-
PDF
- Split View
-
Views
-
Cite
Cite
Anna E Grimenstein, Melissa A Doyle, Bethia H King, Chris J Geden, Longevity, feeding behavior, and egg production of adult house flies (Diptera: Muscidae) provided with potential foods on dairy farms, Journal of Insect Science, Volume 25, Issue 3, May 2025, 1, https://doi.org/10.1093/jisesa/ieaf045
Close - Share Icon Share
Abstract
Adult house flies (Musca domestica L.) are often a major pest at livestock facilities, where oviposition occurs on decomposing organic matter, such as manure. Some potential foods that adult house flies might consume on dairy farms were examined. Relative to when they were given water alone, survival of males and females was greater when they were given water along with liquid whole milk, formulated calf feed, or corn silage, or finely milled sorghum or soy, or buckwheat inflorescences. However, survival was significantly lower with these foods than with sucrose, although not significantly so for males with milk. There was little to no survival advantage when flies were given water along with milled hominy, wet spent brewers grain, or manure than with water alone. Both males and females spent significant time with their labellum in contact with corn silage, dandelion inflorescences, and buckwheat inflorescences, but little time with their labellum contacting manure or white clover inflorescences. Egg production was not increased by access to water along with buckwheat inflorescences or corn silage relative to sucrose; but egg production was increased by access to liquid whole milk. Reaching mature vitellogenesis stages was improved by prior exposure to water and sucrose solution along with a mixture of dry sucrose, milk, and yolk, or along with calf manure or formulated calf feed, but not with milled soy, sorghum, or hominy, or with wet spent brewers grain or citrus pellets. The diet including sucrose–milk–yolk resulted in the most females reaching mature vitellogenesis stages.
Introduction
The house fly, Musca domestica L., is distributed worldwide (Marquez and Krafsur 2002) and is closely associated with human populations (Abbas et al. 2013, Geden et al. 2021). House flies are quite common on cattle farms, poultry farms, and other agricultural areas because adults lay their eggs in manure and other decaying organic matter. Adult house flies are a nuisance pest to people and livestock. Adult house flies are also mechanical, and sometimes biological, vectors for many bacterial and viral diseases of humans and livestock (Nayduch et al. 2023). The current cost of managing house flies for US dairy, poultry, and swine industries with insecticides is estimated at $200 million annually after adjustments for inflation (Geden et al. 2021).
Adult house flies have a sponging labellum that extends and takes up liquid food, although they can eat dry food by first dissolving the food with wet regurgitate (Holl and Gries 2018). Adult house flies feed on many foods in the laboratory, including milk, sucrose, honey, and fruit (Ganda et al. 2020, Neupane et al. 2023). Some sugars, including sucrose, are known to promote longevity in house flies (Galun and Frankel 1957, Lysyk 1991). Females need protein to produce eggs (Adams and Gerst 1991). The most common protein sources for adult females in laboratory-raised colonies, besides milk, are yeast or egg (Yamamoto and Jensen 1967, Pastor et al. 2011). The common presence of lactose in the crops of adult house flies in cattle sheds (Wiesmann 1960) and sensilla that are sensitive to lactose (Schnuch and Seebauer 1998) suggests that flies often ingest milk when it is available. Lactose only occurs in mammal milk (Dominici et al. 2022). Adult house flies seem to live less than 3 wk in their natural habitat, eg 1 to 6, 2 to 7, 3 to 19 d (reviewed in Geden et al. 2021).
Previous research on adult house fly diets has seldom included foods that would be available to house flies on a dairy farm, other than milk. However, products fed to dairy cattle are often kept in large, open commodity barns, providing ample opportunity for house fly visitation. The food kept in the commodity barns not only provides nutrition to the cattle but is potentially nutritious to the house flies as well. Observational studies of insect visitors to flowering plants have noted house flies among the groups of insects seen on the flowers (Basak and Mandal 2018, Paikara and Painkra 2020). Knowing whether house flies use flowers or other dairy farm foods as a food source could guide management of these insects in the future through management of weeds and placement of sticky and other mechanical traps in locations where what adult house flies feed on is common. In addition, making a preferred food for house flies less available or harder to access could give them less incentive to stay (Abbas et al. 2013).
The current study examined the response of adult house flies to various sources of food that may be available on US dairy farms. Flowering plants that were tested were white clover (Trifolium repens), common dandelion (Taraxacum officinale), and common buckwheat (Fagopyrum esculentum). White clover and dandelions are common weeds across the United States (Pornaro et al. 2023) and have been observed as weeds at an Illinois dairy farm (Grimenstein 2024). Common buckwheat is cultivated for its grain-like seed and as a cover crop. Adult house flies might also feed on milk spillage, food intended for cattle, or even on manure.
The present study specifically examined, for adult house flies: (i) how longevity was affected by (a) access to cow manure, corn silage, or milk or (b) access to inflorescences of common buckwheat; (c) access to sucrose–milk–yolk, calf manure, a calf feed formulation, brewers grains, soy, hominy, or sorghum; (ii) feeding duration on (a) manure, corn silage, or inflorescences of white clover or common dandelion, or (b) inflorescences of common buckwheat; (iii) how egg production was affected by access to corn silage or buckwheat inflorescences; and (iv) how ovarian development was affected by access to sucrose–milk–yolk, calf manure, 3 different calf feed formulations, brewers grains, citrus pellets, soy, hominy, or sorghum.
Materials and Methods
House Flies and General Methods
The house fly strain used for the first 2 longevity experiments, the feeding duration experiments, and the egg production experiment was the USDA-mixed strain, also called the whf strain (Burgess et al. 2020). This strain originated from adult flies collected from California, Florida, Minnesota, and Nebraska and is maintained at the USDA-CMAVE center in Gainesville, Florida. The larvae were reared on a wet mixture of wheat bran: Calf-Manna (MannaPro, Illinois): tap water (26:1:15) (White et al. 2021). Colony adults were fed water, sucrose, milk, and egg yolk, except 48 of the 480 flies in the first longevity experiment were from a colony that had not received egg yolk for several generations.
The first 2 longevity experiments, the feeding duration experiments, and the egg production experiment, as well as cages of pupae from which adult flies were collected for these experiments, were at 16:8 h light:dark cycle at about 25 oC. To collect newly emerged adult flies, petri dishes filled with pupae were placed in net cages (BugDorm, Taiwan) (30 × 30 × 30 cm) containing water ad libitum. Petri dishes were removed within 1 to 2 d after the first flies emerged so that all flies would be of similar and known age. To temporarily immobilize adults for the experiments, net cages were placed in a −8 oC freezer for 1 to 2 min. Flies were then placed into a petri dish on ice to maintain immobilization, sorted by sex, and allowed to recover (become upright and start walking) before being used in the experiments. In the first 2 longevity experiments, prior to recovery, one wing was clipped to minimize escape during water and/or food replacement during the 3 wk experiment.
The third longevity experiment and the ovarian development experiment used adult house flies from a colony started with flies collected by sweep net from a north Florida Holsteins dairy (Bell, FL). Larvae were reared on a grain mixture of wheat bran: alfalfa meal: corn meal (5:3:2) with about 1:7 grain:water (Hogsette 1992). Colony adults were fed sucrose–milk–yolk, which was a dry mix of 67% granulated sugar, 27% powdered milk, and 6% powdered egg yolk (by mass), as well as water, both ad libitum. The third longevity experiment and the ovarian development experiment were under constant light at about 27 oC.
