-
PDF
- Split View
-
Views
-
Cite
Cite
Victoria Y. Yokoyama, Olive Fruit Fly (Diptera: Tephritidae) in California Table Olives, USA: Invasion, Distribution, and Management Implications, Journal of Integrated Pest Management, Volume 6, Issue 1, March 2015, 14, https://doi.org/10.1093/jipm/pmv014
Close - Share Icon Share
Abstract
Olive fruit fly, Bactrocera oleae (Rossi), was discovered in California in late 1998. Thereafter, intensive research was conducted to develop pest control methods in table olives grown for canned fruit. The life history of olive fruit fly was elucidated, and the distribution and abundance of the adults determined through trapping programs. Olive samples from noncommercial trees were collected from 2002 through 2013 in different locations to determine the maximum number of larvae per fruit. Larvae were most abundant in September and October and ranged from <0.5 to 10 per fruit. The date of maximum fruit infestations may differ annually due to the amount of fruit set, fruit size, and weather. Very high numbers of larvae were collected from large green fruit (≥4 g), whereas smaller fruit (∼2–3 g) supported fewer insects. High larval numbers were found in fruit from areas with cooler summer weather than in fruit from the hot inland valleys. Olive fruit fly larval populations were prevalent in locations with high summer temperatures when buffered by cool marine air flow or slightly higher elevations from the inland valley floor. High larval infestations were found at an arid location with hot summers, suggesting that olive fruit fly may adapt to such conditions. Infested fruit was collected from the same locations in subsequent years, and the proximity of commercial olive orchards may enhance susceptibility to future infestation. Control methods are summarized, including intensive biological control programs and new techniques such as bait stations considered for pest management programs. Basic cultural practices such as removal of nonharvested fruit that support multiple generations, timing of harvest to avoid adult activity, and elimination of standing water required by olive fruit fly adults for survival would be of major importance in reducing pest populations.
Olive fruit fly, Bactrocera oleae (Rossi), was first found in California on 19 October 1998 (CDFA 1998, CDFA Plant Health and Pest Prevention Services 1998). An unmated, immature female was found in a McPhail wet trap placed on an orange tree along La Grange Avenue in West Los Angeles. The specimen was identified in the biosystematics laboratory at the California Department of Food and Agriculture (CDFA), Plant Health/Pest Prevention Services, Pest Detection/Emergency Projects, Bell, CA, and confirmed by the CDFA Plant Pest Diagnostics Branch, Sacramento, CA. The discovery initiated olive fruit fly eradication procedures and larval surveys because CDFA (1998) maintained that trap finds did not necessarily indicate actual infestations. By November of the same year, malathion bait sprays were applied to all olive trees within 200 m of olive fruit fly trap captures, and soil drench treatments with diazinon were made to properties with larval finds. At the end of 1998, 63 adults had been trapped and nine larvae had been found primarily, in the Westwood area. Locations of these larval collections are shown in Fig. 1, and the collections seem to align along a downwind pattern from the original adult capture. Based on this observation, current detection of olive fruit fly adults in pest-free regions should include additional olive fruit inspections downwind of trap captures.
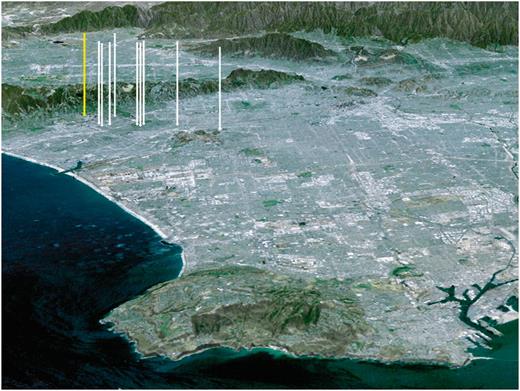
Vertical arrows show locations where olive fruit fly larvae and pupae were collected in the heavily populated Los Angeles basin from 1998 to 1999. The image shows a north by northeast perspective of the city from above the Pacific Ocean. The San Gabriel Mountains are in the distance and the hilly Palos Verdes Peninsula is in the foreground (Los Angeles map, National Aeronautics Space Administration 2000). The far left, yellow arrow points to Westwood where the first adult was found. The locations of other arrows where larvae and pupae were collected appear to be aligned along a prevailing downwind pattern from Westwood. Pupae were also collected in Anaheim and Santa Barbara in September to October 1999 (CDFA 1998, 1999). The land image is about 29 km wide by 70 km in depth.
In 1999, eradication efforts were continued in Los Angeles County through May with >300 adults captured and five larvae found (CDFA 1999, CDFA Plant Health and Pest Prevention Services 1999; Fig. 1). By September 1999, olive fruit fly adults had been captured in Orange, Ventura, Santa Barbara, San Diego, and Tulare counties, and by December adults had been found in Riverside County and in Ensenada, Mexico. The olive fruit fly interior quarantine in the Westwood area of Los Angeles County established in 1998 was repealed on 20 July 2002 (CDFA 2002).
The threat of olive fruit fly to the olive industry after its discovery in California was first addressed by Rice (2000). California is the only U.S. state to produce table olives and exports its products to Canada and Japan. The commodity group California Olive Committee, Clovis, CA, began proactive measures to help evaluate the severity of the pest infestation. Scientists from olive-producing countries including Spain and Greece were invited to assess the invasion and to discuss control procedures (Pollock 2001). Although a vast amount of literature was available from European countries and was recently summarized by Daane and Johnson (2010), Rice (2000) recommended to the olive industry that California needed to develop state-specific biological and pest management information. Subsequent research was performed by California olive fruit fly study centers at the U. S. Department of Agriculture, Agricultural Research Service, San Joaquin Valley Agricultural Sciences Center, Parlier; University of California, Kearney Agricultural Center, Parlier; Departments of Entomology, Davis and Riverside; and the California Department of Food and Agriculture, Sacramento, CA. The listed references represent the major published research findings from these California researchers.
Olive fruit fly larval infestation data had been collected for more than a decade from different California localities and regions. Larval distribution records would be valuable for growers for predicting crop vulnerability to pest damage. Olive fruit fly abundance is related to climatic conditions, and illustrative methods to show pest numbers throughout the growing regions would provide visual information for developing IPM programs.
