mg/m2), median (range) | 84.4 | (10.0–445.0) |
| Actual dose intensity (mg/m2/week), median (range) | 5.6 | (1.5–8.4) |
| Relative dose intensity* (%), median (range) | 67.2 | (17.8–101.0) |
| Number of treatment cycles, median (range) | 4.0 | (1–18) |
| Duration of each treatment cycle (days), median (range) | 28 | (10–202) |
*Calculated as a planned dose intensity of 8.33 mg/m2/week.
| Doses and treatment duration . | Value . | |
|---|---|---|
| N | 660 | |
| Initial dose (mg/m2) | ||
| Median (range) | 20.0 | (10.0–26.3) |
| <15 | 17 | (2.6%) |
| 15 to <20 | 117 | (17.7%) |
| 20 to <25 | 327 | (49.6%) |
| ≥25 | 199 | (30.2%) |
| Dose per cycle (mg/m2/cycle) | ||
| Median (range) | 20.0 | (10.0–25.5) |
| <15 | 15 | (2.3%) |
| 15 to <20 | 151 | (22.9%) |
| 20 to <25 | 376 | (57.0%) |
| ≥25 | 118 | (17.9%) |
| Cumulative dose (mg/m2), median (range) | 84.4 | (10.0–445.0) |
| Actual dose intensity (mg/m2/week), median (range) | 5.6 | (1.5–8.4) |
| Relative dose intensity* (%), median (range) | 67.2 | (17.8–101.0) |
| Number of treatment cycles, median (range) | 4.0 | (1–18) |
| Duration of each treatment cycle (days), median (range) | 28 | (10–202) |
| Doses and treatment duration . | Value . | |
|---|---|---|
| N | 660 | |
| Initial dose (mg/m2) | ||
| Median (range) | 20.0 | (10.0–26.3) |
| <15 | 17 | (2.6%) |
| 15 to <20 | 117 | (17.7%) |
| 20 to <25 | 327 | (49.6%) |
| ≥25 | 199 | (30.2%) |
| Dose per cycle (mg/m2/cycle) | ||
| Median (range) | 20.0 | (10.0–25.5) |
| <15 | 15 | (2.3%) |
| 15 to <20 | 151 | (22.9%) |
| 20 to <25 | 376 | (57.0%) |
| ≥25 | 118 | (17.9%) |
| Cumulative dose (mg/m2), median (range) | 84.4 | (10.0–445.0) |
| Actual dose intensity (mg/m2/week), median (range) | 5.6 | (1.5–8.4) |
| Relative dose intensity* (%), median (range) | 67.2 | (17.8–101.0) |
| Number of treatment cycles, median (range) | 4.0 | (1–18) |
| Duration of each treatment cycle (days), median (range) | 28 | (10–202) |
*Calculated as a planned dose intensity of 8.33 mg/m2/week.
Safety
Common ADRs
Table 3 shows the frequencies of ADRs occurring in 660 patients treated with cabazitaxel. ADRs of all grades and grade ≥3 occurred in 511 patients (77.4%, 1113 events) and 409 patients (62.0%, 644 events), respectively. ADRs (any grade) that were reported in ≥8% of patients included neutropenia (49.1%), febrile neutropenia (18.0%), anemia (15.0%), thrombocytopenia (11.7%), leukopenia (11.2%), and diarrhea (10.0%) (Table 3). Of these, neutropenia, febrile neutropenia, and anemia were evaluated as priority survey items. The rates of the other priority survey items were low (sepsis, 0.6%; septic shock, 0.5%; peripheral neuropathy, 1.5%). Grade ≥3 ADRs occurred in 409 patients. The most frequent grade ≥3 ADRs were neutropenia (39.8%), febrile neutropenia (17.1%), and anemia (8.8%). As shown in Table S1, the frequency of ADRs (any grade and grade ≥3) tended to increase in a dose-dependent manner with initial dose of cabazitaxel, particularly neutropenia and febrile neutropenia.
