-
PDF
- Split View
-
Views
-
Cite
Cite
Go Kimura, Takeshi Ueda, Post hoc analysis of Japanese patients from the placebo-controlled PREVAIL trial of enzalutamide in patients with chemotherapy-naive, metastatic castration-resistant prostate cancer—updated results, Japanese Journal of Clinical Oncology, Volume 47, Issue 3, March 2017, Pages 262–264, https://doi.org/10.1093/jjco/hyw187
Close - Share Icon Share
Abstract
A post hoc analysis of interim results from PREVAIL, a Phase III, double-blind, placebo-controlled trial of men with metastatic castration-resistant prostate cancer, demonstrated that the treatment effects, safety and pharmacokinetics of enzalutamide in Japanese patients were generally consistent with those of the overall population. A recent longer term analysis of PREVAIL demonstrated continued benefit of enzalutamide treatment over placebo. Here, we report results from a post hoc analysis of Japanese patients enrolled in PREVAIL at the prespecified number of deaths for the final analysis. In Japanese patients, enzalutamide reduced the risk of death by 35% (hazard ratio, 0.65; 95% confidence interval, 0.28–1.51) and the risk of investigator-assessed radiographic progression or death by 60% (hazard ratio, 0.40; 95% confidence interval, 0.18–0.90). These results show that treatment effects and safety in Japanese patients in the final analysis of PREVAIL continued to be generally consistent with those of the overall population.
Introduction
Enzalutamide significantly improved radiographic progression-free survival (rPFS) and overall survival (OS) in men with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC) at the prespecified interim analysis of PREVAIL, a Phase III, double-blind, randomized study (1). We recently reported treatment effects and safety from a post hoc analysis of Japanese patients enrolled in PREVAIL at the interim analysis (2). Of the 1717 patients enrolled, 61 (enzalutamide arm, n = 28; placebo arm, n = 33) were from Japanese study sites. At the prespecified interim analysis for OS (at 540 deaths in the overall population; data cutoff, 16 September 2013), the treatment effects of enzalutamide were consistent with those in the overall population (1): enzalutamide reduced the risk of death by 29% [hazard ratio (HR), 0.71; 95% confidence interval (CI), 0.60–0.84] and risk of investigator-assessed radiographic progression or death by 57% (HR, 0.43; 95% CI, 0.18–1.04) in Japanese patients (2). Longer term efficacy and safety up to the prespecified number of deaths in the final analysis were recently reported for the overall PREVAIL population, demonstrating continued benefit of enzalutamide over placebo (3). In the overall population, enzalutamide reduced the risk of death by 23% (HR, 0.32; 95% CI, 0.67–0.88; P = 0.0002) and reduced the risk of radiographic progression or death by 68% (HR, 0.32; 95% CI, 0.28–0.37; P < 0.0001) (3).
Here, we report results using the prespecified number of deaths for the final analysis (784 deaths in the overall population; data cutoff, 1 June 2014) in Japanese patients enrolled in PREVAIL.
Patients and methods
The full methodology of PREVAIL (NCT01212991) has been reported (1). PREVAIL was approved by the independent review board at each participating site, and was carried out according to the provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation.
Eligible patients were those with chemotherapy-naive mCRPC who had progressed despite surgical or medical castration, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 and were asymptomatic or mildly symptomatic per Brief Pain Inventory Short Form question 3 (i.e, pain score 0–3) (4). Patients with visceral disease were eligible. Patients with characteristics that could lower the seizure threshold (i.e, brain metastases, history of seizure and concurrent medications), prior use of chemotherapy or New York Heart Association class III or IV heart failure were excluded.
Patients were enrolled from September 2010 through September 2012 at 207 sites worldwide, 21 of which were in Japan; Japanese sites enrolled patients from November 2011 through May 2012. Patients were randomized 1:1 to receive 160 mg oral enzalutamide or placebo once daily. Randomization was stratified according to study site. Treatment was discontinued for occurrence of unacceptable side effects, or until confirmed radiographic progression or a skeletal-related event and the initiation of cytotoxic therapy or an investigational agent for prostate cancer.
Estimates of the medians and 95% CIs were determined using the Kaplan–Meier method. The HR relative to placebo, with <1 favoring enzalutamide, was determined using an unstratified Cox regression model with treatment as the only covariate.
Endpoints for the extended analysis were evaluated in the intent-to-treat population (all randomly assigned patients). Additional safety analyses were not performed.