The objective of this study is to support California olive producers with an overview of olive fruit fly, which has become the key pest of olives since its introduction in 1998. The biology and life history of the pest are described and illustrated. Current techniques for detecting the adults and immature stages are presented with a comprehensive survey of the distribution and abundance of the immature stages in different localities shown in relief maps. Available control methods for olive fruit fly in California are listed by topic and summarized with suggestions for future olive fruit fly management in commercial olive orchards.
Table Olives
Olives were introduced into California from Mexico by Franciscan padres in the late 1700s, and the fruit was primarily used for oil (Connell 1994). The canned olive industry began in the early 1900s, so California table olives were free of olive fruit fly for almost 100 years. In 1966, about 32,000 acres (12,950 hectares) of table olives were grown in the state, primarily in the Sacramento and San Joaquin Valleys (Hartmann and Opitz 1966). Major information about growing and harvesting California table olives can be found in the technical handbook “Olive Production Manual” (Ferguson et al. 1994). To fulfill consumer demand for olive oil, the current trend in the olive industry is for super-high-density plantings of oil olives and automated oil processing (Warnert 2011, Vossen 2007). To date, olive fruit fly infestations have not been found in high-density olive oil cultivars grown in California.
Pest
Description
Genetic studies have shown that the origin of olive fruit fly in California is eastern Mediterranean (Nardi et al. 2005, Zygouridis et al. 2009), most likely from Cyprus, Israel, and the neighboring coast of Turkey (Nardi et al. 2010).
Genç (2014) described olive fruit fly eggs as creamy white, slightly curved, broader from the middle toward the anterior pole, and slightly tapering toward the posterior pole (Fig. 2). Eggs have an average length of 0.7 mm and an average diameter of 0.2 mm.

Cross section of an olive fruit showing an olive fruit fly egg (about 0.7 mm long) deposited beneath the green surface (Photo by Rollin Coville).
The larval stage has three instars (Fig. 3), white-yellowish in color, slender to stout, elongated, and tapering anteriorly. The mature larva (Fig. 4) is 6.5–7.0 mm long and 1.2–1.7 mm wide. The complete description of the larval stage is provided by Carroll et al. (2004 onwards).

Olive fruit fly second instar (about 4 mm long) surrounded by frass, causing damage by feeding in an olive fruit (Photo by Rollin Coville).

Mature olive fruit fly third-instar larva (about 6 mm long) after leaving the fruit to pupate (Photo by Rollin Coville).
Pupae initially are soft and pale yellow, changing to dark, reddish-brown with age. The pupa is about 5.1 mm long and 2.1 mm wide (Genç and Nation 2008a; Fig. 5).
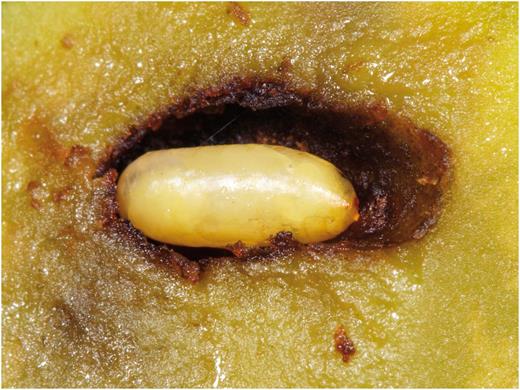
Olive fruit fly pupa (about 5 mm long) found in fruit or in the soil (Photo by Rollin Coville).
Olive fruit fly adults (Figs. 6 and 7) are normally 4–5 mm long with large iridescent eyes and small antennae (Rice 2000). The thorax is brown with two to four gray or black longitudinal stripes. The scutellum is yellow to white with several yellow-white patches on each side of the thorax. The abdomen is brown with variable darker areas on the sides of each segment. The wings of the olive fruit fly are clear except for a small distinct black spot at the tips. Wing veins may also be slightly dark. A technical description of the adult olive fruit fly is provided by White and Hancock (1997).

Olive fruit fly female (4–5 mm long) with ovipositor (Photo by Rollin Coville).

Regulatory agencies have been vigilant about the potential threat of invasion by the olive fruit fly for nearly four decades; the pest was included in the CDFA Plant Pest Detection Manual (Paddock 1976). However, olive fruit fly does not have colored wing bands or patterns typical of many other species of fruit flies such as the Mediterranean fruit fly, Ceratitis capitata (Wiedemann). Lack of distinctive wing patterns and its small size may have enabled olive fruit fly to go unnoticed in trap captures for some time before 1998. After 1998–1999 (Fig. 1), most California counties delineated by the red circle in Fig. 8 reported olive fruit fly adult trap captures, indicating the strong dispersal capacity of the adults. However, the widespread detection of larvae in 2000–2001 surveys (Fig. 8) suggests that the pest may have been a pre-1998 introduction.
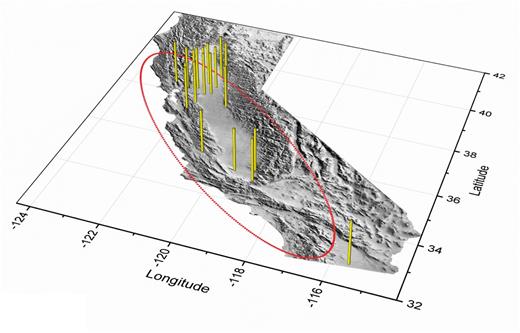
Vertical bars show locations where olive fruit fly larvae and pupae were collected in California from 2000 to 2001 (CDFA 2000, 2001). The red boundary surrounds counties where adults had been captured during this period (CDFA 2001). Map of California by Edwards and Batson (1990).
Life History
The host of olive fruit fly in California is cultivated olives (Olea europaea L.). The life cycle of the olive fruit fly is synchronized with seasonal growth and olive fruit production (Zalom et al. 2009). Annual populations begin in the spring during March, April, and May, with the presence of many adults that originate from overwintering pupae and from insects developing in fruit that remain in the tree from the previous year. Adults are attracted to water in the orchard (Yokoyama and Miller 2007) and feed on available food such as honeydew from black scale, Saissetia oleae (Oliver) (Daane and Caltagirone 1989, Wang et al. 2011a). Adults are strong fliers and can disperse over a distance of 2,000 m (∼1 ¼ mi) and fly for about 2 h (Wang et al. 2009b). Although adults have been captured in other tree species (Athar 2005), larval infestations have not been found in these tree species, indicating the transitory nature of the captured adults.