Potential Foods
Common buckwheat plants were grown in a greenhouse. Delays in blooming led to buckwheat being tested separately from the other foods alongside its own controls, leading to separate longevity experiments and feeding duration experiments. Common dandelion and white clover for the feeding experiments were obtained from lawns on the same day of the experiments, as greenhouse plantings failed to bloom. In the experiments, each individual inflorescence (about 25 mm diameter) was placed with its stem in a water-filled amber glass vial (0.92 ml; 15 mm bottom diameter × 17 mm inner height, then 7.5 mm top diameter × 7 mm neck height) after the inflorescence was cut from the plant, to maximize water uptake and transpiration (van Doorn and Reid 1995). Cattle manure and corn silage were collected from a dairy farm in northern DeKalb County, IL (farm description in Burgess et al. 2019) and stored in a −8 oC laboratory freezer (Eijs et al. 1998). The manure had been deposited by heifers less than 1 d before collection. The milk was whole, pasteurized, homogenized cow’s milk with added vitamin D, as is standard in the United States (Prairie Farms, https://www.prairiefarms.com/) and was stored at −8 oC.
For the third longevity experiment and the ovarian development experiment, potential house fly foods found at Florida dairies were identified based on visual observation of fly congregation: calf manure, Beef Max calf feed, NFH calf feed, Calf-Manna, citrus pellets, brewers grains, soy, hominy, and sorghum. The Beef Max, Calf-Manna, and citrus pellets were only tested in the ovarian development experiment. Also tested in both the third longevity experiment and the ovarian development experiment was the sucrose–milk–yolk, ie what adult house flies were fed in the colony.
Beef Max calf feed (Lakeland Animal Nutrition, Lakeland, FL) and the Calf-Manna Supplement for Cattle (MannaPro Products, LLC, Chesterfield, MO) are commercial pelleted supplements. The NFH calf feed was a pellet feed formulated specifically for North Florida Holsteins Dairy (Bell, FL) by a feed mill and used only with calves less than about 12 wk old that were also receiving milk or a milk replacement; the pellets were sweet (Geden personal observation). Citrus pellets are compressed pellets of dried citrus peels, a waste product from orange juicing facilities, sometimes given as a source of bulk fiber for cattle. The brewers grains were wet spent brewers grains (barley, rice, corn) from a brewery that were delivered daily to open storage bunkers at North Florida Holsteins. The soy, hominy, and sorghum were finely milled products collected from bulk storage banks at the North Florida Holsteins dairy’s commodity barn. The Calf-Manna was purchased from a feed store. The calf manure, NFH calf feed, citrus pellets, and brewers grains were also collected from the North Florida Holsteins dairy or another Florida dairy; Beef Max pellets were collected from the Larson Dairy in Okeechobee, FL.
Longevity Experiments
In the first 2 longevity experiments, each adult house fly (0 to 24 h postemergence) was randomly assigned to one food treatment. The treatments in the first longevity experiment were manure, corn silage, milk, sucrose (+ control), or water (− control). The treatments in the second longevity experiment were buckwheat inflorescences, sucrose (+ control), or water (− control).
Each fly was placed in a glass jar (282 ml; 6.5 width × 6.5 depth × 8.5 cm height) with its respective treatment and water ad libitum. Water and milk were each presented in a 1.5 ml Eppendorf tube attached to the inside wall of the jar with putty (Scotch Removable Mounting Putty, 3M Company, St. Paul, MN) and containing Kimwipe strips to prevent accidental drowning. Granulated sucrose was presented in an amber vial to prevent spills that could make the jar sticky, leading to premature fly death. Manure and silage were each presented in a plastic petri dish (90 mm diameter × 15 mm height). Food and water were replaced every other day to avoid spoilage. Survival was recorded daily for 22 d. Death was counted as lack of movement of the body even in response to external stimuli (eg prodding with forceps). Each experiment was replicated 30 times for every treatment for both male and female flies. Each replicate (block) included a new group of male or female flies, with every fly per group assigned one of the possible food treatments. Flies within a single replicate were haphazardly selected at approximately the same time so that sex, age, cohort, and other environmental factors (eg barometric pressure, relative humidity) matched. On a given day, 3 to 5 replicates were set up (depending on the number of adult flies available).
In the third longevity experiment, each potential food was given ad libitum, along with water ad libitum, to 40 adult house flies (about 1:1 male:female) that had been given only water for 24 to 48 h posteclosion, cold immobilized and placed into a cage (12.5 × 14 × 15 cm, cotton tube gauze stretched around a metal skeleton frame). The control was water ad libitum. Food was replenished every 24 h. Dead flies were sexed and removed daily. Three replicates of each potential food resulted in a total of 120 flies per potential food.
Feeding Duration Experiments
In the first feeding duration experiment, each adult house fly (1 to 2 d postemergence) was haphazardly assigned 1 of 5 food treatments: dandelion, white clover, manure, corn silage, or sucrose (+ control) in a glass jar. For dandelion and white clover, a single inflorescence was laid on the bottom of the jar, and each of the other food treatments was placed in a pile (roughly the same size as an adult house fly’s body) at the bottom of the jar. Each fly was observed for 10 min, with the time spent feeding estimated as time during which the proboscis was in contact with the food. In cases where a house fly fed, briefly left the food, then returned to feeding within the 10 min period, all individual segments of time spent feeding were added together after the end of the experiment to obtain a total time. The experiment was replicated 16 times with males and 16 times with females. Each replicate contained a unique group of male or female flies, with each fly per group assigned one of the treatments. The second feeding duration experiment was executed in the same manner as the first but with common buckwheat and sucrose only.
Egg Production and Ovarian Development Experiments
To measure egg production, groups of 20 house fly pupae, which had been held at about 4 oC for less than 2 wk, were placed in 30 ml plastic cups. Each cup was then placed inside a clear glass jar (282 ml; 6.5 width × 6.5 depth × 8.5 cm height) that was covered with fabric. Each jar was assigned 1 of 4 food treatments: corn silage, buckwheat inflorescence, sucrose (− control because it completely lacks protein), or milk (+ control because it contains protein). Although we refer to sucrose as a negative control, we recognize that adult females with just access to sucrose may lay some eggs using protein obtained in the larval stage (Adams and Nelson 1990). Each jar was provided with water ad libitum and the food source on the same day that the fly pupae were added. The water and food treatments were presented as described in the first 20 longevity experiments.
Four days after the first fly emerged, each jar was supplied with an oviposition bag. Each bag was made with a black cotton cloth square (10 × 10 cm) filled with 7.5 ml moistened fly larva media and 7.5 ml manure. The end of the bag was twisted to form a funnel shape, then closed at the base of the funnel with clear cellophane tape to prevent the media from spilling out. Each bag was placed taped end facing down in the same 30 ml cup first used for the pupae and on top of any puparia or nonviable pupae remaining in the cup. Each cup was filled with 3 ml of water, which was absorbed by the taped end of the bag, keeping the bag moist.
