-
PDF
- Split View
-
Views
-
Cite
Cite
Yuting Wu, Mengchu Wu, Zi Hui, Xiangshun Hu, Xiangli Xu, Polyphenism in Antennal Sensilla Among Different Adult Morphs of Nonhost-Alternating, Holocyclic Sitobion avenae (Hemiptera: Aphididae), Journal of Insect Science, Volume 22, Issue 1, January 2022, 4, https://doi.org/10.1093/jisesa/ieab103
Close - Share Icon Share
Abstract
Aphids, mainly distributed in temperate zones, exhibit seasonal generation-alternating phenomena. Across the life cycle, different morphs are produced. Sitobion avenae (Fabricius 1775) is a major pest of wheat worldwide. To elucidate olfactory perception of morph-specific behavior across their life cycle, we investigated antennal sensilla among seven morphs using scanning electron microscopy. Trichoid, placoid, coeloconic, and campaniform sensilla were identified. Trichoid sensilla, big multiporous placoid sensilla (primary rhinarium), a group of sensilla (primary rhinaria), and campaniform sensilla showed similar distribution and resemblance among morphs, whereas small multiporous placoid sensilla (secondary rhinaria) exhibited obvious differences. Compared to apterous morphs, alate morphs possessed a greater abundance of secondary rhinaria, with the greatest found in males on antennal segments III–V. Alate virginoparae and alate sexuparae ranged from six to fourteen rhinaria on antennal segment III. Fundatrices, apterous virginoparae and apterous sexuparae only had one or two secondary rhinaria on antennal segment III while they disappeared in oviparae. Secondary rhinaria, lying in a cuticle cavity, are convex or concave in their central part. In males, both forms were present, with a greater proportion of convex form than that of the concave form. Fundatrices and virginoparae had the convex form while sexuparae had the concave form. Polyphenism of secondary rhinaria might suggest their association with the olfactory functions of morph-specific behavior. These results have improved our understanding of the adaptive evolution of the antennal sensilla in nonhost-alternating, holocyclic aphids.
More than 5,000 aphid species are known to exist and are mainly distributed in temperate zones (Huang and Qiao 2014). Aphids exhibit distinct seasonal generation-alternating phenomena (Williams and Dixon 2006). During the growth season, aphids reproduce, producing multiple generations by parthenogenesis, whereas, at the beginning of the autumn, in response to shortened light and low temperature, virginoparae produce sexuparae and these produce oviparae and males (Williams and Dixon 2006). After mating, oviparae lay eggs that can overwinter in a severe environment (Huang and Qiao 2014). The next spring, the fertilized eggs hatch and the fundatrices reproduce by parthenogenesis, beginning another generation cycle (Moran 1992). Aphid polymorphism with complex ontogenetic stages plays an important role in ensuring the rapid development and continuous reproduction of the population (Pickett et al. 1992, Powell and Hardie 2001, Williams and Dixon 2006).
Antennae are important in insect sensory systems thanks the role of odorant-binding proteins in host and food searching and mating (Dahanukar et al. 2005, Scieuzo et al. 2021) and this is valid for aphids where antenna sensilla play a central role in dispersal, host location, and mating (Pickett et al. 1992, Zhao and Ban 2011). The aphid antennal sensilla are generally divided into trichoid, placoid, coeloconic, and campaniform sensilla (Zhao and Ban 2011, Kanturski et al. 2018a, Kanturski et al. 2018b, Kanturski et al. 2020). Type I trichoid sensilla are often distributed along antennal segments and serve as mechanoreceptors; type II trichoid sensilla are located at the tip of antennae and serve as mechanoreceptors and chemoreceptors (Ban et al. 2015, Kanturski et al. 2020). Big multiporous placoid sensillum (primary rhinarium) and a group of sensilla (primary rhinaria, consisting of one big multiporous placoid sensillum, small ones, and sunken coeloconic sensilla) have a similar distribution and number of morphs and studies have demonstrated their olfactory functions in response to plant odors and alarm pheromones (Nottingham et al. 1991, Du et al. 1995, Park and Hardie 2002, 2004, Sun et al. 2012, Xue et al. 2016, Bruno et al. 2018). The number and distribution of small multiporous placoid sensilla (secondary rhinaria) vary among the morphs of conspecies, with males possessing the greatest abundance of secondary rhinaria (Du et al. 1995, Zhang and Zhang 2000, Park and Hardie 2002). Alate virginoparae and gynoparae also have a large number of secondary rhinaria, while in apterous virginoparae and oviparae these rhinaria are present in small number or disappear completely (Du et al. 1995, Zhang and Zhang 2000, Park and Hardie 2002, Kanturski et al. 2020, Song et al. 2020). Secondary rhinaria showed a substantially greater response to nepetalactone and nepetalol in males and gynoparae but not in alate virginoparae (Park and Hardie 2002, 2004). Dorsal pedicel bears campaniform sensillum in each morph (Kanturski et al. 2020). These data suggest that the distribution and number of antennal sensilla in each morph might be strongly associated with morph-specific functions and behaviors throughout the life cycle (Pickett et al. 1992, Kanturski et al. 2020).