Olive trees bloom in May, and after fruit set and growth to 1 cm or more in length, sufficient food is available for a larva to complete development to an adult (Yokoyama 2012a). Females (Fig. 6) and males (Fig. 7) mate (Fig. 9) in the late afternoon. When females are 6 d old they begin to lay eggs (Fig. 10). The maximum number of eggs are laid by 13–37-d-old females, and the end of egg laying occurs at 90 d old (Yokoyama 2012a). Females lay eggs about 1 mm beneath the fruit surface (Wang et al. 2009d), creating a depression with necrotic brown tissue (Fig. 2). Under cool and humid conditions of 26°C (79°F) and 63% relative humidity, adults survived for 203 d with food and water, but survived only 10 d when deprived of food and water (Yokoyama 2012a). Mated, gravid females are present at some level throughout the year, with an average of seven eggs in their ovaries (Burrack et al. 2011), and can lay 200 to 500 or more eggs (Rice 2000).

Olive fruit fly male and female mating (Photo by Rollin Coville).

Female olive fruit fly laying eggs in an olive (Photo by Rollin Coville).
High summer temperatures during late June, July, and August have the greatest effect on the abundance and distribution of olive fruit fly in California. Populations are high in cool, humid coastal areas and low in hot, dry areas of the inland valley where olives are grown. When olive fruit fly abundance was related to mean temperatures in California, the hot climates in the Central Valley and deserts were shown to limit pest distribution (Johnson et al. 2011). Mid-day temperatures of 35°C (95°F) in the Central Valley were lethal to eggs and first instars (Wang et al. 2009a). Under these temperatures, adults lived for only 2 d without food and water and only 4 d with provisions (Yokoyama 2012a). Adults exposed to high temperatures had reduced fecundity and longevity, poor flight performance, and high mortality when deprived of food and water (Wang et al. 2009b). The detrimental effects of summer heat should be considered in pest management programs in the Central Valley (Johnson et al. 2006). Eventually, climate warming will shift olive fruit fly populations northward beyond its current range (Gutierrez et al. 2009).
Olive fruit fly larvae hatch from eggs in olive fruit in 1–5 d (Yokoyama and Miller 2007). The larvae feed on the fruit pulp (Fig. 3) and have three instars that can be separated easily by body length (Yokoyama 2012a) or by shape and color of the mandibular stylets (Wang et al. 2009d). Larvae can complete development to the pupal stage in 2 wk at moderate temperatures, e.g., 26°C (79°F). Development is slower at lower temperatures. Pupation can occur in the fruit (Fig. 5), or the larvae leave the fruit to pupate in the soil. Mature third instars (Fig. 4) are capable of moving across smooth surfaces as far as 24 m (79 ft) (Yokoyama 2012b). Once in the soil, the mature larvae are susceptible to adverse high temperatures (Yokoyama 2012a) and mortality from desiccation and low soil moisture (Orsini et al. 2007). Immersion in water simulating orchard flood irrigation causes pupal death after 4 d (Yokoyama and Miller 2007). Pupae require up to 46 d at 14°C (57°F), 19 d at 21°C (70°F), and 12 d at 26°C (79°F) to develop to adults (Yokoyama 2012a). When the adults emerge from the pupal cases in the soil, they are pale (Fig. 11) and capable of climbing to the surface of sand from 51 cm (20 in) deep, walking 13 m (43 ft), and flying after about 3 h (Yokoyama 2012b).

Pale teneral adult after emerging from the pupa and before hardening, darkening, and expanding wings (Photo by Rollin Coville).
Olive fruit fly adults (Figs. 6 and 7) are most abundant during the months of September, October, and early November. Olive fruit fly has multiple generations (at least four) per year (Burrack et al. 2011). The life cycle from egg to adult can be completed in a minimum of 26 d under mild temperatures of 21–26°C (70–79°F) (Yokoyama 2012a, Yokoyama and Miller 2007). All life stages can be found during the winter, especially if fruit is present for oviposition and larval development. Olive fruit fly adults can survive temperature ranges from 5°C (41°F) (Yokoyama 2012a) to greater than 40°C (104°F) (Wang et al. 2009a).
Olive fruit fly females are attracted to large-size fruit (Yokoyama et al. 2006), and table olives of the Sevillano, Manzanillo, and Mission cultivars are more heavily infested than smaller fruit of some olive oil cultivars (Burrack and Zalom 2008). Larger-size fruit allow the larvae to feed and move deeper into the olive pulp as they mature, providing a better food resource than smaller fruit (Wang et al. 2009d).
Monitoring
Adults
The first olive fruit fly adults were collected in McPhail traps in 1998. After the first capture, the McPhail trap density was increased to 80 traps per square mile in the core square mile and 40 McPhail traps per square mile in the eight adjacent square miles (CDFA 1998). ChamP perforated yellow panel, sticky traps (Seabright Laboratories, Emeryville, CA) also were deployed at the same density as the McPhail traps within the delimitation area (CDFA 1999). Olive fruit fly was reported as not responsive to cuelure, a melon fly, Bactrocera cucurbitae (Coquillett), attractant, or methyl eugenol, an oriental fruit fly, Bactrocera dorsalis (Hendel), attractant. Adults also were captured in yellow sticky panel traps with ammonium bicarbonate bait.
Extensive olive fruit fly trapping programs were initiated outside the Los Angeles basin after olive fruit fly was detected. Phillips and Rice (2001) conducted adult surveys using ChamP traps from April to May, 2000, in Santa Barbara and Ojai and noted that more adults were collected in irrigated areas and that the population trends were similar to those in Mediterranean countries. During 2000–2001, Rice et al. (2003) conducted olive fruit fly trap surveys in commercial olive orchards in the northern and southern areas of the San Joaquin Valley and captured adults in every location throughout the year. Adults captured in the spring were attributed to overwintering pupae, the summer decline of adults was related to hot weather, and fall generations resulted from earlier infestations. Zalom et al. (2008) reported olive fruit fly trap captures during 2002–2004 in 15 counties and noted that captures increased dramatically during the 2.5 yr of monitoring. The California Olive Committee, Clovis, CA, has sponsored previous and ongoing olive fruit fly trapping programs since 2001.
