-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroji Iwata, Kenichi Inoue, Koji Kaneko, Yoshinori Ito, Koichiro Tsugawa, Ayumi Hasegawa, Shintaro Nakagawa, Hiroyasu Kuratomi, Kenji Tamura, Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130), Japanese Journal of Clinical Oncology, Volume 49, Issue 12, December 2019, Pages 1083–1091, https://doi.org/10.1093/jjco/hyz135
Close - Share Icon Share
Abstract
In the randomised Phase 3 IMpassion130 trial, atezolizumab combined with nab-paclitaxel (atezo + nab-P) in 902 patients with triple-negative breast cancer (TNBC) showed prolonged progression-free survival (PFS) in both the intention-to-treat (ITT) population and programmed death-ligand 1 (PD-L1)–positive subgroup compared with placebo plus nab-P (plac + nab-P). This study assessed the efficacy and safety of atezo + nab-P in the IMpassion130 Japanese subpopulation.
Eligible patients had unresectable locally advanced or metastatic TNBC previously untreated with chemotherapy for metastatic disease. Patients were randomised 1:1 to receive either atezo + nab-P or plac + nab-P. Co-primary endpoints were investigator-assessed PFS and overall survival (ITT population and PD-L1–positive subgroup). These were also assessed in the Japanese subpopulation.
There were 65 Japanese patients (34 atezo + nab-P; 31 plac + nab-P). The PD-L1–positive subgroup included 25 patients (12 atezo + nab-P; 13 plac + nab-P). Median PFS was 7.4 months (atezo + nab-P) versus 4.6 months (plac + nab-P; hazard ratio [HR], 0.47; 95% CI, 0.25–0.90). In the PD-L1–positive subgroup, median PFS was 10.8 months (atezo + nab-P) versus 3.8 months (plac + nab-P; HR, 0.04; 95% CI, <0.01–0.35). Safety results in the Japanese subgroup were consistent with those in the overall population. The Japanese subgroup had a lower incidence of adverse events leading to treatment withdrawal than the overall population. More patients in the atezo + nab-P arm had neutrophil count decreases and stomatitis than patients in the plac + nab-P arm.
Atezo + nab-P efficacy in Japanese patients was consistent with the overall IMpassion130 population. No new safety signals were observed, and tolerability was consistent with that of the overall population.
Introduction
Triple-negative breast cancer (TNBC) is an aggressive disease with poor clinical outcomes (1). Chemotherapy remains the standard of care for patients with TNBC, with most international guidelines recommending the use of single-agent taxane or anthracyclines for first-line therapy (2, 3). The median overall survival (OS) in patients with TNBC is estimated to be ≤18 months (4, 5) and highlights the need for new treatments.
Programmed death-ligand 1 (PD-L1) expression has been reported to occur mainly on tumour-infiltrating immune cells (IC) in patients with TNBC (6, 7), which leads to inhibition of anticancer immune responses (8, 9). This makes TNBC a good candidate for immune checkpoint inhibition treatment strategies. Atezolizumab (Tecentriq, F. Hoffmann-La Roche/Genentech) is a humanised monoclonal anti–PD-L1 antibody that selectively targets PD-L1 signalling to restore tumour-specific T-cell immunity (9). In the United States, it has been approved for use in the treatment of metastatic urothelial carcinoma as a monotherapy; in non–small-cell lung cancer as a monotherapy or in combination with bevacizumab, paclitaxel and carboplatin; and in small-cell lung cancer in combination with chemotherapy (10).
In patients with TNBC, single-agent atezolizumab has been shown to have a good safety profile and encouraging clinical activity (11). When used in combination with nab-paclitaxel chemotherapy in a Phase 1b study, atezolizumab demonstrated encouraging antitumour activity and a manageable safety profile in patients with metastatic TNBC (12). These findings were further supported by the IMpassion130 trial (ClinicalTrials.gov: NCT02425891), which investigated the efficacy of the first-line atezolizumab plus nab-paclitaxel in patients with locally advance or metastatic TNBC (13). The trial met its co-primary endpoint of progression-free survival (PFS), showing improved PFS in patients who were treated with atezolizumab plus nab-paclitaxel compared with placebo plus nab-paclitaxel in the intention-to-treat (ITT) population and the PD-L1–positive subgroup, and exhibited a safety profile in line with that of the individual agents. Interim OS analysis performed at the time of PFS final analysis showed that OS in the PD-L1–positive subgroup was also improved but not formally tested due to the study’s prespecified hierarchical statistical analysis plan. On the basis of PFS improvement, accelerated approval was granted in the United States for the use of atezolizumab in patients with PD-L1–positive metastatic TNBC. These data have also led to atezolizumab with nab-paclitaxel being listed as a category 2A recommendation in the National Comprehensive Cancer Network guidelines for breast cancer (2).
Numerous studies have documented differences in responses to systemic therapies and toxicities between Asian and Caucasian patients with cancer, including breast cancer (14–18). Recent data indicate that there is no difference in the efficacy and tolerability of atezolizumab between Japanese and non-Japanese patients with non–small-cell lung cancer (19). Thus, it is of clinical interest to assess the efficacy and tolerability of atezolizumab in Asian, particularly Japanese, patients with TNBC compared with those in the global population. This subgroup analysis of the IMpassion130 trial examines the efficacy and safety of atezolizumab in the Japanese population.
Patients and methods
Study design
IMpassion130 is an international, randomised, double-blind, placebo-controlled trial of first-line atezolizumab plus nab-paclitaxel compared with placebo plus nab-paclitaxel in patients with locally advanced or metastatic TNBC. Details of the overall study design were previously reported (13). The trial was conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Ethics approval was obtained from the institutional review board or ethics committee of each participating institution.
Patients
Key eligibility criteria for enrolment included being ≥18 years of age; having histologically confirmed metastatic or unresectable locally advanced TNBC (20, 21) that was measurable according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as evaluated by a local institute; having an Eastern Cooperative Oncology Group performance status of 0 or 1; being eligible to receive taxane monotherapy and having no prior chemotherapy or targeted therapy for metastatic TNBC. However, prior chemotherapy, including taxanes, in the neoadjuvant or adjuvant setting was allowed if it was completed ≥12 months prior to randomisation. Key exclusion criteria were known central nervous system (CNS) disease, except for patients with asymptomatic treated CNS disease; treatment with systemic corticosteroids or other systemic immunosuppressive medications within 2 weeks prior to randomisation or anticipated requirement during the study, history of autoimmune disease and/or previous treatment with immune checkpoint-targeting therapies.
Treatments
Enrolled patients were randomly assigned in a 1:1 ratio to receive either atezolizumab (840 mg) or placebo, administered intravenously on days 1 and 15, combined with nab-paclitaxel (100 mg/m2 body surface area), administered intravenously on days 1, 8 and 15 of every 28-day cycle. Randomisation was carried out using a permuted block method and an interactive voice/web response system. Patients received their assigned interventions until progression per RECIST 1.1 or the occurrence of unacceptable toxicity. In the absence of toxic effects, nab-paclitaxel was administered for at least six cycles. Discontinuation of atezolizumab, placebo or nab-paclitaxel could occur independently in the absence of disease progression, but dose reductions of atezolizumab or placebo were not allowed. To manage chemotherapy-related toxicity, prespecified dose modifications of nab-paclitaxel were permitted.