| Preferred term . | Any grade . | Grade ≥3 . | |||
|---|---|---|---|---|---|
| N | 660 | ||||
| Patients with any ADR | 511 | (77.4) | 409 | (62.0) | |
| Hematologic ADRs | |||||
| Anemia* | 99 | (15.0) | 58 | (8.8) | |
| Leukopenia | 74 | (11.2) | 48 | (7.3) | |
| Febrile neutropenia* | 119 | (18.0) | 113 | (17.1) | |
| Neutropenia * | 324 | (49.1) | 263 | (39.8) | |
| Thrombocytopenia | 77 | (11.7) | 36 | (5.5) | |
| Bone marrow failure | 2 | (0.3) | 2 | (0.3) | |
| Non-hematologic ADRs | |||||
| Pneumonia | 6 | (0.9) | 5 | (0.8) | |
| Pyelonephritis | 3 | (0.5) | 3 | (0.5) | |
| Sepsis* | 4 | (0.6) | 4 | (0.6) | |
| Septic shock* | 3 | (0.5) | 3 | (0.5) | |
| Decreased appetite | 49 | (7.4) | 11 | (1.7) | |
| Peripheral neuropathy* | 10 | (1.5) | 2 | (0.3) | |
| Interstitial lung disease | 8 | (1.2) | 7 | (1.1) | |
| Pneumonitis | 2 | (0.3) | 2 | (0.3) | |
| Diarrhea* | 66 | (10.0) | 21 | (3.2) | |
| Nausea | 22 | (3.3) | 4 | (0.6) | |
| Vomiting | 11 | (1.7) | 3 | (0.5) | |
| Liver disorders | 3 | (0.5) | 3 | (0.5) | |
| Malaise | 40 | (6.1) | 3 | (0.5) | |
| Pyrexia | 22 | (3.3) | 4 | (0.6) | |
| Preferred term . | Any grade . | Grade ≥3 . | |||
|---|---|---|---|---|---|
| N | 660 | ||||
| Patients with any ADR | 511 | (77.4) | 409 | (62.0) | |
| Hematologic ADRs | |||||
| Anemia* | 99 | (15.0) | 58 | (8.8) | |
| Leukopenia | 74 | (11.2) | 48 | (7.3) | |
| Febrile neutropenia* | 119 | (18.0) | 113 | (17.1) | |
| Neutropenia * | 324 | (49.1) | 263 | (39.8) | |
| Thrombocytopenia | 77 | (11.7) | 36 | (5.5) | |
| Bone marrow failure | 2 | (0.3) | 2 | (0.3) | |
| Non-hematologic ADRs | |||||
| Pneumonia | 6 | (0.9) | 5 | (0.8) | |
| Pyelonephritis | 3 | (0.5) | 3 | (0.5) | |
| Sepsis* | 4 | (0.6) | 4 | (0.6) | |
| Septic shock* | 3 | (0.5) | 3 | (0.5) | |
| Decreased appetite | 49 | (7.4) | 11 | (1.7) | |
| Peripheral neuropathy* | 10 | (1.5) | 2 | (0.3) | |
| Interstitial lung disease | 8 | (1.2) | 7 | (1.1) | |
| Pneumonitis | 2 | (0.3) | 2 | (0.3) | |
| Diarrhea* | 66 | (10.0) | 21 | (3.2) | |
| Nausea | 22 | (3.3) | 4 | (0.6) | |
| Vomiting | 11 | (1.7) | 3 | (0.5) | |
| Liver disorders | 3 | (0.5) | 3 | (0.5) | |
| Malaise | 40 | (6.1) | 3 | (0.5) | |
| Pyrexia | 22 | (3.3) | 4 | (0.6) | |
*Priority survey item.
ADR, adverse drug reaction.
Data are shown as n (%).