Daily egg count and number of living females were recorded every day for 7 d, with day 1 being assigned to the first day that eggs were found on a given bag. To minimize escapes when the oviposition bag was removed to count eggs and to improve ease of counting living flies, jars were first placed in a −8 oC freezer just until the flies were no longer able to fly. Dead flies (identified by lack of movement and response to stimuli) were removed and living flies (crawling and/or responding to stimuli) were counted. The jar was typically placed back in the freezer to anesthetize the flies before changing food and water containers or egg bags, to prevent escapes. Any bag containing eggs was then removed for egg counting. To facilitate counting, the eggs were spread over the surface of the bag with a pair of forceps. After the number of eggs was recorded, the old eggs were thoroughly washed or brushed off, media was replaced if necessary, and the bag with the cup was placed back in the jar. Food, water, and egg bags were replaced every other day to minimize mold growth, especially on the egg bags. Water in the cups was refilled as needed to prevent drying out of the egg bags. Dead flies were removed from the jars every day before cold anesthetizing and egg collection to prevent mold and to avoid confusion over whether flies at the bottom of the jar were deceased or not moving from the anesthetization. The experiment was replicated 15 times, ie 15 jars per treatment.
To examine the effect of potential foods on ovarian development, five replicates of 10 female house flies were restricted for 7 d to a 10% sucrose solution, water, and a single potential food. Flies were housed in rectangular plastic jars (25 × 14 × 11 cm) with stockinette closures for replacing foods. The potential foods were the same as in the third longevity experiment but excluding calf manure and including Beef Max, Calf-Manna, and citrus pellets. There was also a water control where females received only water. Food items were replaced daily. The house flies were dissected at the end of 7 d, and their vitellogenesis stage was rated. Stage 1 was previtellogenesis; stage 2 was early vitellogenesis; stage 3 was mid-late vitellogenesis; and stage 4 was post vitellogenesis. (These are modified from Adams’ (1974) and correspond to his stages 1 to 3, 4 to 6, 7 to 9, and 10, respectively.)
Statistical Analysis
Except where noted, statistical analysis was conducted in SPSS version 28 (IBM Corp. 2021). Every statistical test was two-tailed. Alpha was set at 0.05 for each comparison, using planned comparisons to maximize the statistical power of said comparisons (Ruxton and Beauchamp 2008). Males and females were analyzed separately because they are already known to differ in nutritional needs and feeding (Neupane et al. 2023).
Within each longevity experiment, each potential food treatment was first statistically compared to the negative control (water). If significantly different from water, comparison was made to the positive control (sucrose or sucrose–milk–yolk). Survival curves were compared using Cox regression (review of survival analysis in van Alphen et al. 2003). Any fly that was still alive after 22 d, as well as any fly that died accidentally (such as drowning in a water container) was right censored from the analysis. Sixty-six flies from the first longevity experiment (34 male, 32 female) and 61 flies from the second longevity experiment (29 male, 32 female) were right censored. In the feeding duration experiments, because the assumption of normality was violated, Mann–Whitney U tests were used to compare each treatment to sucrose.
For the egg production experiments, the total number of eggs accumulated over 7 d with buckwheat or corn silage was compared to the negative control (sucrose). Then, if different from sucrose, each food was compared to the positive control (milk) using Mann–Whitney U tests. The total number of eggs in a jar is likely to be affected not only by egg production per live female but also by maternal survival. Thus, egg production per live female was also compared among diets for day 1 when sample size was greatest, conducting the same comparisons as with total egg number.
For the ovarian development experiment, the independence of vitellogenesis stage and the prior diet of the females was analyzed by G-test (likelihood ratio χ2 test). Each diet was first compared to the water diet. However, G-tests assume that expected values in all cells are at least 1 and that no more than 20% of cells have expected values less than 5. This assumption was violated for some G-tests of diet treatments. For those tests, vitellogenesis stages were combined in such a way as to meet the assumption (which can be seen in the results by df < 3).
Results
Longevity Experiments
In the first longevity experiment, males and females given milk or silage had greater survival compared to flies given water alone and lower survival with silage compared to flies given sucrose (Fig. 1 and Table 1). When given milk, survival was lower than with sucrose for females, but not significantly so for males. Males and females given manure did not live significantly longer than flies given water alone, with 100% of flies in both treatments dead by day 22.
Comparison of survivorship curves of adult house flies given one of the following potential foods along with water (Fig. 1). Compared to water and, if significantly different, then compared to sucrose
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 20.48, P < 0.001 | |
| Milk | χ21 = 15.56, P < 0.001 | χ21 = 4.21, P = 0.08 | |
| Silage | χ21 = 9.79, P = 0.002 | χ21 = 25.87, P < 0.001 | |
| Manure | χ21 = 0.18, P = 0.68 | ||
| Females | Sucrose | χ21 = 9.05, P = 0.003 | |
| Milk | χ21 = 20.97, P < 0.001 | χ21 = 19.36, P < 0.001 | |
| Silage | χ21 = 18.48, P < 0.001 | χ21 = 38.09, P < 0.001 | |
| Manure | χ21 = 0.1, P = 0.75 |
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 20.48, P < 0.001 | |
| Milk | χ21 = 15.56, P < 0.001 | χ21 = 4.21, P = 0.08 | |
| Silage | χ21 = 9.79, P = 0.002 | χ21 = 25.87, P < 0.001 | |
| Manure | χ21 = 0.18, P = 0.68 | ||
| Females | Sucrose | χ21 = 9.05, P = 0.003 | |
| Milk | χ21 = 20.97, P < 0.001 | χ21 = 19.36, P < 0.001 | |
| Silage | χ21 = 18.48, P < 0.001 | χ21 = 38.09, P < 0.001 | |
| Manure | χ21 = 0.1, P = 0.75 |
Comparison of survivorship curves of adult house flies given one of the following potential foods along with water (Fig. 1). Compared to water and, if significantly different, then compared to sucrose
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 20.48, P < 0.001 | |
| Milk | χ21 = 15.56, P < 0.001 | χ21 = 4.21, P = 0.08 | |
| Silage | χ21 = 9.79, P = 0.002 | χ21 = 25.87, P < 0.001 | |
| Manure | χ21 = 0.18, P = 0.68 | ||
| Females | Sucrose | χ21 = 9.05, P = 0.003 | |
| Milk | χ21 = 20.97, P < 0.001 | χ21 = 19.36, P < 0.001 | |
| Silage | χ21 = 18.48, P < 0.001 | χ21 = 38.09, P < 0.001 | |
| Manure | χ21 = 0.1, P = 0.75 |
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 20.48, P < 0.001 | |
| Milk | χ21 = 15.56, P < 0.001 | χ21 = 4.21, P = 0.08 | |
| Silage | χ21 = 9.79, P = 0.002 | χ21 = 25.87, P < 0.001 | |
| Manure | χ21 = 0.18, P = 0.68 | ||
| Females | Sucrose | χ21 = 9.05, P = 0.003 | |
| Milk | χ21 = 20.97, P < 0.001 | χ21 = 19.36, P < 0.001 | |
| Silage | χ21 = 18.48, P < 0.001 | χ21 = 38.09, P < 0.001 | |
| Manure | χ21 = 0.1, P = 0.75 |
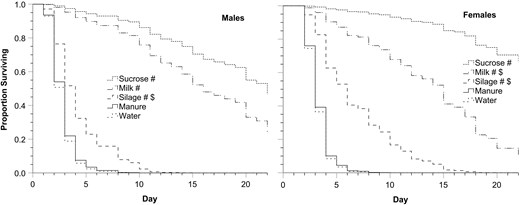
Proportion of male and female house flies alive over 22 d when given water and either sucrose, milk, silage, manure, or water only (n = 30 for each treatment). # indicates a significant difference from water, and $ indicates a significant difference from sucrose, which was tested for only if there was a significant difference from water. Statistical test values, including P values, are in Table 1.