Captures of olive fruit fly adults with ChamP and yellow panel traps were compared with and without ammonium bicarbonate bait packets and plastic dispensers of pheromone lure (1,7-dioxaspiro [5,5] undecane; Vioryl, Athens, Greece; Rice et al. 2003). Yokoyama et al. (2006) showed that the yellow panel Pherocon AM traps (Trécé, Adair, OK) attracted more adults than ChamP traps when both were supplied with a packet of ammonium bicarbonate bait (15–20 g) and a pheromone lure. Burrack et al. (2008) confirmed that yellow panels captured more adults than ChamP traps, but found that McPhail traps outperformed the other types of traps. The water component of the tortula yeast food lure was considered to increase attractiveness, but maintenance of the liquid ingredient is a limitation of the McPhail trap.
Larvae
Olive fruit fly in table olives is not tolerated by canners, and based on industry standards, infested fruit can be rejected upon arrival at processing facilities. Even though olive fruit fly adults are captured in commercial olive orchards, the fruit may not be infested (Yokoyama 2014a). According to European observations, olive fruit is infested at “pit-hardening,” or when a blade slice through immature fruit is resisted by the seed pit (Rice et al. 2003). In California, immature fruit at least 1 mm in length can be infested and will support the development of the larval stages to adults (Yokoyama 2012a). Fruit length is a simple measurement to determine susceptibility to female oviposition and successful development of the larval stages. Additionally, large green fruit can support the development of higher numbers of larvae than small fruit. Adults are most abundant in late summer to fall, especially in October when table olives are harvested. In general, most larvae can be collected in September and October. Thus, timing of harvest may be a factor in avoiding fruit infestations.
Fruit samples are used to determine the presence or absence of olive fruit fly larvae. Samples should be collected in localities and within orchards or trees where olive fruit fly adults have been observed or oviposition in fruit is suspected. Increasing the number of fruit collected increases the chances of detecting an infestation. Fruit samples can be placed in ridged plastic storage containers and covered with sheer cloth such as organdy or chiffon held in place with a rubber band. The cloth cover allows ventilation, and the fruit should be packed loosely to prevent the growth of mold. A raised wire or plastic screen can be placed on the bottom of the container to allow the larvae to exit the fruit and drop to the bottom where they can be observed. The total number of olive fruit fly life stages that emerge divided by the number of olives in the sample is the average number of larvae that developed in each fruit.
Larval densities can range from ≤1 to 11 per fruit, but the presence of two or more larvae per fruit can cause a reduction in individual size and number of pupae (Burrack et al. 2009). In multiple fruit samples from the same area, the highest number of larvae per fruit found in any sample indicates the maximum infestation for the collection date. Figs. 12–18 show the maximum number of larvae observed in multiple samples of about 350 olives from different locations in California from 2002 to 2011. The number of larvae per fruit is shown in a colored graph bar of proportional height on a map of California (Edwards and Batson 1990) to illustrate the abundance and distribution of the pest by region and year. Each map was rotated for the best perspective of the graph bars for the different locations each year. The data are primarily from fruit collections for determining rates of olive fruit fly larval parasitism by Psyttalia humilis (Silvestri) (Yokoyama et al. 2008a,b, 2010, 2011, 2012; Fig. 19). The highest number of olive fruit fly larvae per fruit is shown for the sampling duration at each location, which varied from one to several weeks. The number of insects was dependent on the date the sample was collected, and higher numbers may have been present before or after this date. Fruit was collected primarily from ornamental and windrow olive trees of the Mission and Manzanillo cultivars. These trees can serve as sources of olive fruit fly for nearby commercial table olive orchards, and the threat may be related to the density of larval infestations shown in Figs. 12–18.
Comparison of fruit infestations among years would be difficult without repetitive sampling within the same season. Even so, the date of maximum fruit infestations may differ yearly due to the amount of fruit set, fruit size, weather, and other variables that may shift maximum fruit infestations from late summer to early winter. For example, in Los Angeles at the same location near Westwood where the first olive fruit fly adult was found, the maximum number of larvae collected per fruit was 0.1 in late July 2002 (Fig. 12), 1.4 in late August 2003 (Fig. 13), and 3.8 in early August 2004. Another example of variability in annual larval infestations based on sampling conditions occurred in San Diego where maximum numbers per fruit ranged from 0.5 in 2002 to 3 in 2008 (Figs. 12–15). Therefore, trends in olive fruit fly densities may be more readily evaluated with adult captures, whereas fruit samples suggest potential severity of infestations.
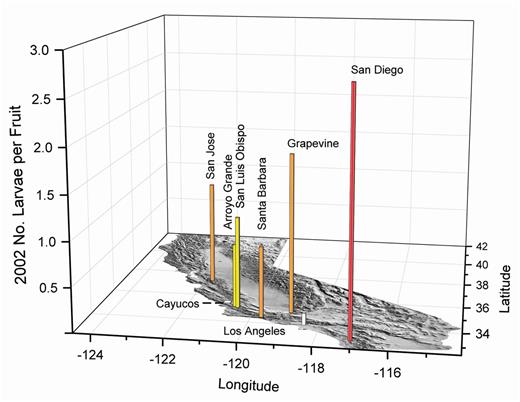
2002 olive fruit fly larval infestations in fruit from the California coast and on the perimeter of the Central Valley. White bars indicate about 0.5, yellow about one, orange about two, and red about three maximum larvae per fruit in multiple samples of about 350 olives.
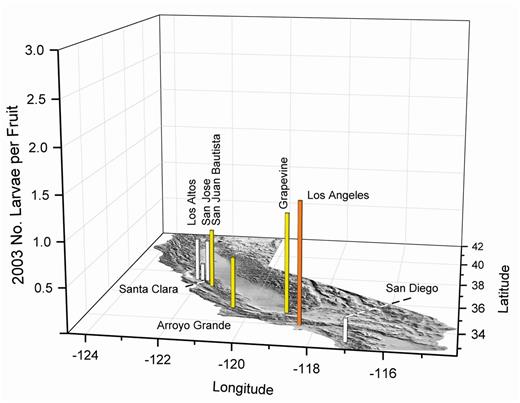
2003 olive fruit fly larval infestations in fruit from the California coast and on the perimeter of the Central Valley. White bars indicate about 0.5, yellow about one, and orange about two maximum larvae per fruit in multiple samples of about 350 olives.