| Preferred term . | Any grade . | Grade ≥3 . | |||
|---|---|---|---|---|---|
| N | 660 | ||||
| Patients with any ADR | 511 | (77.4) | 409 | (62.0) | |
| Hematologic ADRs | |||||
| Anemia* | 99 | (15.0) | 58 | (8.8) | |
| Leukopenia | 74 | (11.2) | 48 | (7.3) | |
| Febrile neutropenia* | 119 | (18.0) | 113 | (17.1) | |
| Neutropenia * | 324 | (49.1) | 263 | (39.8) | |
| Thrombocytopenia | 77 | (11.7) | 36 | (5.5) | |
| Bone marrow failure | 2 | (0.3) | 2 | (0.3) | |
| Non-hematologic ADRs | |||||
| Pneumonia | 6 | (0.9) | 5 | (0.8) | |
| Pyelonephritis | 3 | (0.5) | 3 | (0.5) | |
| Sepsis* | 4 | (0.6) | 4 | (0.6) | |
| Septic shock* | 3 | (0.5) | 3 | (0.5) | |
| Decreased appetite | 49 | (7.4) | 11 | (1.7) | |
| Peripheral neuropathy* | 10 | (1.5) | 2 | (0.3) | |
| Interstitial lung disease | 8 | (1.2) | 7 | (1.1) | |
| Pneumonitis | 2 | (0.3) | 2 | (0.3) | |
| Diarrhea* | 66 | (10.0) | 21 | (3.2) | |
| Nausea | 22 | (3.3) | 4 | (0.6) | |
| Vomiting | 11 | (1.7) | 3 | (0.5) | |
| Liver disorders | 3 | (0.5) | 3 | (0.5) | |
| Malaise | 40 | (6.1) | 3 | (0.5) | |
| Pyrexia | 22 | (3.3) | 4 | (0.6) | |
| Preferred term . | Any grade . | Grade ≥3 . | |||
|---|---|---|---|---|---|
| N | 660 | ||||
| Patients with any ADR | 511 | (77.4) | 409 | (62.0) | |
| Hematologic ADRs | |||||
| Anemia* | 99 | (15.0) | 58 | (8.8) | |
| Leukopenia | 74 | (11.2) | 48 | (7.3) | |
| Febrile neutropenia* | 119 | (18.0) | 113 | (17.1) | |
| Neutropenia * | 324 | (49.1) | 263 | (39.8) | |
| Thrombocytopenia | 77 | (11.7) | 36 | (5.5) | |
| Bone marrow failure | 2 | (0.3) | 2 | (0.3) | |
| Non-hematologic ADRs | |||||
| Pneumonia | 6 | (0.9) | 5 | (0.8) | |
| Pyelonephritis | 3 | (0.5) | 3 | (0.5) | |
| Sepsis* | 4 | (0.6) | 4 | (0.6) | |
| Septic shock* | 3 | (0.5) | 3 | (0.5) | |
| Decreased appetite | 49 | (7.4) | 11 | (1.7) | |
| Peripheral neuropathy* | 10 | (1.5) | 2 | (0.3) | |
| Interstitial lung disease | 8 | (1.2) | 7 | (1.1) | |
| Pneumonitis | 2 | (0.3) | 2 | (0.3) | |
| Diarrhea* | 66 | (10.0) | 21 | (3.2) | |
| Nausea | 22 | (3.3) | 4 | (0.6) | |
| Vomiting | 11 | (1.7) | 3 | (0.5) | |
| Liver disorders | 3 | (0.5) | 3 | (0.5) | |
| Malaise | 40 | (6.1) | 3 | (0.5) | |
| Pyrexia | 22 | (3.3) | 4 | (0.6) | |
*Priority survey item.
ADR, adverse drug reaction.
Data are shown as n (%).
Neutropenia and febrile neutropenia
Figure 1 shows the frequencies of all neutropenia and febrile neutropenia events that occurred in cycle 1 in patients who received prophylactic G-CSF or not. Overall neutropenia events included neutropenia, febrile neutropenia, leukopenia, bone marrow failure, and pancytopenia. Overall, 225 patients (34.1%) started cabazitaxel without prophylactic G-CSF. The frequencies of overall neutropenia events (41.1% vs 79.6%) and febrile neutropenia (10.1% vs 16.0%) were significantly lower in patients who received prophylactic G-CSF than in patients who did not.
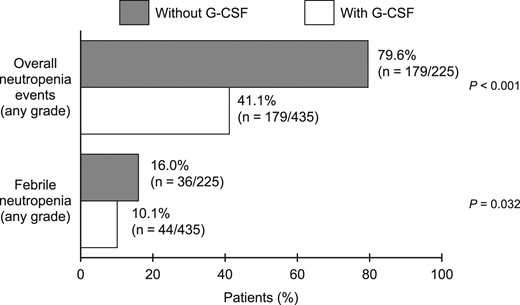
Frequencies of neutropenia (overall) and febrile neutropenia according to prophylactic G-CSF administration in cycle 1. G-CSF, granulocyte colony-stimulating factor; overall neutropenia events: all neutropenia-related events (includes neutropenia, febrile neutropenia, leukopenia, bone marrow failure and pancytopenia).
Figure S1 shows the incidences of overall neutropenia events, overall neutropenia events of grade ≥3, and febrile neutropenia for each cycle, regardless of whether or not G-CSF was administered prophylactically. As shown, the frequencies of overall neutropenia events and febrile neutropenia (any grade) were higher in cycle 1 than in later cycles, and remained relatively stable from cycle 2 onwards.