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 31) . | All patients (N = 65) . |
|---|---|---|---|
| Age | |||
| Median (range), years | 55.0 (31–82) | 64.0 (37–77) | 57.0 (31–82) |
| Distribution, n (%) | |||
| 18–40 years | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| 41–64 years | 22 (64.7) | 17 (54.8) | 39 (60.0) |
| ≥65 years | 9 (26.5) | 13 (41.9) | 22 (33.8) |
| Female sex, n (%) | 34 (100) | 31 (100) | 65 (100) |
| Baseline ECOG PS, n (%) | |||
| 0 | 28 (82.4) | 27 (87.1) | 55 (84.6) |
| 1 | 6 (17.6) | 4 (12.9) | 10 (15.4) |
| Metastatic disease, n (%) | 32 (94.1) | 22 (71.0) | 54 (83.1) |
| No. of sites of metastatic disease, n (%) | |||
| 0–3 | 27 (79.4) | 25 (80.6) | 52 (80.0) |
| ≥ 4 | 7 (20.6) | 6 (19.4) | 13 (20.0) |
| Site of metastatic disease, n (%) | |||
| Livera | 11 (32.4) | 6 (19.4) | 17 (26.2) |
| Bone | 7 (20.6) | 9 (29.0) | 16 (24.6) |
| Brain | 1 (2.9) | 0 | 1 (1.5) |
| Lung | 16 (47.1) | 12 (38.7) | 28 (43.1) |
| Lymph node only | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| Previous therapy, n (%) | |||
| Neoadjuvant or adjuvant therapy | 19 (55.9) | 11 (35.5) | 30 (46.2) |
| Taxanea | 15 (44.1) | 11 (35.5) | 26 (40.0) |
| Anthracycline | 17 (50.0) | 11 (35.5) | 28 (43.1) |
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 31) . | All patients (N = 65) . |
|---|---|---|---|
| Age | |||
| Median (range), years | 55.0 (31–82) | 64.0 (37–77) | 57.0 (31–82) |
| Distribution, n (%) | |||
| 18–40 years | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| 41–64 years | 22 (64.7) | 17 (54.8) | 39 (60.0) |
| ≥65 years | 9 (26.5) | 13 (41.9) | 22 (33.8) |
| Female sex, n (%) | 34 (100) | 31 (100) | 65 (100) |
| Baseline ECOG PS, n (%) | |||
| 0 | 28 (82.4) | 27 (87.1) | 55 (84.6) |
| 1 | 6 (17.6) | 4 (12.9) | 10 (15.4) |
| Metastatic disease, n (%) | 32 (94.1) | 22 (71.0) | 54 (83.1) |
| No. of sites of metastatic disease, n (%) | |||
| 0–3 | 27 (79.4) | 25 (80.6) | 52 (80.0) |
| ≥ 4 | 7 (20.6) | 6 (19.4) | 13 (20.0) |
| Site of metastatic disease, n (%) | |||
| Livera | 11 (32.4) | 6 (19.4) | 17 (26.2) |
| Bone | 7 (20.6) | 9 (29.0) | 16 (24.6) |
| Brain | 1 (2.9) | 0 | 1 (1.5) |
| Lung | 16 (47.1) | 12 (38.7) | 28 (43.1) |
| Lymph node only | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| Previous therapy, n (%) | |||
| Neoadjuvant or adjuvant therapy | 19 (55.9) | 11 (35.5) | 30 (46.2) |
| Taxanea | 15 (44.1) | 11 (35.5) | 26 (40.0) |
| Anthracycline | 17 (50.0) | 11 (35.5) | 28 (43.1) |
atezo, atezolizumab; ECOG PS, Eastern Cooperative Oncology Group performance status; nab-Pac, nab-paclitaxel.
aData were from the case report form.
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 31) . | All patients (N = 65) . |
|---|---|---|---|
| Age | |||
| Median (range), years | 55.0 (31–82) | 64.0 (37–77) | 57.0 (31–82) |
| Distribution, n (%) | |||
| 18–40 years | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| 41–64 years | 22 (64.7) | 17 (54.8) | 39 (60.0) |
| ≥65 years | 9 (26.5) | 13 (41.9) | 22 (33.8) |
| Female sex, n (%) | 34 (100) | 31 (100) | 65 (100) |
| Baseline ECOG PS, n (%) | |||
| 0 | 28 (82.4) | 27 (87.1) | 55 (84.6) |
| 1 | 6 (17.6) | 4 (12.9) | 10 (15.4) |
| Metastatic disease, n (%) | 32 (94.1) | 22 (71.0) | 54 (83.1) |
| No. of sites of metastatic disease, n (%) | |||
| 0–3 | 27 (79.4) | 25 (80.6) | 52 (80.0) |
| ≥ 4 | 7 (20.6) | 6 (19.4) | 13 (20.0) |
| Site of metastatic disease, n (%) | |||
| Livera | 11 (32.4) | 6 (19.4) | 17 (26.2) |
| Bone | 7 (20.6) | 9 (29.0) | 16 (24.6) |
| Brain | 1 (2.9) | 0 | 1 (1.5) |
| Lung | 16 (47.1) | 12 (38.7) | 28 (43.1) |
| Lymph node only | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| Previous therapy, n (%) | |||
| Neoadjuvant or adjuvant therapy | 19 (55.9) | 11 (35.5) | 30 (46.2) |
| Taxanea | 15 (44.1) | 11 (35.5) | 26 (40.0) |
| Anthracycline | 17 (50.0) | 11 (35.5) | 28 (43.1) |
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 31) . | All patients (N = 65) . |
|---|---|---|---|
| Age | |||
| Median (range), years | 55.0 (31–82) | 64.0 (37–77) | 57.0 (31–82) |
| Distribution, n (%) | |||
| 18–40 years | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| 41–64 years | 22 (64.7) | 17 (54.8) | 39 (60.0) |
| ≥65 years | 9 (26.5) | 13 (41.9) | 22 (33.8) |
| Female sex, n (%) | 34 (100) | 31 (100) | 65 (100) |
| Baseline ECOG PS, n (%) | |||
| 0 | 28 (82.4) | 27 (87.1) | 55 (84.6) |
| 1 | 6 (17.6) | 4 (12.9) | 10 (15.4) |
| Metastatic disease, n (%) | 32 (94.1) | 22 (71.0) | 54 (83.1) |
| No. of sites of metastatic disease, n (%) | |||
| 0–3 | 27 (79.4) | 25 (80.6) | 52 (80.0) |
| ≥ 4 | 7 (20.6) | 6 (19.4) | 13 (20.0) |
| Site of metastatic disease, n (%) | |||
| Livera | 11 (32.4) | 6 (19.4) | 17 (26.2) |
| Bone | 7 (20.6) | 9 (29.0) | 16 (24.6) |
| Brain | 1 (2.9) | 0 | 1 (1.5) |
| Lung | 16 (47.1) | 12 (38.7) | 28 (43.1) |
| Lymph node only | 3 (8.8) | 1 (3.2) | 4 (6.2) |
| Previous therapy, n (%) | |||
| Neoadjuvant or adjuvant therapy | 19 (55.9) | 11 (35.5) | 30 (46.2) |
| Taxanea | 15 (44.1) | 11 (35.5) | 26 (40.0) |
| Anthracycline | 17 (50.0) | 11 (35.5) | 28 (43.1) |
atezo, atezolizumab; ECOG PS, Eastern Cooperative Oncology Group performance status; nab-Pac, nab-paclitaxel.
aData were from the case report form.