In 2002 (Fig. 12) and 2003 (Fig. 13), olive fruit samples were collected in the San Francisco Bay area and southern coastal regions as far south as the San Diego area. Olive fruit fly larval infestations in these locations were predictable due to the cool weather conditions. In both years, fruit samples were collected from Grapevine, an isolated location in the southern end of the Central Valley at the base of the Tehachapi Mountains. The ornamental trees in Grapevine were cooled by landscape irrigation, and close proximity to the mountains created a suitable habitat for olive fruit fly.
In 2006 (Fig. 14), extraordinarily high larval infestations in olives were found in the San Diego area. The fruit size in these samples was large, about 4.3 g, capable of supporting the development of several larvae per fruit. Most other samples were commonly made with smaller (2–3 g) fruit. Small numbers of olive fruit fly larvae were found in Orland on the northern end of the Central Valley, the first inland valley location to be sampled. Olive fruit fly larvae were collected in fruit from Sylmar, a historic and former major olive-growing region, and the inland coast location of Riverside.
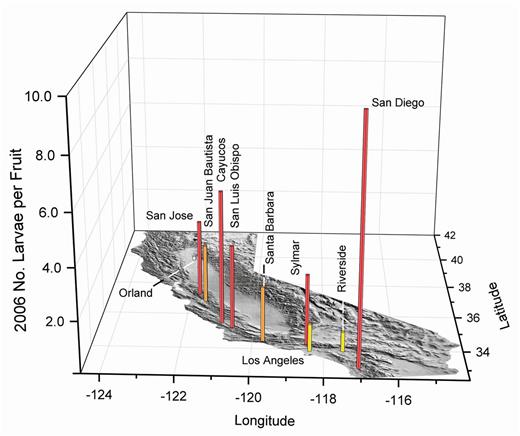
2006 olive fruit fly larval infestations in fruit from the California coast and at the northern end of the Central Valley. Sylmar is a historic olive-growing region. The vertical scale has been increased to show the extremely high infestation found in San Diego in large-size olives. White bars indicate about 0.5, yellow about one, orange about two, and red about three or more maximum larvae per fruit in multiple samples of about 350 olives.
In 2008 (Fig. 15), fruit collections were made from two new inland valley locations, Davis and Lodi. Lodi had very high numbers of larvae per fruit, even though summer temperatures in the location typically would cause high mortality of adults and immature stages. Survival in Lodi was attributed to cooling conditions created by the marine influence of the Bay Area (Yokoyama et al. 2010). Olive fruit fly larvae also were collected from Napa where olives commonly are planted around vineyards.
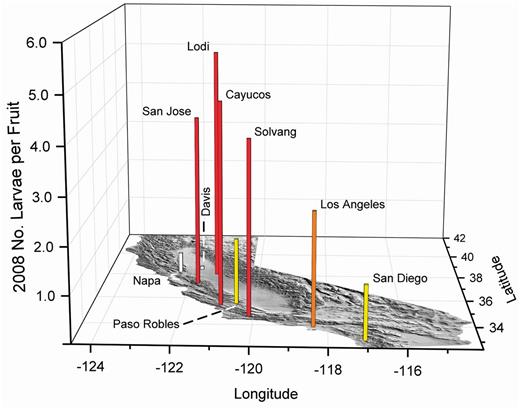
2008 olive fruit fly larval infestations in fruit from the California coast and two Central Valley locations, Davis and Lodi. The vertical scale has been increased to include the high infestation found in Lodi. White bars indicate about 0.5, yellow about one, orange about two, and red with three or more maximum larvae per fruit in multiple samples of about 350 olives.
By 2009 (Fig. 16), fruit sampling for olive fruit fly infestations was extended into the foothill areas on the western side of the Central Valley in Bakersfield, Lemon Cove, Oroville, and Porterville. Infestation levels in these locations should have been adversely affected by high summer temperatures. However, the slightly higher elevations of these locations above the valley floor tempered the lethal effects of heat. Low levels of fruit infestations were found in Orland and Oroville close to the foothills of the northern part of the Central Valley. Based on these findings, olive fruit fly can be expected to occur as an economic pest of olives in California except under the most arid and extremely hot conditions during the summer. Deviations from these conditions, including cooling created by marine air flow or higher elevation, will buffer the effect of summer mortality typically associated with high temperatures.

2009 olive fruit fly larval infestations in fruit from the California coast, foothill, and Central Valley locations. The larval infestation in Orland was less than 0.5 per fruit. Green or white bars indicate about 0.5, yellow about one, orange about two, and red about three maximum larvae per fruit in multiple samples of about 350 olives.
In 2010 (Fig. 17), fruit infestations were also evaluated in the Central Valley in Exeter, Merced, and Woodland. Exeter is located near the foothills on the southern end of the valley, and Woodland would be cooled by marine air during the summer, conditions that would sustain olive fruit fly populations. However, Merced has very high summer temperatures, so the high level of infested fruit was unexpected, suggesting that olive fruit fly can adapt to California inland valley summer conditions. As in 2008 (Fig. 15), very high numbers of larvae were found in Lodi.
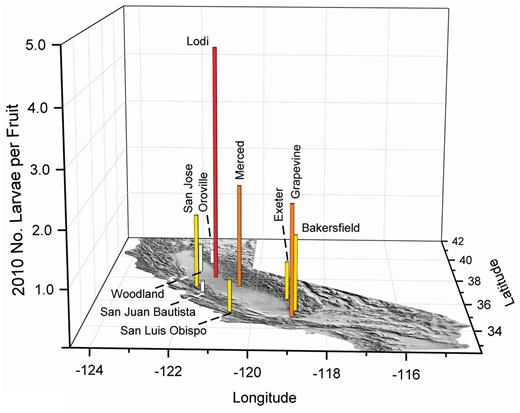
2010 olive fruit fly larval infestations in fruit from the California coast, foothill, and Central Valley locations. The vertical scale has been increased to include the high infestation found in Lodi. White bars indicate about 0.5, yellow about one, orange about two, and red with three or more maximum larvae per fruit in multiple samples of about 350 olives.
By 2011 (Fig. 18), olive fruit fly-infested fruit was readily collected from sites where earlier fruit collections had been made. As in 2010, high populations were found in fruit sampled from Lodi and Merced. Growers need to be vigilant of potential olive fruit fly movement into new high-density plantings of oil olives now grown in the Lodi region.