The frequencies of leukopenia, thrombocytopenia, anemia, and diarrhea in each treatment cycle are shown in Figure S2. The frequencies of these ADRs were low throughout cycles 2–10, although there was a slight increase in the frequency of anemia in cycles 8–10.
The frequencies of neutropenia, febrile neutropenia and grade 4/5 febrile neutropenia tended to increase with increasing initial dose of cabazitaxel (from <15 to ≥25 mg; Table S2). However, the frequencies of these events were consistently lower in patients who received prophylactic G-CSF.
Treatment-related deaths
There were 15 treatment-related deaths (2.3% of patients) in this study; seven of these patients received prophylactic G-CSF (Table S3). The most frequent causes of death were febrile neutropenia (8 patients) and interstitial lung disease (4 patients). In most cases, febrile neutropenia (7/8 patients) and interstitial lung disease (2/4 patients) occurred relatively early, in cycles 1–3 and cycles 1–2, respectively.
ADRs leading to discontinuation of cabazitaxel
ADRs resulted in treatment discontinuation in 91 patients (any grade: 13.8%), which included 70 patients (10.6%) with grade ≥3 ADRs (Table S4). The most frequent ADRs (any grade and grade ≥3) that led to treatment discontinuation were febrile neutropenia (3.0% and 3.0%, respectively), neutropenia (2.4% and 2.1%, respectively), thrombocytopenia (2.0% and 1.4%, respectively) and anemia (1.8% and 1.7%, respectively).
Efficacy
PSA response rates
Table 4 shows the PSA response rates according to the baseline PSA reference value (≥20 or ≥5 ng/ml) and the magnitude of change (≥30% or ≥50% decrease). Among patients with a baseline PSA level of ≥20 ng/ml, PSA decreased by ≥30% and ≥50% in 148 (27.9%) and 91 (17.1%) patients, respectively. Similar response rates were observed among patients with a baseline PSA of ≥5 ng/ml.
| Criteria . | n . | (%) . |
|---|---|---|
| Reference (1) | ||
| Number of patients with baseline PSA ≥20 ng/ml | 531 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 148 | (27.9) |
| ≥50% decrease | 91 | (17.1) |
| Reference (2) | ||
| Number of patients with baseline PSA ≥5 ng/ml | 601 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 169 | (28.1) |
| ≥50% decrease | 105 | (17.5) |
| Criteria . | n . | (%) . |
|---|---|---|
| Reference (1) | ||
| Number of patients with baseline PSA ≥20 ng/ml | 531 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 148 | (27.9) |
| ≥50% decrease | 91 | (17.1) |
| Reference (2) | ||
| Number of patients with baseline PSA ≥5 ng/ml | 601 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 169 | (28.1) |
| ≥50% decrease | 105 | (17.5) |
PSA, prostate specific antigen.
| Criteria . | n . | (%) . |
|---|---|---|
| Reference (1) | ||
| Number of patients with baseline PSA ≥20 ng/ml | 531 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 148 | (27.9) |
| ≥50% decrease | 91 | (17.1) |
| Reference (2) | ||
| Number of patients with baseline PSA ≥5 ng/ml | 601 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 169 | (28.1) |
| ≥50% decrease | 105 | (17.5) |
| Criteria . | n . | (%) . |
|---|---|---|
| Reference (1) | ||
| Number of patients with baseline PSA ≥20 ng/ml | 531 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 148 | (27.9) |
| ≥50% decrease | 91 | (17.1) |
| Reference (2) | ||
| Number of patients with baseline PSA ≥5 ng/ml | 601 | |
| Patients with a PSA response of: | ||
| ≥30% decrease | 169 | (28.1) |
| ≥50% decrease | 105 | (17.5) |
PSA, prostate specific antigen.
OS and TTF
Figures 2 and 3 show the Kaplan–Meier curves for OS and TTF, respectively. The median OS was 319.0 days (95% CI: 293.0–361.0 days; Fig. 2) and the median TTF was 116.0 days (95% CI: 108.0–135.0; Fig. 3).
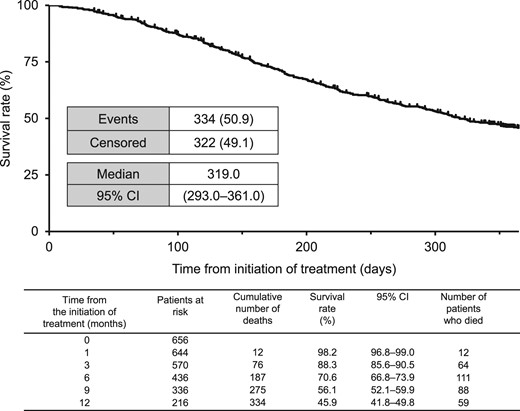
Kaplan–Meier plot of OS. CI, confidence interval; OS, overall survival.