In the second longevity experiment, males and females given sucrose or buckwheat inflorescences had greater survival compared to flies given water alone and lower survival compared to flies given sucrose (Fig. 2 and Table 2). Differences in longevity for a given treatment, eg for sucrose, between the first 2 longevity experiments likely reflect differences among experiment in, eg temperature, relative humidity, barometric pressure, and fly age when first given food. Such differences among experiments are why comparisons of treatments are most appropriate within each experiment, not among experiments. Each experiment was separately blocked, ie one replicate of each treatment of that experiment performed on the same day with the same environmental conditions, and with flies from the same emergence group. The same is true for the feeding duration experiments.
Comparison of survivorship curves of adult house flies given one of the following potential foods along with water (Fig. 2). Compared to water and, if significantly different, then compared to sucrose
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 10.837, P < 0.001 | |
| Buckwheat | χ21 = 4.583, P = 0.032 | χ21 = 28.48, P < 0.001 | |
| Females | Sucrose | χ21 = 9.565, P = 0.002 | |
| Buckwheat | χ21 = 4.372, P = 0.037 | χ21 = 29.51, P < 0.001 |
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 10.837, P < 0.001 | |
| Buckwheat | χ21 = 4.583, P = 0.032 | χ21 = 28.48, P < 0.001 | |
| Females | Sucrose | χ21 = 9.565, P = 0.002 | |
| Buckwheat | χ21 = 4.372, P = 0.037 | χ21 = 29.51, P < 0.001 |
Comparison of survivorship curves of adult house flies given one of the following potential foods along with water (Fig. 2). Compared to water and, if significantly different, then compared to sucrose
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 10.837, P < 0.001 | |
| Buckwheat | χ21 = 4.583, P = 0.032 | χ21 = 28.48, P < 0.001 | |
| Females | Sucrose | χ21 = 9.565, P = 0.002 | |
| Buckwheat | χ21 = 4.372, P = 0.037 | χ21 = 29.51, P < 0.001 |
| Sex . | Potential food . | vs water alone . | vs sucrose . |
|---|---|---|---|
| Males | Sucrose | χ21 = 10.837, P < 0.001 | |
| Buckwheat | χ21 = 4.583, P = 0.032 | χ21 = 28.48, P < 0.001 | |
| Females | Sucrose | χ21 = 9.565, P = 0.002 | |
| Buckwheat | χ21 = 4.372, P = 0.037 | χ21 = 29.51, P < 0.001 |
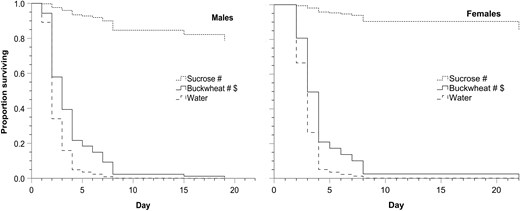
Proportion of male and female house flies alive over 22 d when given water and either sucrose, buckwheat, or water only (n = 30 for each treatment). # indicates a significant difference from water, and $ indicates a significant difference from sucrose, which was tested for only if there was a significant difference from water. Statistical test values, including P values, are in Table 2.
In the third longevity experiment, males and females given NFH calf feed, soy, sorghum, hominy, or manure had significantly greater survival compared to flies given water alone and significantly lower survival compared to flies given sucrose–milk–yolk (Fig. 3 and Table 3). The magnitude of the survival benefit was small with manure and hominy versus water for both sexes. For brewers grain, the magnitude of the survival benefit compared to water was small for females and nonsignificant for males.
Comparison of survivorship curves of adult house flies given one of the following potential foods along with water (Fig. 3). Compared to water and, if significantly different, then compared to sucrose–milk–yolk.
| Sex . | Potential food . | vs water alone . | vs sucrose–milk–yolk . |
|---|---|---|---|
| Males | Sucrose–milk–yolk | χ21 = 19.75, P < 0.001 | |
| NFH calf feed | χ21 = 30.56, P < 0.001 | χ21 = 34.91, P < 0.001 | |
| Soy | χ21 = 46.87, P < 0.001 | χ21 = 49.21, P < 0.001 | |
| Sorghum | χ21 = 46.82, P < 0.001 | χ21 = 42.68, P < 0.001 | |
| Hominy | χ21 = 34.34, P < 0.001 | χ21 = 22.62, P < 0.001 | |
| Manure | χ21 = 7.39, P = 0.007 | χ21 = 42.33, P < 0.001 | |
| Brewers’ grain | χ21 = 1.67, P = 0.20 | ||
| Females | Sucrose–milk–yolk | χ21 = 38.05, P < 0.001 | |
| NFH calf feed | χ21 = 27.84, P < 0.001 | χ21 = 19.73, P < 0.001 | |
| Sorghum | χ21 = 52.10, P < 0.001 | χ21 = 66.29, P < 0.001 | |
| Soy | χ21 = 31.62, P < 0.001 | χ21 = 70.79, P < 0.001 | |
| Hominy | χ21 = 13.67, P < 0.001 | χ21 = 50.66, P < 0.001 | |
| Manure | χ21 = 5.74, P = 0.017 | χ21 = 43.03, P < 0.001 | |
| Brewers’ grain | χ21 = 4.00, P = 0.046 | χ21 = 39.30, P < 0.001 |
| Sex . | Potential food . | vs water alone . | vs sucrose–milk–yolk . |
|---|---|---|---|
| Males | Sucrose–milk–yolk | χ21 = 19.75, P < 0.001 | |
| NFH calf feed | χ21 = 30.56, P < 0.001 | χ21 = 34.91, P < 0.001 | |
| Soy | χ21 = 46.87, P < 0.001 | χ21 = 49.21, P < 0.001 | |
| Sorghum | χ21 = 46.82, P < 0.001 | χ21 = 42.68, P < 0.001 | |
| Hominy | χ21 = 34.34, P < 0.001 | χ21 = 22.62, P < 0.001 | |
| Manure | χ21 = 7.39, P = 0.007 | χ21 = 42.33, P < 0.001 | |
| Brewers’ grain | χ21 = 1.67, P = 0.20 | ||
| Females | Sucrose–milk–yolk | χ21 = 38.05, P < 0.001 | |
| NFH calf feed | χ21 = 27.84, P < 0.001 | χ21 = 19.73, P < 0.001 | |
| Sorghum | χ21 = 52.10, P < 0.001 | χ21 = 66.29, P < 0.001 | |
| Soy | χ21 = 31.62, P < 0.001 | χ21 = 70.79, P < 0.001 | |
| Hominy | χ21 = 13.67, P < 0.001 | χ21 = 50.66, P < 0.001 | |
| Manure | χ21 = 5.74, P = 0.017 | χ21 = 43.03, P < 0.001 | |
| Brewers’ grain | χ21 = 4.00, P = 0.046 | χ21 = 39.30, P < 0.001 |
Comparison of survivorship curves of adult house flies given one of the following potential foods along with water (Fig. 3). Compared to water and, if significantly different, then compared to sucrose–milk–yolk.