Assessments and endpoints
Tumour assessment was conducted at baseline, then every 8 weeks for 12 months and every 12 weeks thereafter. Follow-up for survival occurred every 3 months after treatment discontinuation. The primary endpoints were investigator-assessed PFS and OS and were assessed in both the ITT population and PD-L1–positive (PD-L1 ≥ 1% on IC) subgroup, using the VENTANA SP142 PD-L1 assay. Secondary endpoints were investigator-assessed objective response rate (ORR) and duration of response (DOR) per RECIST 1.1. Safety was evaluated per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Details of the statistical analysis have been previously described (13). Summaries of PFS and OS, including unstratified hazard ratios (HRs), estimated from Cox proportional hazards models and Kaplan–Meier estimates of the median were produced separately for each treatment group in the Japanese ITT and PD-L1–positive subgroups. ORR was summarised for the Japanese ITT and PD-L1–positive subgroups. As the present analyses of the Japanese subgroup are exploratory, P values are not reported for comparisons between treatment arms.
Results
Patients and treatments
Of the 902 patients randomised in the IMpassion130 trial, 65 were enrolled at Japanese centres between August 2016 and May 2017. Thirty-four were randomised to the atezolizumab arm and 31 to the placebo arm (Table 1). One patient in the placebo arm discontinued the trial before administration of placebo and was therefore removed from the safety-evaluable population. The PD-L1–positive subgroup included 25 patients (12 in the atezolizumab plus nab-paclitaxel group and 13 in the placebo plus nab-paclitaxel group). The median duration of treatment with atezolizumab or placebo was 30.1 weeks (range, 4–81 weeks) and 18.6 weeks (range, 6–75 weeks), respectively (Supplementary Table S1). The median duration of treatment with nab-paclitaxel was 28.6 weeks (range, 5–81 weeks) and 18.6 weeks (range, 6–75 weeks) in the atezolizumab- and placebo-treated groups, respectively (Supplementary Table S1). Baseline characteristics in the Japanese subgroup were largely balanced between both treatment groups except for age, presence and site of metastatic disease and previous therapy (Table 1).
Efficacy
In Japanese patients from the overall ITT population, median investigator-assessed PFS was 7.4 months (95% CI, 5.4–10.8) in the atezolizumab plus nab-paclitaxel group compared with 4.6 months (95% CI, 3.7–7.2) in the placebo plus nab-paclitaxel group (HR, 0.47 [95% CI, 0.25–0.90]) (Fig. 1A). Median OS was not estimable (NE) in the atezolizumab group compared with 16.8 months (95% CI, 13.3–NE) in the placebo group (HR, 0.44 [95% CI, 0.16–1.24]) (Fig. 2A).
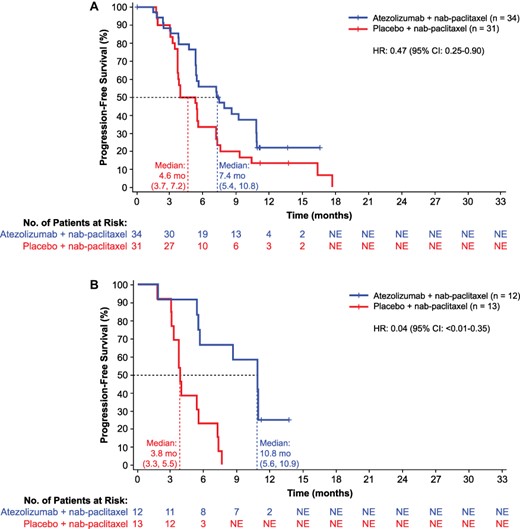
(A) Investigator-assessed progression-free survival in Japanese patients (ITT) and (B) PD-L1–positive patients. Atezo, atezolizumab; ITT, intention-to-treat; nab-Pac, nab-paclitaxel; NE, not estimable; PD-L1, programmed death-ligand 1.
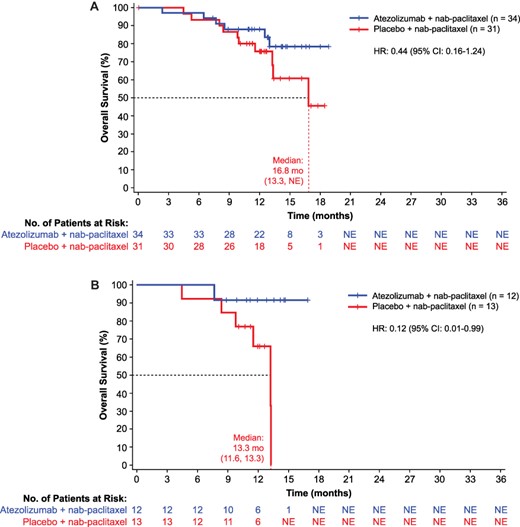
(A) Overall survival in Japanese patients (ITT) and (B) PD-L1–positive patients. Atezo, atezolizumab.
In the PD-L1–positive subgroup, median PFS (investigator assessed) was 10.8 months (95% C], 5.6–10.9) in the atezolizumab plus nab-paclitaxel group compared with 3.8 months (95% CI, 3.3–5.5) in the placebo plus nab-paclitaxel group (HR, 0.04 [95% CI, <0.01–0.35]) (Fig. 1B). Median OS was NE in the atezolizumab group compared with 13.3 months (95% CI, 11.6–13.3) in the placebo group (HR, 0.12 [95% CI, 0.01–0.99]) (Fig. 2B).
In the ITT population of the Japanese subgroup, the investigator-assessed ORR was 67.6% (95% CI, 49.5–82.6) in the atezolizumab plus nab-paclitaxel group compared with 51.6% (95% CI, 33.1–69.9) in the placebo plus nab-paclitaxel group (Table 2; Supplementary Fig. S1). Investigator-assessed ORR in the PD-L1–positive subgroup was 75.0% (95% CI, 42.8–94.5) in the atezolizumab plus nab-paclitaxel group compared with 53.8% (95% CI, 25.1–80.8) in the placebo plus nab-paclitaxel group. The median DOR in the ITT population of the Japanese subgroup was 5.6 months (95% CI, 3.7–9.1) and 3.7 months (95% CI, 3.6–5.6) in the atezolizumab plus nab-paclitaxel and placebo plus nab-paclitaxel groups, respectively (Table 2; Supplementary Fig. S2). Median DOR in the PD-L1–positive subgroup was 9.1 months (95% CI, 7.3–NE) and 3.7 months (95% CI, 1.9–5.5) in the atezolizumab plus nab-paclitaxel and placebo plus nab-paclitaxel groups, respectively.