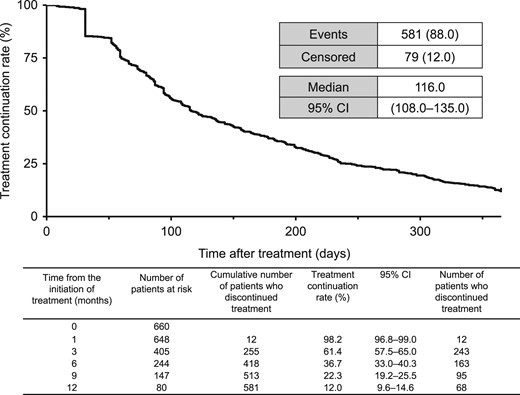
Kaplan–Meier plot of TTF. CI, confidence interval; TTF, time to treatment failure.
Discussion
This study was performed in real-world settings to evaluate the safety and efficacy of cabazitaxel in Japanese patients with mCRPC previously treated with a docetaxel-containing regimen. To the best of our knowledge, this is the largest observational study to assess cabazitaxel use in real-world settings. The safety profile of cabazitaxel observed in this study was generally similar to those observed in previous reports in the clinical trial setting (12,14,20,22), and new safety concerns were not reported. This study also revealed that cabazitaxel was effective in terms of the PSA response and median OS (319 days) and TTF (116 days) in patients with mCRPC.
Regarding safety, ADRs occurred in 77.4% of the patients. As expected, the most frequent ADRs were bone marrow toxicities, including neutropenia, febrile neutropenia, and thrombocytopenia. These hematologic and gastrointestinal ADRs that were associated with cabazitaxel in this study are generally consistent with the AEs reported for paclitaxel, docetaxel and cabazitaxel in previous studies (12,14,16,17,22). As might be expected, the frequencies of neutropenia-related ADRs and febrile neutropenia in cycle 1 were significantly lower in patients who received prophylactic G-CSF (41.1% and 10.1%, respectively) than in patients who did not (79.6% and 16.0%, respectively). In an open-label study, 21 Japanese mCRPC patients were treated with 25 mg/m2 cabazitaxel every 3 weeks (RDI 67.4%, range 53.2–91.3%) and pegfilgrastim (pegylated G-CSF) was administered prophylactically at a dose of 3.6 mg at least 24 h after cabazitaxel administration (23). The primary endpoint, the incidence of febrile neutropenia in cycle 1, occurred in 9.5% of patients (2/21 patients) and no additional events occurred in later cycles. Therefore, we suspect that prophylactic use of G-CSF in cycle 1 might have contributed to the lower incidence of overall neutropenia events and febrile neutropenia in the present study as compared with those in the previous Japanese Phase I studies (13,14). Although numerous hematologic and gastrointestinal ADRs were observed in this study, the results of previous studies suggest that these AEs could be managed with careful monitoring and dose reduction (18,19). The recommended initial dose of cabazitaxel is 25 mg/m2. However, we note that the initial dose was <25 mg/m2 in 461 patients (69.8%) and the dose per cycle was <25 mg/m2 in 542 patients (82.1%). Unfortunately, the reason for selecting a lower initial dose was not recorded, and we do not know whether the dose was reduced to control ADRs. Nevertheless, it is possible that lower doses were used for reasons of safety.
A total of 377 patients died during the study period, and most deaths were due to the primary disease (92.6%); 4.0% of deaths were due to ADRs. Meanwhile, 10.6% of patients discontinued cabazitaxel treatment due to grade ≥3 ADRs, such as febrile neutropenia (3.0%), neutropenia (2.1%), thrombocytopenia (1.4%), and anemia (1.7%). These safety results suggest that cabazitaxel is generally tolerated by Japanese patients with mCRPC, and no new safety concerns were identified.
Regarding efficacy, almost 30% of patients showed a PSA response with a decrease of ≥30% and nearly 18% showed a PSA decrease of ≥50% regardless of whether the baseline PSA level was ≥5 or ≥20 ng/ml. These PSA response rates compare favorably with those observed in the prior Phase I study, in which PSA decreased by ≥50% from a baseline level of ≥20 ng/ml in 29.3% of patients (14). In that study, the slightly higher response rate may be due to the administration of cabazitaxel at the maximum tolerated dose of 25 mg/m2; only about one-third of patients received cabazitaxel at an initial dose of ≥25 mg/m2 in the present study.