| Sex . | Potential food . | vs water alone . | vs sucrose–milk–yolk . |
|---|---|---|---|
| Males | Sucrose–milk–yolk | χ21 = 19.75, P < 0.001 | |
| NFH calf feed | χ21 = 30.56, P < 0.001 | χ21 = 34.91, P < 0.001 | |
| Soy | χ21 = 46.87, P < 0.001 | χ21 = 49.21, P < 0.001 | |
| Sorghum | χ21 = 46.82, P < 0.001 | χ21 = 42.68, P < 0.001 | |
| Hominy | χ21 = 34.34, P < 0.001 | χ21 = 22.62, P < 0.001 | |
| Manure | χ21 = 7.39, P = 0.007 | χ21 = 42.33, P < 0.001 | |
| Brewers’ grain | χ21 = 1.67, P = 0.20 | ||
| Females | Sucrose–milk–yolk | χ21 = 38.05, P < 0.001 | |
| NFH calf feed | χ21 = 27.84, P < 0.001 | χ21 = 19.73, P < 0.001 | |
| Sorghum | χ21 = 52.10, P < 0.001 | χ21 = 66.29, P < 0.001 | |
| Soy | χ21 = 31.62, P < 0.001 | χ21 = 70.79, P < 0.001 | |
| Hominy | χ21 = 13.67, P < 0.001 | χ21 = 50.66, P < 0.001 | |
| Manure | χ21 = 5.74, P = 0.017 | χ21 = 43.03, P < 0.001 | |
| Brewers’ grain | χ21 = 4.00, P = 0.046 | χ21 = 39.30, P < 0.001 |
| Sex . | Potential food . | vs water alone . | vs sucrose–milk–yolk . |
|---|---|---|---|
| Males | Sucrose–milk–yolk | χ21 = 19.75, P < 0.001 | |
| NFH calf feed | χ21 = 30.56, P < 0.001 | χ21 = 34.91, P < 0.001 | |
| Soy | χ21 = 46.87, P < 0.001 | χ21 = 49.21, P < 0.001 | |
| Sorghum | χ21 = 46.82, P < 0.001 | χ21 = 42.68, P < 0.001 | |
| Hominy | χ21 = 34.34, P < 0.001 | χ21 = 22.62, P < 0.001 | |
| Manure | χ21 = 7.39, P = 0.007 | χ21 = 42.33, P < 0.001 | |
| Brewers’ grain | χ21 = 1.67, P = 0.20 | ||
| Females | Sucrose–milk–yolk | χ21 = 38.05, P < 0.001 | |
| NFH calf feed | χ21 = 27.84, P < 0.001 | χ21 = 19.73, P < 0.001 | |
| Sorghum | χ21 = 52.10, P < 0.001 | χ21 = 66.29, P < 0.001 | |
| Soy | χ21 = 31.62, P < 0.001 | χ21 = 70.79, P < 0.001 | |
| Hominy | χ21 = 13.67, P < 0.001 | χ21 = 50.66, P < 0.001 | |
| Manure | χ21 = 5.74, P = 0.017 | χ21 = 43.03, P < 0.001 | |
| Brewers’ grain | χ21 = 4.00, P = 0.046 | χ21 = 39.30, P < 0.001 |

Proportion of male and female house flies surviving when given water and, from highest survival to lowest survival (top right to bottom left), sucrose–milk–yolk (n = 46 males, 68 females), NFH feed (n = 56 males, 49 females), soy (n = 56 males, 51 females), sorghum (n = 52 males, 62 females), hominy (n = 62 males, 55 females), calf manure (n = 68 males, 46 females), brewers grain (n = 60 males, 57 females), or water only (n = 60 males, 53 females). # indicates a significant difference from water, and $ indicates a significant difference from sucrose–milk–yolk, which was tested for only if there was a significant difference from water. Statistical test values, including P values, are in Table 3.
Feeding Duration Experiments
In the first feeding duration experiment, males and females spent much less time with their proboscis in contact with manure or white clover than sucrose, whereas there was no significant difference with sucrose versus silage or versus common dandelion inflorescences (Fig. 4 and Table 4). In the second feeding duration experiment, there was no significant difference in duration of feeding on buckwheat inflorescences versus sucrose for males or females (Fig. 4 and Table 4).
Feeding duration of adult house flies given one of the following potential foods, compared to sucrose (Fig. 4), n = 16 flies for each treatment for each sex
| Sex . | Potential food . | vs sucrose . |
|---|---|---|
| First feeding duration experiment | ||
| Males | Common dandelion | U = 111.00, P = 0.51 |
| Silage | U = 104.00, P = 0.35 | |
| White clover | U = 61.00, P = 0.005 | |
| Manure | U = 60.00, P = 0.005 | |
| Females | Common dandelion | U = 100.00, P = 0.28 |
| Silage | U = 84.00, P = 0.08 | |
| White clover | U = 64.00, P = 0.008 | |
| Manure | U = 63.00, P = 0.007 | |
| Second feeding duration experiment | ||
| Males | Buckwheat | U = 121.50, P = 0.77 |
| Females | Buckwheat | U = 106.50, P = 0.37 |
| Sex . | Potential food . | vs sucrose . |
|---|---|---|
| First feeding duration experiment | ||
| Males | Common dandelion | U = 111.00, P = 0.51 |
| Silage | U = 104.00, P = 0.35 | |
| White clover | U = 61.00, P = 0.005 | |
| Manure | U = 60.00, P = 0.005 | |
| Females | Common dandelion | U = 100.00, P = 0.28 |
| Silage | U = 84.00, P = 0.08 | |
| White clover | U = 64.00, P = 0.008 | |
| Manure | U = 63.00, P = 0.007 | |
| Second feeding duration experiment | ||
| Males | Buckwheat | U = 121.50, P = 0.77 |
| Females | Buckwheat | U = 106.50, P = 0.37 |
Feeding duration of adult house flies given one of the following potential foods, compared to sucrose (Fig. 4), n = 16 flies for each treatment for each sex
| Sex . | Potential food . | vs sucrose . |
|---|---|---|
| First feeding duration experiment | ||
| Males | Common dandelion | U = 111.00, P = 0.51 |
| Silage | U = 104.00, P = 0.35 | |
| White clover | U = 61.00, P = 0.005 | |
| Manure | U = 60.00, P = 0.005 | |
| Females | Common dandelion | U = 100.00, P = 0.28 |
| Silage | U = 84.00, P = 0.08 | |
| White clover | U = 64.00, P = 0.008 | |
| Manure | U = 63.00, P = 0.007 | |
| Second feeding duration experiment | ||
| Males | Buckwheat | U = 121.50, P = 0.77 |
| Females | Buckwheat | U = 106.50, P = 0.37 |
| Sex . | Potential food . | vs sucrose . |
|---|---|---|
| First feeding duration experiment | ||
| Males | Common dandelion | U = 111.00, P = 0.51 |
| Silage | U = 104.00, P = 0.35 | |
| White clover | U = 61.00, P = 0.005 | |
| Manure | U = 60.00, P = 0.005 | |
| Females | Common dandelion | U = 100.00, P = 0.28 |
| Silage | U = 84.00, P = 0.08 | |
| White clover | U = 64.00, P = 0.008 | |
| Manure | U = 63.00, P = 0.007 | |
| Second feeding duration experiment | ||
| Males | Buckwheat | U = 121.50, P = 0.77 |
| Females | Buckwheat | U = 106.50, P = 0.37 |
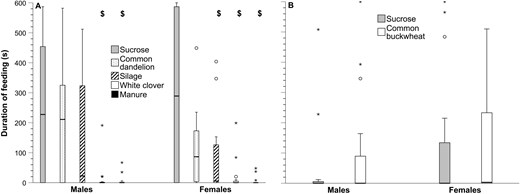
Duration (s) that male and female house flies spent feeding during 10 min observations in: A) the experiment where flies were given, from left to right, either sucrose, common dandelion, silage, white clover, or manure and B) the experiment where flies were given either sucrose or common buckwheat (n = 16 for each treatment for each sex in each experiment). The box and whisker plots show median (horizontal line inside box), interquartile range (box), whiskers (minimum and maximum that are not outliers), mild outliers (circles: > 1.5 < 3× above the top of, or below the bottom of, the interquartile range), and extreme outliers (*: >3× above the top of, or below the bottom of, the interquartile range). $ indicates a significant difference from sucrose; significance patterns were the same for males and females. Statistical test values, including P values, are in Table 4.