| . | Japanese ITT . | PD-L1 positive . | ||
|---|---|---|---|---|
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 31) . | Atezolizumab + nab-paclitaxel (n = 12) . | Placebo + nab-paclitaxel (n = 13) . |
| Objective confirmed response, n (%) | ||||
| Objective response (95% CI) | 23 (67.6) (49.5–82.6) | 16 (51.6) (33.1–69.9) | 9 (75.0) (42.8–94.5) | 7 (53.8) (25.1–80.8) |
| Complete response | 1 (2.9) | 0 | 0 | 0 |
| Partial response | 22 (64.7) | 16 (51.6) | 9 (75.0) | 7 (53.8) |
| Stable disease | 9 (26.5) | 11 (35.5) | 2 (16.7) | 5 (38.5) |
| Progressive disease | 2 (5.9) | 3 (9.7) | 1 (8.3) | 1 (7.7) |
| Missing or unevaluable | 0 | 1 (3.2) | 0 | 0 |
| Duration of responsea | ||||
| Median (95% CI), months | 5.6 (3.7–9.1) | 3.7 (3.6–5.6) | 9.1 (7.3–NE) | 3.7 (1.9–5.5) |
| . | Japanese ITT . | PD-L1 positive . | ||
|---|---|---|---|---|
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 31) . | Atezolizumab + nab-paclitaxel (n = 12) . | Placebo + nab-paclitaxel (n = 13) . |
| Objective confirmed response, n (%) | ||||
| Objective response (95% CI) | 23 (67.6) (49.5–82.6) | 16 (51.6) (33.1–69.9) | 9 (75.0) (42.8–94.5) | 7 (53.8) (25.1–80.8) |
| Complete response | 1 (2.9) | 0 | 0 | 0 |
| Partial response | 22 (64.7) | 16 (51.6) | 9 (75.0) | 7 (53.8) |
| Stable disease | 9 (26.5) | 11 (35.5) | 2 (16.7) | 5 (38.5) |
| Progressive disease | 2 (5.9) | 3 (9.7) | 1 (8.3) | 1 (7.7) |
| Missing or unevaluable | 0 | 1 (3.2) | 0 | 0 |
| Duration of responsea | ||||
| Median (95% CI), months | 5.6 (3.7–9.1) | 3.7 (3.6–5.6) | 9.1 (7.3–NE) | 3.7 (1.9–5.5) |
ITT, intention-to-treat; PD-L1, programmed death-ligand 1.
aInvestigator assessed.
| . | Japanese ITT . | PD-L1 positive . | ||
|---|---|---|---|---|
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 31) . | Atezolizumab + nab-paclitaxel (n = 12) . | Placebo + nab-paclitaxel (n = 13) . |
| Objective confirmed response, n (%) | ||||
| Objective response (95% CI) | 23 (67.6) (49.5–82.6) | 16 (51.6) (33.1–69.9) | 9 (75.0) (42.8–94.5) | 7 (53.8) (25.1–80.8) |
| Complete response | 1 (2.9) | 0 | 0 | 0 |
| Partial response | 22 (64.7) | 16 (51.6) | 9 (75.0) | 7 (53.8) |
| Stable disease | 9 (26.5) | 11 (35.5) | 2 (16.7) | 5 (38.5) |
| Progressive disease | 2 (5.9) | 3 (9.7) | 1 (8.3) | 1 (7.7) |
| Missing or unevaluable | 0 | 1 (3.2) | 0 | 0 |
| Duration of responsea | ||||
| Median (95% CI), months | 5.6 (3.7–9.1) | 3.7 (3.6–5.6) | 9.1 (7.3–NE) | 3.7 (1.9–5.5) |
| . | Japanese ITT . | PD-L1 positive . | ||
|---|---|---|---|---|
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 31) . | Atezolizumab + nab-paclitaxel (n = 12) . | Placebo + nab-paclitaxel (n = 13) . |
| Objective confirmed response, n (%) | ||||
| Objective response (95% CI) | 23 (67.6) (49.5–82.6) | 16 (51.6) (33.1–69.9) | 9 (75.0) (42.8–94.5) | 7 (53.8) (25.1–80.8) |
| Complete response | 1 (2.9) | 0 | 0 | 0 |
| Partial response | 22 (64.7) | 16 (51.6) | 9 (75.0) | 7 (53.8) |
| Stable disease | 9 (26.5) | 11 (35.5) | 2 (16.7) | 5 (38.5) |
| Progressive disease | 2 (5.9) | 3 (9.7) | 1 (8.3) | 1 (7.7) |
| Missing or unevaluable | 0 | 1 (3.2) | 0 | 0 |
| Duration of responsea | ||||
| Median (95% CI), months | 5.6 (3.7–9.1) | 3.7 (3.6–5.6) | 9.1 (7.3–NE) | 3.7 (1.9–5.5) |
ITT, intention-to-treat; PD-L1, programmed death-ligand 1.
aInvestigator assessed.
Safety
The incidence of all-cause adverse events in Japanese patients was comparable to that in patients from the overall IMpassion130 population (Table 3). There was a numerically lower rate of all-cause death, serious adverse events and adverse events leading to any treatment withdrawal in Japanese patients than in the overall population. There were no treatment-related deaths or adverse events leading to the withdrawal of atezolizumab or placebo in Japanese patients. However, there was a higher rate of adverse events leading to any dose reduction or study treatment interruption in both treatment arms in the Japanese subgroup (atezolizumab plus nab-paclitaxel: 64.7%; placebo plus nab-paclitaxel: 56.7%).
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 30) . |
|---|---|---|
| All-cause AEs, n (%) | 34 (100) | 30 (100) |
| Treatment-related AEs | 34 (100) | 30 (100) |
| AEs of special interest | 21 (61.8) | 15 (50.0) |
| All-cause grade 3–4 AEs, n (%) | 13 (38.2) | 12 (40.0) |
| Treatment-related grade 3–4 AEs | 12 (35.3) | 11 (36.7) |
| Grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| All deaths, n (%) | 6 (17.6) | 10 (33.3) |
| Treatment-related deaths | 0 | 0 |
| Serious AEs, n (%) | 4 (11.8) | 3 (10.0) |
| Serious AEs of special interest | 1 (2.9) | 1 (3.3) |
| AEs leading to withdrawal from any treatment, n (%) | 2 (5.9) | 0 |
| AEs leading to withdrawal from atezolizumab or placebo | 0 | 0 |
| AEs leading to withdrawal from nab-paclitaxel | 2 (5.9) | 0 |
| AEs leading to any dose reduction or study treatment interruption, n (%) | 22 (64.7) | 17 (56.7) |
| AEs leading to any dose interruption of atezolizumab or placebo | 15 (50.0) | 10 (33.3) |
| AEs leading to dose reduction or interruption of nab-paclitaxel | 22 (64.7) | 17 (56.7) |
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 30) . |
|---|---|---|
| All-cause AEs, n (%) | 34 (100) | 30 (100) |
| Treatment-related AEs | 34 (100) | 30 (100) |
| AEs of special interest | 21 (61.8) | 15 (50.0) |
| All-cause grade 3–4 AEs, n (%) | 13 (38.2) | 12 (40.0) |
| Treatment-related grade 3–4 AEs | 12 (35.3) | 11 (36.7) |
| Grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| All deaths, n (%) | 6 (17.6) | 10 (33.3) |
| Treatment-related deaths | 0 | 0 |
| Serious AEs, n (%) | 4 (11.8) | 3 (10.0) |
| Serious AEs of special interest | 1 (2.9) | 1 (3.3) |
| AEs leading to withdrawal from any treatment, n (%) | 2 (5.9) | 0 |
| AEs leading to withdrawal from atezolizumab or placebo | 0 | 0 |
| AEs leading to withdrawal from nab-paclitaxel | 2 (5.9) | 0 |
| AEs leading to any dose reduction or study treatment interruption, n (%) | 22 (64.7) | 17 (56.7) |
| AEs leading to any dose interruption of atezolizumab or placebo | 15 (50.0) | 10 (33.3) |
| AEs leading to dose reduction or interruption of nab-paclitaxel | 22 (64.7) | 17 (56.7) |
AE, adverse event; atezo, atezolizumab; nab-Pac, nab-paclitaxel.