The median OS and TTF in the present study were 319 and 116 days, respectively. In the TROPIC study, cabazitaxel at a dose of 25 mg/m2 significantly increased OS compared with mitoxantrone (15.1 vs 12.7 months, P < 0.0001); both drugs were administered in combination with prednisone/prednisolone (12). Median OS and TTF were 13.2 months and 4.4 months in the CAPRISTANA study, in which patients with mCRPC previously treated with docetaxel were administered cabazitaxel at a dose of 25 mg/m2 every 3 weeks, as second-line therapy in 84.7% of patients or as third-/later-line in 15.3% (20). There are several possible explanations for the differences in OS among these studies. In particular, the patient characteristics differed. In our study, the Gleason score was 8–10 in 78.2% of patients and 88.0% had bone metastases. Additionally, nearly all of the patients (97.9%) had previously been treated with docetaxel, while 86.5% had been treated with the new AR inhibitors (abiraterone and/or enzalutamide). These features suggest that most of the patients included in this study had severe and highly progressed PC with poor differentiation and high malignancy. Furthermore, patients in our study received lower initial and median doses of cabazitaxel, most likely in consideration of safety.
Indeed, these differences are partly supported by the results of an international Phase III study, which compared the safety and efficacy of a reduced-dose regimen of cabazitaxel (20 mg/m2) versus the standard-dose regimen (25 mg/m2). Notably, the reduced-dose regimen was noninferior to the standard-dose regimen (25 mg/m2) in terms of median OS (13.4 vs 14.5 months, HR 1.024) but the PSA response rate was significantly lower with the reduced-dose regimen (29.5% vs 42.9%, P < 0.001). The frequencies of AEs were also lower with the reduced-dose group (22).
However, we must also acknowledge that the observation period of our study was 1 year, and 216 patients were alive and evaluable at the end of the observation period based on the Kaplan–Meier plot, and 80 patients were still on treatment. Therefore, it might be valuable to investigate the characteristics of these patients to determine possible prognostic factors for survival beyond 1 year.
This study has some limitations to discuss. First, this was a single-arm, non-interventional, observational study conducted in real-world settings. The doses/treatment periods of cabazitaxel and patient selection were determined by the investigators based on their clinical judgment, and it is possible that some patients received lower doses than perhaps might be necessary. Second, the patients registered in this study had heterogenous characteristics, including previous treatment history, baseline PS, and laboratory data. Owing to the study design, it was not possible to assess progression-freesurvival (PFS) in this cohort of patients, although PSA response rates and TTF should provide useful information on treatment decisions and likely patient outcomes in lieu of radiologic imaging in patients with mCRPC. Indeed, our data could provide a valuable reference for the use of cabazitaxel in daily clinical practice.
Conclusion
In this observational study, we evaluated the safety and efficacy of cabazitaxel in Japanese patients with mCRPC in real-world settings. The safety profile of cabazitaxel was generally consistent with those observed in previous clinical studies, and no new safety concerns were identified. The efficacy results are also consistent with those of the registration clinical trials. These safety and efficacy results obtained in real-world settings indicate that cabazitaxel is tolerated and effective for the treatment of Japanese patients with mCRPC, most of whom were previously treated with docetaxel and AR inhibitors.
Acknowledgments
This PMS was funded by Sanofi K.K., Tokyo, Japan. The authors thank Koichi Shirakawa (Sanofi K.K.) for support with reviewing data. Medical writing support was provided by Honyaku Center Inc. and Nicholas D. Smith (EMC K.K.) funded by Sanofi K.K. All authors have read and agreed with the content of this manuscript.
Conflict of interest statement
NM has received lecture fees from Janssen, AstraZeneca, Sanofi, Taiho, Novartis, Bayer, and Chugai, and research grants from Janssen, Sanofi, Bayer, Shionogi, Chugai, and MSD. HM has received travel grants, advisory board contract or speaker’s honorarium from Sanofi. KS has received lecture fees from Sanofi, Takeda, Astellas, Daiichi-Sankyo, and Bayer, and research grants from Takeda, Astellas, Daiichi-Sankyo, Bayer, and AstraZeneca. HK, ST and TS are employees of Sanofi.