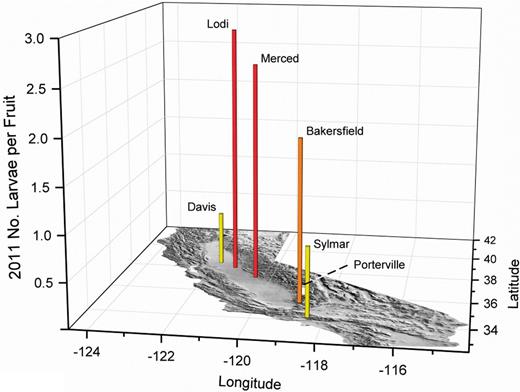
2011 olive fruit fly larval infestations in fruit from California foothill and Central Valley locations, and the Sylmar historic olive-growing region. Yellow bars indicate about one, orange about two, and red about three maximum larvae per fruit in multiple samples of about 350 olives.

Olive fruit fly larval parasitoid, Psyttalia humilis (Silvestri) (female, forewing length about 4 mm), imported from the USDA-APHIS-PPQ, Guatemala, and released in California (Photo by Peggy Greb).
Only years with multiple sampling locations are shown in Figs. 12–18, and in some years fruit collections were made in only a few locations. Maximum larvae per fruit for these years were: 3.81 in Los Angeles in 2004; 2.0, 2.2, and 1.83 in Grapevine, Los Angeles, and San Jose, respectively, in 2005; and 2.51 in San Jose in 2007. Fruit collections from Davis and Lodi in 2013 had moderate infestations.
Management
Introduction of olive fruit fly in California initiated regulatory agency action programs to delineate, contain, and eradicate the pest. Quarantine treatments to prevent the spread of the newly introduced insect through fruit shipped to canners located in different regions of the state were expeditiously developed for immediate implementation. Quarantine treatments, integrated pest management (IPM), cultural control, trapping, biocontrol, and rearing are presented as separate management topics based on broad or specialized studies, unique approaches, or continuing development to control olive fruit fly within the state. Successful pest reduction in table olive orchards utilizing these different control techniques and procedures will vary by region, climate, and yearly growing conditions. IPM recommendations by the University of California Cooperative Extension include chemical and all alternate and compatible control methods that can be used to suppress the pest in table olives. Cultural control tactics are the most economical and should be considered in cultivation of all olives. Olive fruit fly attract-and-kill traps are widely used in European countries, but is still under development in California. Selection of biological control agents is a continuing process, and even with limited success against olive fruit fly at this time, biocontrol is the most preferable means of reducing pest numbers in table olives. The capacity to rear olive fruit fly in the laboratory is a major consideration in future research to develop control methods, and the possibility of mass rearing may eventually result in a sterile insect technique (SIT) program.
Quarantine Treatments
Soon after olive fruit fly was discovered, quarantine control strategies were under development to prevent the pest’s dispersal from infested areas of the state to northern California where major olive canneries are located (Yokoyama et al. 2004). Initially, in the spring of 2000 a laboratory was established at the USDA, APHIS, PPQ in Bell, CA, to develop quarantine treatments, because the Southern California facility was within the olive fruit fly infestation area. Later, the restriction that required olive fruit fly to be studied exclusively in areas with known infestations was alleviated. Thereafter, two quarantine treatments were developed to control any larvae in fruit to prevent distribution resulting from fruit shipped to pest-free areas. Low-temperature storage was designed to hold fruit in cold storage before or after shipment, and brine solutions were intended as a part of the table-olive curing process; both treatments would cause insect mortality (Yokoyama and Miller 2004).
Integrated Pest Management
Integrated control measures for olive fruit fly were proposed soon after the pest was found in the olive production areas of California (Collier and Van Steenwyk 2003). A number of University of California extension publications provided olive fruit fly management procedures and are periodically updated. The original publications are cited, with links to the current online versions. In IPM guidelines for olives, Van Steenwyk et al. (2002) addressed the problem of ornamental olives as a pest source and suggested planting fruitless varieties, application of plant growth regulators to abort flowers, and sanitation by removing unused fruit. Recommendations for commercial orchards included use of attract-and-kill traps, sanitation, and bait spray with GF-120 NF Naturalyte Fruit Fly Bait (Dow AgroSciences, Indianapolis, IN). These recommendations were also presented in “Pest Notes” (Zalom et al. 2003).
Johnson et al. (2006) developed the most comprehensive management guidelines, which included information from statewide workshops and findings from olive fruit fly researchers throughout California. Although olive fruit fly larvae are not tolerated in fruit used for canning, 10–30% infested fruit can be tolerated in olives from coastal orchards that are pressed for oil. McPhail traps and yellow panel sticky traps with female baits and male pheromone lures (two traps per 2–4 ha [5–10 acres]) were recommended to monitor adult activity and population trends. Traps should be installed in orchards by March 1 and placed in trees in a shaded open area on the north side at mid-canopy. Numbers of adult males and females should be monitored weekly, and baits and lures should be replenished as needed. Trap captures can be used to time and evaluate spray applications, but trap captures do not necessarily correlate with level of fruit damage.
GF-120 bait spray was used for olive fruit fly control under an Environmental Protection Agency Section 18 emergency exemption from registration until the product received federal registration in 2004. Applications were recommended at 414 ml per 0.4 ha (14 oz per acre) in dilutions of 1:1.5 to 1:4 with 7 d between treatments (Johnson et al. 2006). Ground applications with large droplets of 4–5 mm to prevent drying should be applied to the upper half of each tree in every other row per week. The alternate unsprayed row would be sprayed on the next treatment. First sprays are recommended as early as before bloom and before June 1, and applications should be made weekly, thereafter.
Even though GF-120 bait spray has been the primary control method for olive fruit fly in California olive orchards, the beginning of resistance has been found in bioassays of GF-120 on adults from areas with numerous bait spray applications (Kakani et al. 2010). A reduction in the number of spray applications may be possible as Collier and Van Steenwyk (2003) noted that only two to three applications were made in some hot, dry locations in Europe. Yokoyama (2014a) observed a small reduction in adult numbers in the spring after two applications of bait spray were made on the underside of bait stations.
A fundamental control recommendation includes postharvest sanitation (Johnson et al. 2006) by removal of all unused fruit (Van Steenwyk et al. 2002). Yokoyama et al. (2006) found in March before bloom more than six olive fruit fly larvae emerging from 10 g of fruit in an orchard that had not been harvested the previous year, stressing the need to prevent annual re-infestations.