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 30) . |
|---|---|---|
| All-cause AEs, n (%) | 34 (100) | 30 (100) |
| Treatment-related AEs | 34 (100) | 30 (100) |
| AEs of special interest | 21 (61.8) | 15 (50.0) |
| All-cause grade 3–4 AEs, n (%) | 13 (38.2) | 12 (40.0) |
| Treatment-related grade 3–4 AEs | 12 (35.3) | 11 (36.7) |
| Grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| All deaths, n (%) | 6 (17.6) | 10 (33.3) |
| Treatment-related deaths | 0 | 0 |
| Serious AEs, n (%) | 4 (11.8) | 3 (10.0) |
| Serious AEs of special interest | 1 (2.9) | 1 (3.3) |
| AEs leading to withdrawal from any treatment, n (%) | 2 (5.9) | 0 |
| AEs leading to withdrawal from atezolizumab or placebo | 0 | 0 |
| AEs leading to withdrawal from nab-paclitaxel | 2 (5.9) | 0 |
| AEs leading to any dose reduction or study treatment interruption, n (%) | 22 (64.7) | 17 (56.7) |
| AEs leading to any dose interruption of atezolizumab or placebo | 15 (50.0) | 10 (33.3) |
| AEs leading to dose reduction or interruption of nab-paclitaxel | 22 (64.7) | 17 (56.7) |
| . | Atezo + nab-Pac (n = 34) . | Placebo + nab-Pac (n = 30) . |
|---|---|---|
| All-cause AEs, n (%) | 34 (100) | 30 (100) |
| Treatment-related AEs | 34 (100) | 30 (100) |
| AEs of special interest | 21 (61.8) | 15 (50.0) |
| All-cause grade 3–4 AEs, n (%) | 13 (38.2) | 12 (40.0) |
| Treatment-related grade 3–4 AEs | 12 (35.3) | 11 (36.7) |
| Grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs of special interest | 0 | 2 (6.7) |
| All deaths, n (%) | 6 (17.6) | 10 (33.3) |
| Treatment-related deaths | 0 | 0 |
| Serious AEs, n (%) | 4 (11.8) | 3 (10.0) |
| Serious AEs of special interest | 1 (2.9) | 1 (3.3) |
| AEs leading to withdrawal from any treatment, n (%) | 2 (5.9) | 0 |
| AEs leading to withdrawal from atezolizumab or placebo | 0 | 0 |
| AEs leading to withdrawal from nab-paclitaxel | 2 (5.9) | 0 |
| AEs leading to any dose reduction or study treatment interruption, n (%) | 22 (64.7) | 17 (56.7) |
| AEs leading to any dose interruption of atezolizumab or placebo | 15 (50.0) | 10 (33.3) |
| AEs leading to dose reduction or interruption of nab-paclitaxel | 22 (64.7) | 17 (56.7) |
AE, adverse event; atezo, atezolizumab; nab-Pac, nab-paclitaxel.
The most frequent all-grade adverse events were alopecia (atezolizumab plus nab-paclitaxel: 85.3%; placebo plus nab-paclitaxel: 86.7%), peripheral sensory neuropathy (atezolizumab plus nab-paclitaxel: 58.8%; placebo plus nab-paclitaxel: 50.0%), nausea (atezolizumab plus nab-paclitaxel: 47.1%; placebo plus nab-paclitaxel: 40.0%) and decreased neutrophil count (atezolizumab plus nab-paclitaxel: 44.1%; placebo plus nab-paclitaxel: 33.3%) (Table 4). Grade ≥ 3 adverse events were reported in 13 patients (28.2%) in the atezolizumab plus nab-paclitaxel group and 12 patients (40.0%) in the placebo plus nab-paclitaxel group. The treatment-related serious adverse events were hepatitis (atezolizumab plus nab-paclitaxel: 2.9%; placebo plus nab-paclitaxel: 0%), dyspnoea (atezolizumab plus nab-paclitaxel: 2.9%; placebo plus nab-paclitaxel: 0%), inappropriate antidiuretic hormone secretion (atezolizumab plus nab-paclitaxel: 2.9%; placebo plus nab-paclitaxel: 0%), cholecystocholangitis (atezolizumab plus nab-paclitaxel: 0%; placebo plus nab-paclitaxel: 3.3%) and dermatomyositis (atezolizumab plus nab-paclitaxel: 0%; placebo plus nab-paclitaxel: 3.3%) (Supplementary Table S3).
Adverse events occurring in ≥10% of patients in the Japanese safety-evaluable subgroup
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 30) . | ||
|---|---|---|---|---|
| . | Any grade in ≥10% of patients . | Grade ≥ 3 . | Any grade in ≥10% of patients . | Grade ≥ 3 . |
| Patients with ≥1 event, n (%) | 34 (100) | 13 (28.2) | 30 (100) | 12 (40.0) |
| Alopecia | 29 (85.3) | 0 | 26 (86.7) | 0 |
| Peripheral sensory neuropathy | 20 (58.8) | 0 | 15 (50.0) | 0 |
| Nausea | 16 (47.1) | 0 | 12 (40.0) | 1 (3.3) |
| Neutrophil count decreased | 15 (44.1) | 6 (17.6) | 10 (33.3) | 5 (16.7) |
| Nasopharyngitis | 11 (32.4) | 0 | 3 (10.0) | 0 |
| Rash | 10 (29.4) | 0 | 6 (20.0) | 0 |
| White blood cell count decreased | 10 (29.4) | 4 (11.8) | 3 (10.0) | 2 (6.7) |
| Stomatitis | 9 (26.5) | 0 | 3 (10.0) | 0 |
| Constipation | 8 (23.5) | 0 | 10 (33.3) | 0 |
| Dysgeusia | 8 (23.5) | 0 | 9 (30.0) | 0 |
| Pyrexia | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Fatigue | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Peripheral oedema | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Nail discoloration | 7 (20.6) | 0 | 9 (30.0) | 0 |
| Decreased appetite | 7 (20.6) | 0 | 8 (26.7) | 1 (3.3) |
| Vomiting | 7 (20.6) | 1 (2.9) | 3 (10.0) | 0 |
| Paronychia | 7 (20.6) | 0 | 0 | 0 |
| Myalgia | 6 (17.6) | 0 | 7 (23.3) | 1 (3.3) |
| Anaemia | 6 (17.6) | 0 | 7 (23.3) | 0 |
| Dry skin | 6 (17.6) | 0 | 6 (20.0) | 0 |
| Diarrhoea | 6 (17.6) | 0 | 5 (16.7) | 0 |
| Headache | 6 (17.6) | 0 | 4 (13.3) | 0 |
| Pruritis | 6 (17.6) | 0 | 2 (6.7) | 0 |
| Malaise | 5 (14.7) | 0 | 10 (33.3) | 0 |
| Alanine aminotransferase increased | 5 (14.7) | 0 | 8 (26.7) | 1 (3.3) |
| Aspartate aminotransferase increased | 4 (11.8) | 0 | 5 (16.7) | 1 (3.3) |
| Arthralgia | 4 (11.8) | 0 | 4 (13.3) | 0 |
| Urinary tract infection | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Insomnia | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Neutropenia | 4 (11.8) | 3 (8.8) | 0 | 0 |
| Hypothyroidism | 4 (11.8) | 0 | 0 | 0 |
| Oedema | 3 (8.8) | 0 | 3 (10.0) | 0 |
| Peripheral neuropathy | 2 (5.9) | 0 | 5 (16.7) | 0 |
| Cough | 2 (5.9) | 0 | 4 (13.3) | 0 |
| Pharyngitis | 2 (5.9) | 0 | 3 (10.0) | 0 |
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 30) . | ||
|---|---|---|---|---|
| . | Any grade in ≥10% of patients . | Grade ≥ 3 . | Any grade in ≥10% of patients . | Grade ≥ 3 . |