Results
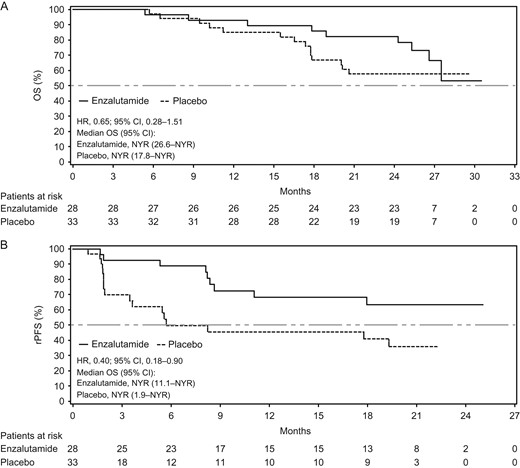
Kaplan–Meier estimates of updated (A) OS for Japanese patients, including 9 months of additional follow-up and 5 months of data after patients crossed over from placebo to enzalutamide (data cutoff 1 June 2014) and (B) rPFS for Japanese patients, including 4 months of additional follow-up (data cutoff 15 January 2014). The dashed horizontal line indicates median. HRs are based on unstratified Cox regression models with treatment as the only covariate, with values <1.00 favoring enzalutamide. CI, confidence interval; HR, hazard ratio; OS, overall survival; NYR, not yet reached; rPFS, radiographic progression-free survival.
| . | Japanese subgroup (n = 61) . | |
|---|---|---|
| Enzalutamide (n = 28) . | Placebo (n = 33) . | |
| Median age, years (range) | 73 (57–93) | 69 (49–89) |
| Median body weight (kg) | 64.8 | 68.9 |
| Median body mass index (kg/m2) | 23.5 | 24.4 |
| Gleason score ≥8 at initial diagnosis (%) | 82.1 | 87.1 |
| ECOG PS = 0 (%) | 89.3 | 81.8 |
| Baseline pain score 0–1 on BPI-SF Q3 (%) | 89.3 | 87.9 |
| Median PSA (ng/ml) | 20.3 | 23.2 |
| Median lactate dehydrogenase (IU/l) | 193.0 | 207.0 |
| Baseline use of corticosteroids (%) | 17.9 | 18.2 |
| Prior anti-androgen use (%) | 100 | 100 |
| No. of prior unique anti-androgen therapies (%) | ||
| ≤1 | 57.1 | 42.4 |
| ≥2 | 42.9 | 57.6 |
| No. of prior unique hormone therapies (%) | ||
| ≤2 | 42.9 | 33.3 |
| ≥3 | 57.1 | 66.7 |
| Prior radical prostatectomy (%) | 3.6 | 3.0 |
| Bone disease (%) | 96.4 | 100 |
| ≥10 bone metastases | 50.0 | 36.4 |
| Soft-tissue disease—lymph node, visceral or other (%) | 46.4 | 36.4 |
| . | Japanese subgroup (n = 61) . | |
|---|---|---|
| Enzalutamide (n = 28) . | Placebo (n = 33) . | |
| Median age, years (range) | 73 (57–93) | 69 (49–89) |
| Median body weight (kg) | 64.8 | 68.9 |
| Median body mass index (kg/m2) | 23.5 | 24.4 |
| Gleason score ≥8 at initial diagnosis (%) | 82.1 | 87.1 |
| ECOG PS = 0 (%) | 89.3 | 81.8 |
| Baseline pain score 0–1 on BPI-SF Q3 (%) | 89.3 | 87.9 |
| Median PSA (ng/ml) | 20.3 | 23.2 |
| Median lactate dehydrogenase (IU/l) | 193.0 | 207.0 |
| Baseline use of corticosteroids (%) | 17.9 | 18.2 |
| Prior anti-androgen use (%) | 100 | 100 |
| No. of prior unique anti-androgen therapies (%) | ||
| ≤1 | 57.1 | 42.4 |
| ≥2 | 42.9 | 57.6 |
| No. of prior unique hormone therapies (%) | ||
| ≤2 | 42.9 | 33.3 |
| ≥3 | 57.1 | 66.7 |
| Prior radical prostatectomy (%) | 3.6 | 3.0 |
| Bone disease (%) | 96.4 | 100 |
| ≥10 bone metastases | 50.0 | 36.4 |
| Soft-tissue disease—lymph node, visceral or other (%) | 46.4 | 36.4 |
BPI-SF Q3, Brief Pain Inventory Short Form question 3; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen.
aReproduced from Kimura et al. (2), under the Attribution-NonCommercial-NoDerivatives 4.0 International license. Copyright 2016, The Authors and John Wiley & Sons Australia, Ltd, on behalf of the Japanese Urological Association.