Essential Cultural Control
Availability of water ensures survival of olive fruit fly adults, especially during the hot periods of the year. Therefore, irrigation systems must not leak, which leaves standing water in orchards, providing adults with an abundance of water. Timing harvest to remove most of the fruit before very high adult populations develop in the orchard helps prevent damage and losses. Sanitation is a major consideration after harvest. Any fruit that remains in trees is susceptible to infestation and would sustain additional pest generations, potentially until the following year’s crop. These fundamental cultural control procedures help reduce pest numbers and fruit damage in commercial olive orchards.
Attract-and-Kill Traps, Bait Stations, and Novel Approaches
An attract-and-kill trap, Eco-Trap (Vioryl) containing deltamethrin, was studied for olive fruit fly control in 2001–2002 in an isolated infested area (Yokoyama et al. 2004), but the trap was not marketed in the United States. Other manufactured attract-and-kill traps were not registered for use in California (Collier and Van Steenwyk 2003) at that time. Attract-and-kill traps used as bait stations showed promise for olive fruit fly control (Yokoyama 2014a,b). Such devices may reduce the amount of bait spray applications in olive orchards because they attract the pest to the device that contains the toxicant. Attractive bait stations need further refinement for maximum efficacy and should be developed so growers can construct practical versions. A registered manufactured attract-and-kill trap, Magnet OLI (Suterra, Bend, Oregon), is currently available (Johnson et al. 2006).
Studies of novel methods for controlling olive fruit fly control include use of adult repellant from seed extracts of Peganum harmala L. as a potential fruit protectant and insect growth inhibitor (Rehman et al. 2009). In studies in urban settings, olive fruit fly in infested fruit did not survive after 2 wk in chipped yard-waste piles, allowing the mulch to be used for beneficial purposes (Crohn et al. 2008). Other suggested control techniques include application of Kaolin clay and mass trapping with OLIPE wet traps made from bottles (Van Steenwyk et al. 2002, Johnson et al. 2006).
Biological Control
The development of biological control for olive fruit fly in California was a large, modern, international project (Johnson et al. 2012). Use of natural enemies could potentially reduce high pest population densities along the coast where other means of control are not practical, especially in areas where olive trees are abundant in rough terrain. These areas are reservoirs of olive fruit fly that could potentially re-infest commercial orchards where control had been previously achieved. Parasitoid species that have been evaluated against olive fruit fly in California are shown in Table 1. Parasitoids in the genus Psyttalia were released in California, and genetic studies resulted in a change of names of P. cf. concolor to P. humilis (Rugman-Jones et al. 2009; Fig. 19). Other parasitoids originating from other countries with potential for use in California have been reviewed by Hoelmer et al. (2011).

A naturally occurring parasitoid, Pteromalus nr. myopitae (female, about 4 mm entire length), found on olive fruit fly in California (Photo by Rollin Coville).
Hymenopteran, braconid, and one pteromalid wasp parasitoids, studied for biological control of olive fruit fly in California, of which three species were introduced and one was found naturally in olives
| Species . | Released . | References . |
|---|---|---|
| Bracon celer Szépligeti | No | Sime et al. 2006a, Nadel et al. 2009 |
| Diachasmimorpha kraussii (Fullaway) | No | Sime et al. 2006c, Daane et al. 2011 |
| Diachasmimorpha longicaudata (Ashmead) | No | Sime et al. 2006c, Daane et al. 2011 |
| Fopius arisanus (Sonan) | No | Sime et al. 2008, Daane et al. 2011 |
| Psyttalia concolor (Szépligeti) | Yes | Sime et al. 2006b, Wang et al. 2009c |
| Psyttalia humilis (Silvestri) (Fig. 19) formerly P. cf. concolor (Szépligeti) | Yes | Daane et al. 2013, 2015; Wang et al. 2011b,,2012, 2013; Yokoyama et al. 2008a,b, 2010, 2011, 2012 |
| Psyttalia lounsburyi (Silvestri) | Yes | Daane et al. 2008, 2015; Wang et al. 2009d, 2011, 2012, 2013 |
| Psyttalia ponerophaga (Silvestri) | No | Daane et al. 2013, Sime et al. 2007 |
| Pteromalus nr. myopitae (Fig. 20) | Resident | Kapaun et al. 2010 |
| Utetes africanus (Silvestri) | No | Daane et al. 2011 |
| Species . | Released . | References . |
|---|---|---|
| Bracon celer Szépligeti | No | Sime et al. 2006a, Nadel et al. 2009 |
| Diachasmimorpha kraussii (Fullaway) | No | Sime et al. 2006c, Daane et al. 2011 |
| Diachasmimorpha longicaudata (Ashmead) | No | Sime et al. 2006c, Daane et al. 2011 |
| Fopius arisanus (Sonan) | No | Sime et al. 2008, Daane et al. 2011 |
| Psyttalia concolor (Szépligeti) | Yes | Sime et al. 2006b, Wang et al. 2009c |
| Psyttalia humilis (Silvestri) (Fig. 19) formerly P. cf. concolor (Szépligeti) | Yes | Daane et al. 2013, 2015; Wang et al. 2011b,,2012, 2013; Yokoyama et al. 2008a,b, 2010, 2011, 2012 |
| Psyttalia lounsburyi (Silvestri) | Yes | Daane et al. 2008, 2015; Wang et al. 2009d, 2011, 2012, 2013 |
| Psyttalia ponerophaga (Silvestri) | No | Daane et al. 2013, Sime et al. 2007 |
| Pteromalus nr. myopitae (Fig. 20) | Resident | Kapaun et al. 2010 |
| Utetes africanus (Silvestri) | No | Daane et al. 2011 |
Hymenopteran, braconid, and one pteromalid wasp parasitoids, studied for biological control of olive fruit fly in California, of which three species were introduced and one was found naturally in olives
| Species . | Released . | References . |
|---|---|---|
| Bracon celer Szépligeti | No | Sime et al. 2006a, Nadel et al. 2009 |
| Diachasmimorpha kraussii (Fullaway) | No | Sime et al. 2006c, Daane et al. 2011 |
| Diachasmimorpha longicaudata (Ashmead) | No | Sime et al. 2006c, Daane et al. 2011 |
| Fopius arisanus (Sonan) | No | Sime et al. 2008, Daane et al. 2011 |
| Psyttalia concolor (Szépligeti) | Yes | Sime et al. 2006b, Wang et al. 2009c |
| Psyttalia humilis (Silvestri) (Fig. 19) formerly P. cf. concolor (Szépligeti) | Yes | Daane et al. 2013, 2015; Wang et al. 2011b,,2012, 2013; Yokoyama et al. 2008a,b, 2010, 2011, 2012 |
| Psyttalia lounsburyi (Silvestri) | Yes | Daane et al. 2008, 2015; Wang et al. 2009d, 2011, 2012, 2013 |