| Patients with ≥1 event, n (%) | 34 (100) | 13 (28.2) | 30 (100) | 12 (40.0) |
| Alopecia | 29 (85.3) | 0 | 26 (86.7) | 0 |
| Peripheral sensory neuropathy | 20 (58.8) | 0 | 15 (50.0) | 0 |
| Nausea | 16 (47.1) | 0 | 12 (40.0) | 1 (3.3) |
| Neutrophil count decreased | 15 (44.1) | 6 (17.6) | 10 (33.3) | 5 (16.7) |
| Nasopharyngitis | 11 (32.4) | 0 | 3 (10.0) | 0 |
| Rash | 10 (29.4) | 0 | 6 (20.0) | 0 |
| White blood cell count decreased | 10 (29.4) | 4 (11.8) | 3 (10.0) | 2 (6.7) |
| Stomatitis | 9 (26.5) | 0 | 3 (10.0) | 0 |
| Constipation | 8 (23.5) | 0 | 10 (33.3) | 0 |
| Dysgeusia | 8 (23.5) | 0 | 9 (30.0) | 0 |
| Pyrexia | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Fatigue | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Peripheral oedema | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Nail discoloration | 7 (20.6) | 0 | 9 (30.0) | 0 |
| Decreased appetite | 7 (20.6) | 0 | 8 (26.7) | 1 (3.3) |
| Vomiting | 7 (20.6) | 1 (2.9) | 3 (10.0) | 0 |
| Paronychia | 7 (20.6) | 0 | 0 | 0 |
| Myalgia | 6 (17.6) | 0 | 7 (23.3) | 1 (3.3) |
| Anaemia | 6 (17.6) | 0 | 7 (23.3) | 0 |
| Dry skin | 6 (17.6) | 0 | 6 (20.0) | 0 |
| Diarrhoea | 6 (17.6) | 0 | 5 (16.7) | 0 |
| Headache | 6 (17.6) | 0 | 4 (13.3) | 0 |
| Pruritis | 6 (17.6) | 0 | 2 (6.7) | 0 |
| Malaise | 5 (14.7) | 0 | 10 (33.3) | 0 |
| Alanine aminotransferase increased | 5 (14.7) | 0 | 8 (26.7) | 1 (3.3) |
| Aspartate aminotransferase increased | 4 (11.8) | 0 | 5 (16.7) | 1 (3.3) |
| Arthralgia | 4 (11.8) | 0 | 4 (13.3) | 0 |
| Urinary tract infection | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Insomnia | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Neutropenia | 4 (11.8) | 3 (8.8) | 0 | 0 |
| Hypothyroidism | 4 (11.8) | 0 | 0 | 0 |
| Oedema | 3 (8.8) | 0 | 3 (10.0) | 0 |
| Peripheral neuropathy | 2 (5.9) | 0 | 5 (16.7) | 0 |
| Cough | 2 (5.9) | 0 | 4 (13.3) | 0 |
| Pharyngitis | 2 (5.9) | 0 | 3 (10.0) | 0 |
Adverse events occurring in ≥10% of patients in the Japanese safety-evaluable subgroup
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 30) . | ||
|---|---|---|---|---|
| . | Any grade in ≥10% of patients . | Grade ≥ 3 . | Any grade in ≥10% of patients . | Grade ≥ 3 . |
| Patients with ≥1 event, n (%) | 34 (100) | 13 (28.2) | 30 (100) | 12 (40.0) |
| Alopecia | 29 (85.3) | 0 | 26 (86.7) | 0 |
| Peripheral sensory neuropathy | 20 (58.8) | 0 | 15 (50.0) | 0 |
| Nausea | 16 (47.1) | 0 | 12 (40.0) | 1 (3.3) |
| Neutrophil count decreased | 15 (44.1) | 6 (17.6) | 10 (33.3) | 5 (16.7) |
| Nasopharyngitis | 11 (32.4) | 0 | 3 (10.0) | 0 |
| Rash | 10 (29.4) | 0 | 6 (20.0) | 0 |
| White blood cell count decreased | 10 (29.4) | 4 (11.8) | 3 (10.0) | 2 (6.7) |
| Stomatitis | 9 (26.5) | 0 | 3 (10.0) | 0 |
| Constipation | 8 (23.5) | 0 | 10 (33.3) | 0 |
| Dysgeusia | 8 (23.5) | 0 | 9 (30.0) | 0 |
| Pyrexia | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Fatigue | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Peripheral oedema | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Nail discoloration | 7 (20.6) | 0 | 9 (30.0) | 0 |
| Decreased appetite | 7 (20.6) | 0 | 8 (26.7) | 1 (3.3) |
| Vomiting | 7 (20.6) | 1 (2.9) | 3 (10.0) | 0 |
| Paronychia | 7 (20.6) | 0 | 0 | 0 |
| Myalgia | 6 (17.6) | 0 | 7 (23.3) | 1 (3.3) |
| Anaemia | 6 (17.6) | 0 | 7 (23.3) | 0 |
| Dry skin | 6 (17.6) | 0 | 6 (20.0) | 0 |
| Diarrhoea | 6 (17.6) | 0 | 5 (16.7) | 0 |
| Headache | 6 (17.6) | 0 | 4 (13.3) | 0 |
| Pruritis | 6 (17.6) | 0 | 2 (6.7) | 0 |
| Malaise | 5 (14.7) | 0 | 10 (33.3) | 0 |
| Alanine aminotransferase increased | 5 (14.7) | 0 | 8 (26.7) | 1 (3.3) |
| Aspartate aminotransferase increased | 4 (11.8) | 0 | 5 (16.7) | 1 (3.3) |
| Arthralgia | 4 (11.8) | 0 | 4 (13.3) | 0 |
| Urinary tract infection | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Insomnia | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Neutropenia | 4 (11.8) | 3 (8.8) | 0 | 0 |
| Hypothyroidism | 4 (11.8) | 0 | 0 | 0 |
| Oedema | 3 (8.8) | 0 | 3 (10.0) | 0 |
| Peripheral neuropathy | 2 (5.9) | 0 | 5 (16.7) | 0 |
| Cough | 2 (5.9) | 0 | 4 (13.3) | 0 |
| Pharyngitis | 2 (5.9) | 0 | 3 (10.0) | 0 |
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel (n = 30) . | ||
|---|---|---|---|---|
| . | Any grade in ≥10% of patients . | Grade ≥ 3 . | Any grade in ≥10% of patients . | Grade ≥ 3 . |
| Patients with ≥1 event, n (%) | 34 (100) | 13 (28.2) | 30 (100) | 12 (40.0) |
| Alopecia | 29 (85.3) | 0 | 26 (86.7) | 0 |
| Peripheral sensory neuropathy | 20 (58.8) | 0 | 15 (50.0) | 0 |
| Nausea | 16 (47.1) | 0 | 12 (40.0) | 1 (3.3) |
| Neutrophil count decreased | 15 (44.1) | 6 (17.6) | 10 (33.3) | 5 (16.7) |
| Nasopharyngitis | 11 (32.4) | 0 | 3 (10.0) | 0 |
| Rash | 10 (29.4) | 0 | 6 (20.0) | 0 |
| White blood cell count decreased | 10 (29.4) | 4 (11.8) | 3 (10.0) | 2 (6.7) |
| Stomatitis | 9 (26.5) | 0 | 3 (10.0) | 0 |
| Constipation | 8 (23.5) | 0 | 10 (33.3) | 0 |
| Dysgeusia | 8 (23.5) | 0 | 9 (30.0) | 0 |
| Pyrexia | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Fatigue | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Peripheral oedema | 8 (23.5) | 0 | 6 (20.0) | 0 |
| Nail discoloration | 7 (20.6) | 0 | 9 (30.0) | 0 |
| Decreased appetite | 7 (20.6) | 0 | 8 (26.7) | 1 (3.3) |
| Vomiting | 7 (20.6) | 1 (2.9) | 3 (10.0) | 0 |
| Paronychia | 7 (20.6) | 0 | 0 | 0 |
| Myalgia | 6 (17.6) | 0 | 7 (23.3) | 1 (3.3) |
| Anaemia | 6 (17.6) | 0 | 7 (23.3) | 0 |
| Dry skin | 6 (17.6) | 0 | 6 (20.0) | 0 |
| Diarrhoea | 6 (17.6) | 0 | 5 (16.7) | 0 |
| Headache | 6 (17.6) | 0 | 4 (13.3) | 0 |
| Pruritis | 6 (17.6) | 0 | 2 (6.7) | 0 |
| Malaise | 5 (14.7) | 0 | 10 (33.3) | 0 |
| Alanine aminotransferase increased | 5 (14.7) | 0 | 8 (26.7) | 1 (3.3) |
| Aspartate aminotransferase increased | 4 (11.8) | 0 | 5 (16.7) | 1 (3.3) |