The difference in magnitude of proboscis contact duration on sucrose in the first versus the second feeding duration experiment (Fig. 4) is likely related to unknown differences in methodology or conditions. The difference underscores the value of blocking when designing experiments (PSU 2024).
Egg Production and Ovarian Development Experiments
The total number of eggs across the 7 d of the experiment with the silage diet was not different from the total with the sucrose diet but was significantly less than the total with the milk diet (Fig. 5 and Table 5). Total egg production with the diet of buckwheat was not greater than the total with the diet of sucrose or milk. Total egg production was not significantly greater with the diet of milk versus sucrose.
Effect of potential foods on egg production (n = 15 jars for each treatment) (Fig. 5). Compared to sucrose and, if significantly different, then compared to milk
| Potential food . | vs sucrose . | vs milk . |
|---|---|---|
| Total no. eggs produced over 7 d per jar | ||
| Milk | U = 70.00, P = 0.078 | |
| Silage | U = 83.00, P = 0.22 | U = 51.00, P = 0.01 |
| Buckwheat | U = 41.00, P < 0.002 | U = 24.000, P < 0.001 |
| Egg production per live female at the end of day 1 | ||
| Milk | U = 52.50, P = 0.001 | |
| Silage | U = 112.50, P = 1.00 | U = 52.50, P = 0.001 |
| Buckwheat | U = 105.00, P = 1.00 | U = 49.00, P = 0.002 |
| Potential food . | vs sucrose . | vs milk . |
|---|---|---|
| Total no. eggs produced over 7 d per jar | ||
| Milk | U = 70.00, P = 0.078 | |
| Silage | U = 83.00, P = 0.22 | U = 51.00, P = 0.01 |
| Buckwheat | U = 41.00, P < 0.002 | U = 24.000, P < 0.001 |
| Egg production per live female at the end of day 1 | ||
| Milk | U = 52.50, P = 0.001 | |
| Silage | U = 112.50, P = 1.00 | U = 52.50, P = 0.001 |
| Buckwheat | U = 105.00, P = 1.00 | U = 49.00, P = 0.002 |
Effect of potential foods on egg production (n = 15 jars for each treatment) (Fig. 5). Compared to sucrose and, if significantly different, then compared to milk
| Potential food . | vs sucrose . | vs milk . |
|---|---|---|
| Total no. eggs produced over 7 d per jar | ||
| Milk | U = 70.00, P = 0.078 | |
| Silage | U = 83.00, P = 0.22 | U = 51.00, P = 0.01 |
| Buckwheat | U = 41.00, P < 0.002 | U = 24.000, P < 0.001 |
| Egg production per live female at the end of day 1 | ||
| Milk | U = 52.50, P = 0.001 | |
| Silage | U = 112.50, P = 1.00 | U = 52.50, P = 0.001 |
| Buckwheat | U = 105.00, P = 1.00 | U = 49.00, P = 0.002 |
| Potential food . | vs sucrose . | vs milk . |
|---|---|---|
| Total no. eggs produced over 7 d per jar | ||
| Milk | U = 70.00, P = 0.078 | |
| Silage | U = 83.00, P = 0.22 | U = 51.00, P = 0.01 |
| Buckwheat | U = 41.00, P < 0.002 | U = 24.000, P < 0.001 |
| Egg production per live female at the end of day 1 | ||
| Milk | U = 52.50, P = 0.001 | |
| Silage | U = 112.50, P = 1.00 | U = 52.50, P = 0.001 |
| Buckwheat | U = 105.00, P = 1.00 | U = 49.00, P = 0.002 |
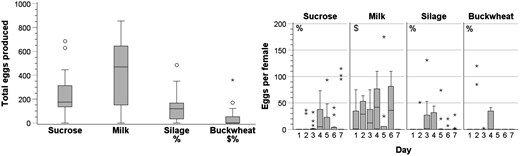
Total number of eggs produced over 7 d and number of eggs produced per female house fly alive at the end of each of 7 d when house flies were given either sucrose, milk, corn silage, or buckwheat inflorescences along with water (n = 15 jars for each treatment). $ indicates a significant difference from sucrose, and % indicates a significant difference from milk. Statistical test values, including P values, are in Table 5.
Egg production per live female at the end of day 1 with the silage diet or the buckwheat diet was not different from egg production with the sucrose diet (Fig. 5 and Table 5) but was less than egg production with the milk diet. Egg production per live female at the end of day 1 was significantly greater with milk than with sucrose.
In the ovarian development experiment, a greater proportion of females exhibited the more mature vitellogenesis stages after exposure to the diet with sucrose–milk–yolk, calf manure, Beef Max, NFH feed, or Calf-Manna than after exposure to water only (Fig. 6 and Table 6). In contrast, after access to the diet with soy, brewers grain, hominy, citrus, or sorghum, the proportion of females that exhibited different vitellogenesis stages was not significantly different from the proportion of females when given water only. The diet with calf manure, Beef Max, NFH feed, and Calf-Manna resulted in a greater proportion of females exhibiting the less mature vitellogenesis stages compared to the diet with sucrose–milk–yolk.