The grain aphid, Sitobion avenae (Fabricius 1775) (Hemiptera: Aphididae), is one of the most important pests, often occurring on cereal crops worldwide (Morales-Hojas et al. 2020, Xing et al. 2021). This species causes direct infestation through feeding and leads to indirect losses as vectors of plant virus transmission (Liu et al. 2014). S. avenae has a nonhost-alternating, holocyclic life cycle and exhibits polymorphism (Dedryver et al. 2001, Morales-Hojas et al. 2020). During the switching of reproductive modes, seven morphs are produced, including virginoparae (alate and apterous), sexuparae (alate and apterous), males (alate), oviparae (apterous), and fundatrices (apterous) (Shi 2019). To the authors’ knowledge, aphid related research about antennal sensilla has focused on some morphs in the life cycle of host-alternating holocyclic aphids (Du et al. 1995, Zhang and Zhang 2000, Park and Hardie 2002, Kanturski et al. 2020). However, little information is available on differences in antennal sensilla among various morphs of nonhost-alternating, holocyclic aphids. A detailed study of polyphenism in antennal sensilla is essential to elucidate olfactory perception to assist with pest control in all phases of the life cycle.
In the present study, the distribution, number, and size of antennal sensilla, and the external morphology of small multiporous placoid sensillum (secondary rhinaria) were investigated in seven adult morphs of S. avenae using scanning electron microscopy (SEM), and the results were systematically compared with those of other aphid species. The possible functions of sensilla among morphs were discussed with reference to their morphology and spatial distribution. The results are expected to provide a basis for explaining the adaptive evolution of the antennal sensilla during the switching of reproduction modes in aphids, and also provide a reference for related research on other aphids.
Materials and Methods
Experimental Insects
In late May 2019, S. avenae virginoparae were collected from wheat fields in Gulang County (102.97° E, 37.62° N), Gansu Province, China. The laboratory population was raised using the water-cultured wheat seedling method (Triticum aestivum L. CV. Xinong 979) in a climate chamber at 21 ± 1°C, 65 ± 10% relative humidity (RH), and a photoperiod of 16 h light: 8 h dark (Xu et al. 2019).
Induction and Collection of Different Aphid Morphs
The life cycle of nonhost-alternating, holocyclic S. avenae is displayed in Fig. 1. Alate virginoparae that molted between days 2 and 4 were individually placed in Petri dishes containing one wheat seedling, at 14 ± 1°C, a photoperiod of 16:8 h L:D, and 65 ± 10% RH. After 12 h, adult aphids were removed and only newborn nymphs were raised individually to maturity. Alate and apterous virginoparae adults that molted within 48 h were collected.
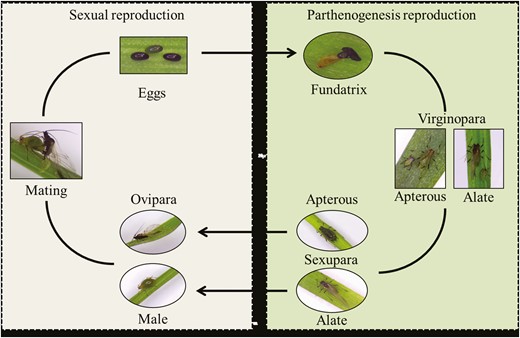
Sexual reproduction of S. avenae was induced at 14 ± 1°C, a photoperiod of 8:16 h L:D, and 65 ± 10% RH, according to the method of Helden and Dixon (2002). The 4th instar alate virginoparae (G0) were placed in Petri dishes with one wheat seedling. When virginoparae matured and produced several offspring (G1), these were individually transferred to Petri dishes containing wheat seedlings and raised to maturity. They produced offspring (G2) which were also raised continuously to maturity. Subsequently, these adults were individually transferred to Petri dishes with wheat seedlings and produced offspring (G3), which were then raised continuously to maturity. According to the morphological characteristics of the adults and the offspring produced, alate and apterous sexuparae were collected from generation G1 within 48 h of the final molt, and oviparae and males were collected within 48 h of the final molt from generations G2 and G3.
To obtain fertilized eggs, oviparae, and males were collected and were paired 1:1 in a Petri dish containing one wheat seedling. The fertilized eggs laid by the oviparae were raised in a chamber at 14 ± 1°C, a photoperiod of 16:8 h L:D, and 65 ± 10% RH. After the eggs hatched, they were raised continuously to maturity and the adults (fundatrices) were collected within 48 h of the final molt. Each aphid morphs had more than 15 individuals.