| Arthralgia | 4 (11.8) | 0 | 4 (13.3) | 0 |
| Urinary tract infection | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Insomnia | 4 (11.8) | 0 | 1 (3.3) | 0 |
| Neutropenia | 4 (11.8) | 3 (8.8) | 0 | 0 |
| Hypothyroidism | 4 (11.8) | 0 | 0 | 0 |
| Oedema | 3 (8.8) | 0 | 3 (10.0) | 0 |
| Peripheral neuropathy | 2 (5.9) | 0 | 5 (16.7) | 0 |
| Cough | 2 (5.9) | 0 | 4 (13.3) | 0 |
| Pharyngitis | 2 (5.9) | 0 | 3 (10.0) | 0 |
All-grade adverse events of special interest were reported in 21 patients (61.8%) in the atezolizumab plus nab-paclitaxel group and 15 patients (50.0%) in the placebo plus nab-paclitaxel group (Table 3). Most adverse events of special interest were grade 1–2, regardless of the treatment group. There were no patients in the atezolizumab plus nab-paclitaxel arm and two patients (6.7%) in the placebo plus nab-paclitaxel arm with grade 3–4 adverse events of special interest. Similarly, treatment-related grade 3–4 adverse events of special interest were only observed in two patients (6.7%) in the placebo plus nab-paclitaxel arm. The most frequently occurring adverse events of special interest were rash (atezolizumab plus nab-paclitaxel: 44.1%; placebo plus nab-paclitaxel: 30.0%), hypothyroidism (atezolizumab plus nab-paclitaxel: 17.6%; placebo plus nab-paclitaxel: 3.3%) and hepatitis based on laboratory abnormalities (atezolizumab plus nab-paclitaxel: 14.7%; placebo plus nab-paclitaxel: 26.7%) (Table 5).
Summary of adverse events of special interest occurring the in the Japanese safety-evaluable subgroup
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel(n = 30) . |
|---|---|---|
| Patients with ≥ 1 event, n (%) | 21 (61.8) | 15 (50.0) |
| Rash | 15 (44.1) | 9 (30.0) |
| Hypothyroidism | 6 (17.6) | 1 (3.3) |
| Hepatitis (laboratory abnormalities) | 5 (14.7) | 8 (26.7) |
| Hepatitis (diagnosis) | 2 (5.9) | 0 |
| Hyperthyroidism | 2 (5.9) | 0 |
| Pneumonitis | 1 (2.9) | 0 |
| Myositis | 0 | 1 (3.3) |
| Grade 3–4 AEs, n (%) | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs | 0 | 2 (6.7) |
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel(n = 30) . |
|---|---|---|
| Patients with ≥ 1 event, n (%) | 21 (61.8) | 15 (50.0) |
| Rash | 15 (44.1) | 9 (30.0) |
| Hypothyroidism | 6 (17.6) | 1 (3.3) |
| Hepatitis (laboratory abnormalities) | 5 (14.7) | 8 (26.7) |
| Hepatitis (diagnosis) | 2 (5.9) | 0 |
| Hyperthyroidism | 2 (5.9) | 0 |
| Pneumonitis | 1 (2.9) | 0 |
| Myositis | 0 | 1 (3.3) |
| Grade 3–4 AEs, n (%) | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs | 0 | 2 (6.7) |
Summary of adverse events of special interest occurring the in the Japanese safety-evaluable subgroup
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel(n = 30) . |
|---|---|---|
| Patients with ≥ 1 event, n (%) | 21 (61.8) | 15 (50.0) |
| Rash | 15 (44.1) | 9 (30.0) |
| Hypothyroidism | 6 (17.6) | 1 (3.3) |
| Hepatitis (laboratory abnormalities) | 5 (14.7) | 8 (26.7) |
| Hepatitis (diagnosis) | 2 (5.9) | 0 |
| Hyperthyroidism | 2 (5.9) | 0 |
| Pneumonitis | 1 (2.9) | 0 |
| Myositis | 0 | 1 (3.3) |
| Grade 3–4 AEs, n (%) | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs | 0 | 2 (6.7) |
| . | Atezolizumab + nab-paclitaxel (n = 34) . | Placebo + nab-paclitaxel(n = 30) . |
|---|---|---|
| Patients with ≥ 1 event, n (%) | 21 (61.8) | 15 (50.0) |
| Rash | 15 (44.1) | 9 (30.0) |
| Hypothyroidism | 6 (17.6) | 1 (3.3) |
| Hepatitis (laboratory abnormalities) | 5 (14.7) | 8 (26.7) |
| Hepatitis (diagnosis) | 2 (5.9) | 0 |
| Hyperthyroidism | 2 (5.9) | 0 |
| Pneumonitis | 1 (2.9) | 0 |
| Myositis | 0 | 1 (3.3) |
| Grade 3–4 AEs, n (%) | 0 | 2 (6.7) |
| Treatment-related grade 3–4 AEs | 0 | 2 (6.7) |
Discussion
Efficacy results (PFS and OS) in the Japanese subgroup were numerically consistent with those in the overall IMpassion130 population (Supplementary Table S2) (13). Additionally, the median PFS observed in the placebo plus nab-paclitaxel arm of the Japanese subgroup was comparable to that previously reported in other studies with taxane monotherapy (22–25). ORR results in both treatment arms of the Japanese subgroup were numerically higher than the ORRs reported in the overall IMpassion130 population (atezolizumab plus nab-paclitaxel: 56.0%; placebo plus nab-paclitaxel: 45.9%) (13). A smaller proportion of Japanese patients received prior neoadjuvant or adjuvant therapy than the overall IMpassion130 population (atezolizumab group: 55.9% versus 63.0%, respectively; placebo group: 35.5% versus 63.4%, respectively) (13). However, this was not expected to affect the interpretation of our data, as subgroup analysis data from the overall population showed similar treatment effects in patients who did and did not have prior therapy. Median DOR in both treatment arms (atezolizumab plus nab-paclitaxel: 5.6 months [95% CI, 3.7–9.1]; placebo plus nab-paclitaxel: 3.7 months [95% CI, 3.6–5.6]) was shorter than that reported in the overall IMpassion130 ITT population (atezolizumab plus nab-paclitaxel: 7.4 months [95% CI, 6.9–9.0]; placebo plus nab-paclitaxel: 5.6 months [95% CI, 5.5–6.9]). However, the median DOR in the atezolizumab-treated PD-L1–positive subgroup was similar to that in the overall IMpassion130 population (9.1 months [95% CI, 7.3–NE] versus 8.5 months [95% CI, 7.3–9.7], respectively), while the median DOR in the placebo-treated PD-L1–positive subgroup was shorter than that in the overall IMpassion130 population (3.7 months [95% CI, 1.9–5.5] versus 5.5 months [95% CI, 3.7–7.1], respectively) (13). Notably, the median DOR observed in PD-L1–positive patients who received atezolizumab plus nab-paclitaxel in this subanalysis was longer than that observed in both the placebo-treated arm and other studies of taxane monotherapy in patients with metastatic TNBC (22, 25).