Effect on stage of ovarian development of female house flies being given one of the following potential foods along with a 10% sucrose solution and water for 7 d (Fig. 6). Compared to water and, if significantly different, then compared to sucrose–milk–yolk
| Potential food . | vs water . | vs sucrose–milk–yolk . |
|---|---|---|
| Sucrose–milk–yolk | χ23 = 88.87, P < 0.001 | |
| Calf-Manna | χ23 = 38.23, P < 0.001 | χ23 = 47.41, P < 0.001 |
| Beef Max | χ23 = 51.93, P < 0.001 | χ23 = 27.57, P < 0.001 |
| NFH calf feed | χ23 = 27.37, P < 0.001 | χ23 = 45.07, P < 0.001 |
| Manure | χ23 = 60.92, P < 0.001 | χ23 = 15.44, P < 0.001 |
| Soy | χ22 = 4.22, P = 0.12 | |
| Brewers’ grain | χ22 = 3.65, P = 0.16 | |
| Hominy | χ21 = 0.37, P = 0.54 | |
| Citrus pellets | χ21 = 0.11, P = 0.74 | |
| Sorghum | χ21 = 0.15, P = 0.90 |
| Potential food . | vs water . | vs sucrose–milk–yolk . |
|---|---|---|
| Sucrose–milk–yolk | χ23 = 88.87, P < 0.001 | |
| Calf-Manna | χ23 = 38.23, P < 0.001 | χ23 = 47.41, P < 0.001 |
| Beef Max | χ23 = 51.93, P < 0.001 | χ23 = 27.57, P < 0.001 |
| NFH calf feed | χ23 = 27.37, P < 0.001 | χ23 = 45.07, P < 0.001 |
| Manure | χ23 = 60.92, P < 0.001 | χ23 = 15.44, P < 0.001 |
| Soy | χ22 = 4.22, P = 0.12 | |
| Brewers’ grain | χ22 = 3.65, P = 0.16 | |
| Hominy | χ21 = 0.37, P = 0.54 | |
| Citrus pellets | χ21 = 0.11, P = 0.74 | |
| Sorghum | χ21 = 0.15, P = 0.90 |
That df differed among the χ2df resulted from the need to combine vitellogenesis stages for some comparisons to meet the assumption of the statistical test, as explained in the Statistical analysis section of the Methods.
Effect on stage of ovarian development of female house flies being given one of the following potential foods along with a 10% sucrose solution and water for 7 d (Fig. 6). Compared to water and, if significantly different, then compared to sucrose–milk–yolk
| Potential food . | vs water . | vs sucrose–milk–yolk . |
|---|---|---|
| Sucrose–milk–yolk | χ23 = 88.87, P < 0.001 | |
| Calf-Manna | χ23 = 38.23, P < 0.001 | χ23 = 47.41, P < 0.001 |
| Beef Max | χ23 = 51.93, P < 0.001 | χ23 = 27.57, P < 0.001 |
| NFH calf feed | χ23 = 27.37, P < 0.001 | χ23 = 45.07, P < 0.001 |
| Manure | χ23 = 60.92, P < 0.001 | χ23 = 15.44, P < 0.001 |
| Soy | χ22 = 4.22, P = 0.12 | |
| Brewers’ grain | χ22 = 3.65, P = 0.16 | |
| Hominy | χ21 = 0.37, P = 0.54 | |
| Citrus pellets | χ21 = 0.11, P = 0.74 | |
| Sorghum | χ21 = 0.15, P = 0.90 |
| Potential food . | vs water . | vs sucrose–milk–yolk . |
|---|---|---|
| Sucrose–milk–yolk | χ23 = 88.87, P < 0.001 | |
| Calf-Manna | χ23 = 38.23, P < 0.001 | χ23 = 47.41, P < 0.001 |
| Beef Max | χ23 = 51.93, P < 0.001 | χ23 = 27.57, P < 0.001 |
| NFH calf feed | χ23 = 27.37, P < 0.001 | χ23 = 45.07, P < 0.001 |
| Manure | χ23 = 60.92, P < 0.001 | χ23 = 15.44, P < 0.001 |
| Soy | χ22 = 4.22, P = 0.12 | |
| Brewers’ grain | χ22 = 3.65, P = 0.16 | |
| Hominy | χ21 = 0.37, P = 0.54 | |
| Citrus pellets | χ21 = 0.11, P = 0.74 | |
| Sorghum | χ21 = 0.15, P = 0.90 |
That df differed among the χ2df resulted from the need to combine vitellogenesis stages for some comparisons to meet the assumption of the statistical test, as explained in the Statistical analysis section of the Methods.

Proportion of female flies in different stages of ovarian development, with stage 4 being the most mature, after 7 d with access to a 10% sucrose solution, water, and a potential food (42 to 49 females per potential food). # indicates a significant difference from water, and $ indicates a significant difference from sucrose–milk–yolk, which was tested for only if there was a significant difference from water. Statistical test values, including P values, are in Table 6.
Discussion
In the present study, whole milk increased fly longevity relative to water alone. A previous study showed that “condensed milk” increased longevity of house flies (Lysyk 1991). However, condensed milk typically has sugar added. Milk contains lactose as its primary natural sugar, which in house flies is primarily utilized by lactose-fermenting bacteria (Neupane et al. 2020). The ability of house flies to taste and metabolize lactose is not a typical feature of flies and has been hypothesized to be a specific adaptation to the milk availability that results from association with humans (Hansen et al. 2006). Male and female house flies tend to live longer with sucrose than with either condensed or whole milk, although not significantly longer than for males with whole milk (Lysyk 1991, the present study). This may be related to the lactose in milk versus sucrose or to other compounds present in milk (Scano et al. 2014). For adult house flies, a 5% lactose solution increases longevity about 80% as much as a 5% sucrose solution, and the volume consumed of the lactose solution is also about 80% that of the sucrose solution (Galun and Fraenkel 1957). For house flies with access to both sucrose and water, the addition of powdered milk to the diet increases female, but not male, longevity (Gray and Berberian 1971).
Both male and female house flies appear to feed on corn silage. Both sexes spent a fair amount of time with their labellum in contact with the silage. Access to the silage increased longevity relative to water only and not relative to sucrose. That corn silage increased longevity relative to water may be related to its high starch content. In corn silage, starch has a dry mass content of about 20% to 35% (Der Bedrosian et al. 2012). Sorghum and hominy also increased longevity relative to water, and both have high starch content, 56% to 75% (Jambunathan and Subramanian 1987) and 46% to 63% by dry mass, respectively (Zhang et al. 2005). Brewers grain has a very low starch content, 2% to 8% by dry mass (Westendorf et al. 2014); and brewers grain did not significantly increase male longevity and minimally increased female longevity relative to water. However, starch content alone is not enough to predict a benefit to house fly longevity. Soy also increased longevity relative to water, but soy has a lower starch content than sorghum, or hominy, about 5% by dry mass (Heuzé et al. 2020). Perhaps the sugar content of soy compensates for its low starch content, as soy contains about 10% total sugars by dry mass versus <1% for brewers grain (Heuzé et al. 2017).