| . | Japanese subgroup (n = 61) . | |
|---|---|---|
| Enzalutamide (n = 28) . | Placebo (n = 33) . | |
| Median age, years (range) | 73 (57–93) | 69 (49–89) |
| Median body weight (kg) | 64.8 | 68.9 |
| Median body mass index (kg/m2) | 23.5 | 24.4 |
| Gleason score ≥8 at initial diagnosis (%) | 82.1 | 87.1 |
| ECOG PS = 0 (%) | 89.3 | 81.8 |
| Baseline pain score 0–1 on BPI-SF Q3 (%) | 89.3 | 87.9 |
| Median PSA (ng/ml) | 20.3 | 23.2 |
| Median lactate dehydrogenase (IU/l) | 193.0 | 207.0 |
| Baseline use of corticosteroids (%) | 17.9 | 18.2 |
| Prior anti-androgen use (%) | 100 | 100 |
| No. of prior unique anti-androgen therapies (%) | ||
| ≤1 | 57.1 | 42.4 |
| ≥2 | 42.9 | 57.6 |
| No. of prior unique hormone therapies (%) | ||
| ≤2 | 42.9 | 33.3 |
| ≥3 | 57.1 | 66.7 |
| Prior radical prostatectomy (%) | 3.6 | 3.0 |
| Bone disease (%) | 96.4 | 100 |
| ≥10 bone metastases | 50.0 | 36.4 |
| Soft-tissue disease—lymph node, visceral or other (%) | 46.4 | 36.4 |
| . | Japanese subgroup (n = 61) . | |
|---|---|---|
| Enzalutamide (n = 28) . | Placebo (n = 33) . | |
| Median age, years (range) | 73 (57–93) | 69 (49–89) |
| Median body weight (kg) | 64.8 | 68.9 |
| Median body mass index (kg/m2) | 23.5 | 24.4 |
| Gleason score ≥8 at initial diagnosis (%) | 82.1 | 87.1 |
| ECOG PS = 0 (%) | 89.3 | 81.8 |
| Baseline pain score 0–1 on BPI-SF Q3 (%) | 89.3 | 87.9 |
| Median PSA (ng/ml) | 20.3 | 23.2 |
| Median lactate dehydrogenase (IU/l) | 193.0 | 207.0 |
| Baseline use of corticosteroids (%) | 17.9 | 18.2 |
| Prior anti-androgen use (%) | 100 | 100 |
| No. of prior unique anti-androgen therapies (%) | ||
| ≤1 | 57.1 | 42.4 |
| ≥2 | 42.9 | 57.6 |
| No. of prior unique hormone therapies (%) | ||
| ≤2 | 42.9 | 33.3 |
| ≥3 | 57.1 | 66.7 |
| Prior radical prostatectomy (%) | 3.6 | 3.0 |
| Bone disease (%) | 96.4 | 100 |
| ≥10 bone metastases | 50.0 | 36.4 |
| Soft-tissue disease—lymph node, visceral or other (%) | 46.4 | 36.4 |
BPI-SF Q3, Brief Pain Inventory Short Form question 3; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen.
aReproduced from Kimura et al. (2), under the Attribution-NonCommercial-NoDerivatives 4.0 International license. Copyright 2016, The Authors and John Wiley & Sons Australia, Ltd, on behalf of the Japanese Urological Association.
Discussion
The OS results after an additional 9 months of follow-up and rPFS results with an additional 4 months of follow-up were consistent with those from the interim analysis and from the longer term analysis in the overall study population, demonstrating treatment benefit of enzalutamide compared with placebo in asymptomatic or mildly symptomatic men with chemotherapy-naive mCRPC. This post hoc analysis was limited by the small number of patients enrolled in Japan and the fact that PREVAIL was not powered to detect differences between enzalutamide and placebo in Japanese patients. However, the results from Japanese patients in PREVAIL have been highly consistent with those in overall study population.
Conclusions
These longer term efficacy results in Japanese patients enrolled in PREVAIL demonstrate continued treatment benefit of enzalutamide compared with placebo in men with asymptomatic or mildly symptomatic mCRPC.
Acknowledgements
The authors would like to thank the patients and acknowledge all of the investigators working on the PREVAIL trial in Japan. Medical writing and editorial support funded by both sponsor companies was provided by Stephanie Vadasz, PhD, and Shannon Davis of Ashfield Healthcare Communications. Statistical analyses were performed by Ad Theeuwes, PhD, an employee of Astellas.
Funding
PREVAIL was funded by Medivation, Inc., and Astellas Pharma, Inc., the co-developers of enzalutamide. Medivation was acquired by Pfizer, Inc., in September 2016.
Conflict of interest statement
Go Kimura reports honoraria and clinical study fees from Astellas, Takeda, Pfizer, Bayer, Novartis, GlaxoSmithKline and Ono Pharmaceutical Company. Takeshi Ueda reports grants from Astellas.