There were no specific safety concerns with the use of atezolizumab in the Japanese population. Safety results in the Japanese subgroup population were consistent with the overall population, although there was a higher incidence of alopecia, peripheral sensory neuropathy, decreased neutrophil count, nasopharyngitis and decreased white blood cell count and a lower incidence of fatigue than in the overall population. Higher incidences of neutropenia and peripheral sensory neuropathy in Japanese and Asian patients have also been reported in other clinical trials (24, 26–29). Overall, atezolizumab was well tolerated with no treatment-related deaths, although more patients in the atezolizumab arm had neutrophil count decreases, nasopharyngitis, white blood cell count decreases, stomatitis, vomiting and pruritis than patients in the placebo arm. There were no treatment-related deaths or adverse events leading to atezolizumab withdrawal in Japanese patients. The frequency of all-grade adverse events of special interest in both arms of this subanalysis (atezolizumab: 61.8%, placebo 50.0%) was similar to that in the overall population (57.3% and 41.8%, respectively) (13). However, in the Japanese subgroup, no grade 3–4 adverse events of special interest were observed in the atezolizumab arm and two events (6.7%) were observed in the placebo arm compared with 34 events (7.5%) and 19 events (4.3%), respectively, in the overall population. Compared with the overall IMpassion130 population, the Japanese subgroup had lower rates of adverse events leading to treatment discontinuation and higher rates of adverse events leading to any dose reduction or study treatment interruption. This suggests that these adverse events could be mitigated with appropriate dose intervals for treatments. The dose of nab-paclitaxel used in this study was different from that recommended in the Japanese treatment guidelines for TNBC (i.e. 260 mg/m2 every 3 weeks). Notably, the observed incidence of peripheral sensory neuropathy in this subanalysis (50–58.8%, all-grade) is lower than previously reported rates from other Phase 2 and 3 trials in Japanese patients (76–88%, all-grade) (24, 30), so nab-paclitaxel 100 mg/m2 weekly appears to be tolerable in Japanese patients.
Limitations
This study is limited by its small sample size, which limits the interpretation of the comparisons between the Japanese subpopulation and the overall patient population in the IMpassion130 trial. Also, the current subgroup analysis was not powered for efficacy comparisons. In this subanalysis, the number of patients who had received prior therapy was unbalanced between the atezolizumab and placebo treatment arms. However, the subgroup analysis of the overall IMpassion130 population showed similar treatment effects between patients who did or did not received previous therapy (13). Thus, the imbalance observed in the Japanese subgroup is not expected to have affected the efficacy results observed.
Conclusion
This report has provided new insights on the efficacy and safety of atezolizumab in Japanese patients with TNBC and the comparability of the Japanese subgroup data with that of overall IMpassion130 population. These findings may help to optimise the use of atezolizumab plus nab-paclitaxel as the standard of care for Japanese patients with TNBC.
Acknowledgements
We thank the patients, their families and the clinical study site investigators and staff for their contributions to the study. Medical writing assistance for this manuscript was provided by Jonathan Lee, PhD, of Health Interactions, Inc and funded by Chugai Pharmaceutical Co, Ltd.
Funding
This work was supported by F. Hoffmann-La Roche/Genentech/Chugai, a member of the Roche Group. Medical writing assistance for this manuscript was funded by Chugai Pharmaceutical Co, Ltd.
Conflict of interest statement
Hiroji Iwata reports grant/research support from Chugai, MSD, AstraZeneca, Eisai, Kyowa Hakko Kirin, GSK, Daiichi-Sankyo, Lilly, Novartis, Bayer and Pfizer; consultancy for Chugai, Daiichi Sankyo, Pfizer, AstraZeneca, Lilly, Kyowa Hakko Kirin and Novartis; and honorarium from Chugai, AstraZeneca, Eisai, Pfizer, Daiichi Sankyo and Novartis. Kenichi Inoue reports grant/research support from Chugai, Novartis, Pfizer, Daiichi Sankyo, Parexel, Puma Biotechnology, MSD, Bayer, Lilly and Eisai and honorarium from Chugai, Eisai, Pfizer, AstraZeneca and Lilly. Koji Kaneko and Kenji Tamura report grant/research support from Chugai. Yoshinori Ito reports grant/research support from Chugai, Daiichi Sankyo, Novartis, Parexel, EPS, MSD, AstraZeneca, Lilly, Kyowa Hakko Kirin, Covance, Taiho, A2 Healthcare, IQVIA Services Japan and Cimic and speaker’s bureau for Chugai, Eisai, Taiho, Lilly, Novartis and Pfizer. Koichiro Tsugawa reports grant/research support from Chugai, Taiho, Eisai and Lilly and speaker’s bureau for Chugai, Pfizer and Novartis. Ayumi Hasegawa, Shintaro Nakagawa and Hiroyasu Kuratomi are employees of Chugai.
Presented in part in Schmid et al., N Engl J Med. 2018; 379 (22): 2108–2121.
Suggested reviewers:
1. Yamashita Toshinari, Department of Breast and Endocrine Surgery, Kanagawa Cancer Center, Yokohama, Japan. Email: [email protected]
2. Tokunaga Eriko, Department of Breast Oncology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan. Email: [email protected]
Clinical trial registration: NCT02425891.