House flies seem to gain no to relatively little longevity benefit from exposure to manure. Flies spent almost no time with their labellum in contact with manure. For both males and females, access to manure was not significantly better for longevity than access to just water in one experiment and added minimally to longevity in another experiment. Lysyk (1991) found that neither cattle manure nor poultry manure increased longevity of house flies relative to water only. Nutrients in cattle manure include about 15% crude protein by dry mass, 1% crude fat content by dry mass, and many minerals (Hussein et al. 2017). Possible explanations for why cattle manure did not act as a strong phagostimulant to house flies in the present study include: (i) the fly populations in the present study and in Lysyk (1991) may have evolved to utilize the sucrose, milk, and eggs provided in colony in the laboratory, though this alone would not explain why the fly populations in the present study fed from corn silage; (ii) perhaps manure contains house fly enteric pathogens, so house flies have evolved to minimize feeding on it, though this raises questions as to whether house flies reared in the laboratory over multiple generations can retain the evolved ability to detect pathogens they may not be regularly exposed to. Adult house flies, females especially, consume manure in the laboratory at least when it is mixed with phosphate buffer saline (Thomson et al. 2017). Adult house flies also consume some manure incidentally when grooming (Nayduch and Burrus 2017).
House flies showed little interest in white clover inflorescences in the present study, but buckwheat inflorescences provided some benefit. The labellum of male and female house flies was seldom in contact with white clover but was in contact with buckwheat inflorescences as long as with sucrose. Also, exposure to buckwheat increased longevity of males and females relative to water. Buckwheat did not increase longevity or egg production beyond levels seen with sucrose, but the volume of food provided from an inflorescence was less than the volume provided by the sucrose in these experiments. House flies have been found on buckwheat flowers on buckwheat fields in Nepal and India (Rijal et al 2018, Manhare and Painkra 2020). Manhare and Painkra (2020) found a peak of about 3 house flies per 5 min per m2, although this was much less than bee visits. House flies have been found on buckwheat flowers on farm fields in New York, although in very small numbers relative to honey bees (Björkman 1995). Buckwheat nectar contains soluble sugars, especially fructose, glucose, and sucrose (Nešović et al. 2021), and increases longevity of a parasitoid wasp that parasitizes house flies (Taylor et al. 2022). Buckwheat pollen contains protein, but not much compared to other pollens, and so is considered a poor quality pollen (Somerville 2001). Other flowers that house flies have been found to visit include sunflowers (Basak and Mandal 2018), coriander (Paikara and Painkra 2020), and smartweed (Grimenstein 2024). Whether house flies are using these flowers for feeding is unclear, eg they might be taking shelter (Woodcock et al. 2014). Future tests of benefits of buckwheat and other flowering plants should test arenas large enough to supply flies with opportunities to visit larger groups of inflorescences or even whole flowering plants. Field trials of flowering plant supplementation might compare fly card spot counts or other measures of pest level. This would also address whether any benefit to natural enemies of house flies outweigh any advantage that house flies receive from the plants.
Ovarian development was more advanced with sucrose–milk–yolk than even with the other foods that were better than water at leading to ovarian maturation, ie the 3 calf feeds and manure. Whether this outcome was related to the adult house flies being sourced from colonies in which the adults are fed sucrose–milk–yolk is unclear. Prior exposure to not only the milk and yolk in the sucrose–milk–yolk but also prior exposure to the calf feed led to ovarian maturation. This may be related to the protein in the feeds, about 15% crude protein by dry mass in Beef Max (Vendramini and Arthington 2007) and a minimum of 25% in Calf-Manna Supplement for Cattle (MannaPro 2024), and to other nutrients. Total protein content alone is not enough to know; soy has even greater crude protein (Heuzé et al. 2020) but did not increase ovarian maturation relative to water only.
As noted above, cattle manure includes about 15% crude protein (Hussein et al. 2017), but we found little evidence that house flies feed on plain manure. Calf manure increased ovarian development but not longevity, and flies seldom contacted it with their labellum. Perhaps house flies have evolved to females using the odor, or some other feature, of manure, not as nutrition, but rather as just a cue to advance ovarian development because of its suitability as an oviposition substrate. Another possibility is that flies feed on manure intermittently and can sequester sufficient protein for egg maturation following brief feeding events on manure.
The egg production experiment should be viewed as looking at whether either buckwheat or corn silage affect egg production relative to laboratory colony foods (milk and sucrose) when adult female house flies also have access to feeding from liquid from a wet mixture of fly larva media (wheat bran and Calf-Manna) and cattle manure (from the experiment’s oviposition bags). Such feeding could have masked any egg production differences that might have occurred if females had only been able to feed on the buckwheat, corn silage, sucrose, or milk. That some eggs were produced even when females were given sucrose might have been from such oviposition bag feeding, or because some house flies are still able to produce some eggs even with no protein eaten as an adult (Adams and Nelson 1990).
In the present study, egg production was significantly greater with milk than with sucrose when egg production per female on day 1 was analyzed, but not when total egg production over the 7 d of the experiment was analyzed, despite equivalent sample sizes for the two measures of egg production. Perhaps this was because the longevity advantage of sucrose over milk allowed females to live long enough to lay more eggs. House flies produce a greater total mass of eggs over 34 d when given milk and sucrose than when given just sucrose (eg Pastor et al. 2011). Surprisingly, Ganda et al. (2020) found egg production per female to be about as great with pineapple as with a milk–sugar combination. One possibility is that pineapple as well as their milk–sugar combination contain some other important nutrient for egg production other than protein. Alternatively, pineapple is known to contain proteases, which break down proteins; if dead flies were not removed from the cages, perhaps the proteases made amino acids in the flies available for the remaining living flies to feed on.
Results of the present study confirm that flies spend more time visiting farm food resources that provide survival and reproductive benefits. Using exclusion methods to limit fly access to preferred feedstuffs and/or targeting placement of scatter baits, UV light traps, giant sticky ribbons, and other controls in such hot spots could be helpful for fly management in commodity barns. Producers should pay particular to limiting fly visitation to pelleted feeds given to young calves to mitigate the risk of flies transferring microorganisms that cause scours, as this is a substantial contributor to mortality, poor weight gain, and antibiotic use in the replacement herd. Future studies should examine the role of vitamins and minerals in adult house fly feeding, as vitamins and minerals are added to commercial calf feeds (MannaPro 2024). Much research on house fly nutrition has concentrated on carbohydrates as a source of energy (calories) and a need for protein, lipids for egg production (eg Neupane et al. 2023), and other macronutrients. There may be other nutrients, such as vitamins and minerals, that may play a role in house fly nutrition, but this has yet to be explored in depth. Future studies should also evaluate additional animal production systems for food sources fed on by house flies and how such feeding affects house fly longevity and egg production. Dairies likely provide flies with a richer variety of high-value foods than, for example, poultry, where food options may often be limited to chicken manure and spilled feed. Variation in food quality and availability could partly account for the wide discrepancies in published estimates of fly longevity in the field (reviewed in Geden et al. 2021).
Acknowledgments
We thank the farm owners for their generosity in allowing us to use their farm for our research. We also thank A. Senobari and G. Watkins for assistance with experiments and colony rearing, and R. King and H. Jones for helpful suggestions and edits of the manuscript before submission. The observations at a dairy farm used buckwheat plants that were part of a project supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under agreement #H008917141 to B. King and E. Taylor through the North Central Region SARE program under project #GNC22-358. USDA is an equal opportunity employer and service provider. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Author contributions
Anna Grimenstein (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Resources [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Bethia King (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [Equal], Resources [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—review & editing [equal]), Melissa Doyle (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Resources [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—original draft [equal]), and Chris Geden (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Resources [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—review & editing [equal])
Conflicts of interest. None declared.