| Psyttalia ponerophaga (Silvestri) | No | Daane et al. 2013, Sime et al. 2007 |
| Pteromalus nr. myopitae (Fig. 20) | Resident | Kapaun et al. 2010 |
| Utetes africanus (Silvestri) | No | Daane et al. 2011 |
| Species . | Released . | References . |
|---|---|---|
| Bracon celer Szépligeti | No | Sime et al. 2006a, Nadel et al. 2009 |
| Diachasmimorpha kraussii (Fullaway) | No | Sime et al. 2006c, Daane et al. 2011 |
| Diachasmimorpha longicaudata (Ashmead) | No | Sime et al. 2006c, Daane et al. 2011 |
| Fopius arisanus (Sonan) | No | Sime et al. 2008, Daane et al. 2011 |
| Psyttalia concolor (Szépligeti) | Yes | Sime et al. 2006b, Wang et al. 2009c |
| Psyttalia humilis (Silvestri) (Fig. 19) formerly P. cf. concolor (Szépligeti) | Yes | Daane et al. 2013, 2015; Wang et al. 2011b,,2012, 2013; Yokoyama et al. 2008a,b, 2010, 2011, 2012 |
| Psyttalia lounsburyi (Silvestri) | Yes | Daane et al. 2008, 2015; Wang et al. 2009d, 2011, 2012, 2013 |
| Psyttalia ponerophaga (Silvestri) | No | Daane et al. 2013, Sime et al. 2007 |
| Pteromalus nr. myopitae (Fig. 20) | Resident | Kapaun et al. 2010 |
| Utetes africanus (Silvestri) | No | Daane et al. 2011 |
Hymenopteran parasitoids that have been approved for release for biological control of olive fruit fly in California include P. concolor, P. humilis (Fig. 19), and P. lounsburyi (Table 1). The impact of introduced parasitoids on nontarget fruit fly species, some which are native to California (Foote and Blanc 1963), received special consideration and investigation before their releases. Much of the available literature from California researchers involves studies of parasitoid biology and life history, response to physical and biological factors such as temperature and food sources, and rates of olive fruit fly parasitism. One introduced parasitoid, P. lounsburyi, has become established in some central California coastal regions (Daane et al. 2015). A naturally occurring olive fruit fly parasitoid, Pteromalus nr. myopitae, (Fig. 20) was discovered by Kapaun et al. (2010) in San Luis Obispo. Other potential biological control factors include predation of pupae in the soil by ants (Orsini et al. 2007) and control of the olive fruit fly endosymbiont, Candidatus Erwinia dacicola (Estes et al. 2012). Further studies would help determine the impact of the latter three agents on olive fruit fly in table olive orchards.
Biological control of olive fruit fly with larval parasitoids has been limited by the inability of introduced parasitoids to survive without the host during the period when olives have dropped from the trees. The presence of fruit throughout the year ensures a constant infestation of olive fruit fly and availability of immature stages in the fruit. However, under very high infestations, drought conditions, or extremely cold weather, the fruit falls from the tree. Even though olive fruit fly can survive until the next season when fruit becomes available, most parasitoids have not been able survive without the larval host. The capacity of a biological control agent to overwinter in the absence of the host may resolve this issue. The search continues for parasitoids with this characteristic (Wang et al. 2013) and the ability to reduce olive fruit fly populations to subeconomic levels.
Laboratory Rearing and SIT
The sterile insect technique could be used to control olive fruit fly in California if a method is developed to mass-rear the pest. Olive fruit fly has been reared on a small scale using a laboratory-formulated diet (Yokoyama 2012a) according to techniques described by Genç and Nation (2008b). However, rearing of the immature stages on formulated diet is more expensive than rearing on olive fruit, and rearing on olives is limited by the need for a constant source of fruit throughout the year. Wang et al. (2009c) have maintained an olive fruit fly colony since 2003 on stored olives from orchards with prolonged fruit set. Successful establishment of an olive fruit fly colony has provided a constant source of larvae for host-specific parasitoids such as Psyttalia lounsburyi (Silvestri). Although olive fruit fly has not yet been mass-reared, laboratory colonies have been the foundation for comprehensive studies of the biology, life history, and biological control of the pest in California.
Summary
Active monitoring for olive fruit fly is the most important tactic to detect and evaluate pest numbers before the occurrence of economic outbreaks or widespread distribution. Larval infestations of fruit are indicators of immediate control considerations. The areas, locations, and regions with climates similar to those found with high larval infestations in this study need special monitoring and implementation of appropriate control tactics to suppress, contain, or eradiate the pest. Proactive programs are especially needed in regions and countries where olives are a new crop. In California, the larval distribution data delineate areas where new olive plantings may be at risk for olive fruit fly infestations.
IPM control tactics for olive fruit fly including bait sprays, attract-and-kill traps, and other methods, used alone or in combination, can eradicate or reduce pest numbers to subeconomic levels. Cultural control is the most economical and essential procedure to mitigate the risk of olive fruit fly infestations of fruit, especially in hot and arid regions where the pest is not abundant.
In the future, new pest management techniques may evolve from California studies that actively pursue biological control agents and novel control tactics, or from European countries where olive fruit fly is an ancient pest. The information in this paper serves as a resource for all domestic and international olive fruit fly studies and control programs, especially in areas where olive fruit fly has been newly discovered.
Acknowledgments
The late Dr. Richard E. Rice is acknowledged for his original studies of the California olive fruit fly invasion. I am grateful to Dr. Xin-geng Wang, University of California, Parlier, for reviewing the manuscript, and to the many California olive growers, processors, and entomologists who contributed their resources to conduct this investigation. Due to the number of technical reports concerning olive fruit fly in California, only research publications and historical records were referenced. This project was supported in part by the California Olive Committee.