Scanning Electron Microscopy
The samples of seven different morphs were directly fixed in 70% ethanol and were cleaned three times, each time for 10 s in an ultrasonic bath, and then dehydrated in ascending concentrations of ethanol (70, 80, 90, 95, and 100% for 10, 15, 20, 25, and 30 min, respectively). After air drying at 20°C, the intact antennae from different morphs were mounted on aluminum stubs with double-sided tape and sputter-coated with gold. At least fifteen antennae from each aphid morph were observed using SEM (Hitachi S-3400N, Japan) with an accelerating voltage of 5–15 kV. Sensilla on antennae of different morphs were identified, counted, and measured. Classification of the sensillum types was based mainly on morphological characters described by Bromley et al. (1979, 1980), Kanturski et al. (2017a), and Kantureki and Bezděk (2019). The lengths of antennae, as well as lengths, and basal diameters of sensilla were measured using Hitachi’s SEM Data Manager.
Statistical Analysis
Differences in antennae length and number and size of sensilla among the different morphs were analyzed using a one-way analysis of variance (ANOVA), followed by Tukey multiple range test. Independent sample t-tests were used to determine differences in size and number. The data were mean ± standard error. All data analyses were performed using IBM SPSS Statistics 22.0 (IBM Corp. 2013).
Results
General Description of the Antennae
The antennae of various S. avenae morphs were linear-shaped, consisting of the scape, pedicel, and flagellum which contain four flagellomeres (Fig. 2A). The average antenna length of the fundatrix is the shortest among different morphs. Apterous virginoparae, apterous sexuparae, oviparae, and fundatrices have significantly shorter antennae than in the alate virginoparae, alate sexuparae, and males (F = 32.61; df = 6, 150; P < 0.001; Fig. 3A).
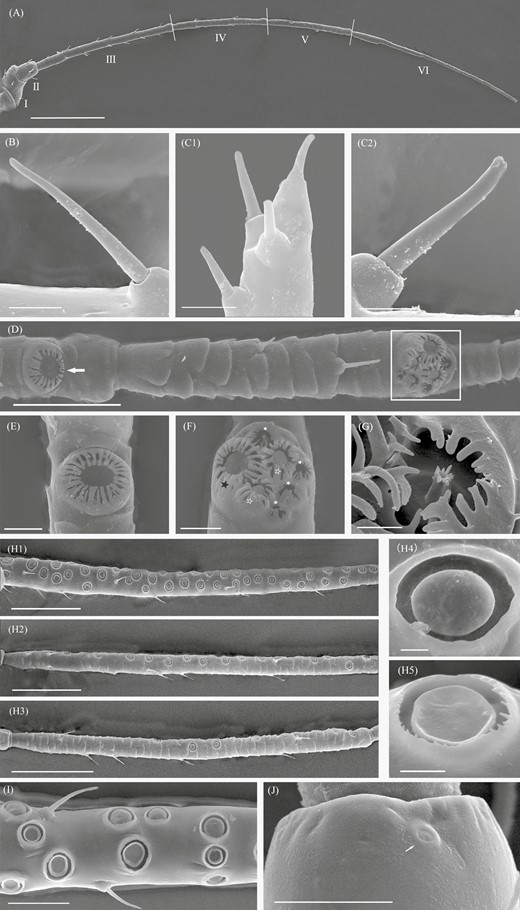
Scanning electron microscopy micrographs of antenna and sensilla on the antennal surface of Sitobion avenae. A, A whole linear antenna comprises six segments, I–VI (antennal segments I–VI). B, Type I trichoid sensilla on antennal segment III of a male. C1, Four type II trichoid sensilla at the antenna tip of a male. C2, An enlarged view of type II trichoid sensilla. D, Big multiporous placoid sensillum (primary rhinarium) on antennal segment V (white arrowhead) and a group of sensilla (primary rhinaria) on antennal segment VI (framed) of a male. E, An enlarged view of primary rhinarium. F, An enlarged view of primary rhinaria which contain major rhinarium (one big multiporous placoid sensillum (black solid asterisk)) and accessory rhinaria (two small multiporous placoid sensilla (white hallow asterisk) and sunken coeloconic sensilla (white solid asterisk)). G, An enlarged view of coeloconic sensillum. H1, H2, and H3, Numerous secondary rhinaria (small multiporous placoid sensillum) on antennal segment III, IV, and V of a male. H4 and H5, An enlarged view of convex and concave forms of secondary rhinaria. I, Convex and concave forms of secondary rhinaria on antennal segment III of a male. J, Campaniform sensillum (white arrowhead) on the dorsal pedicel of a male. Scale bars: A = 250 μm; B, C1, C2, and H5 = 5 μm, D = 50 μm, E and F = 10 μm, G and H4 = 2.5 μm, H1, H2, and H3 = 100 μm, I and J = 25 μm.
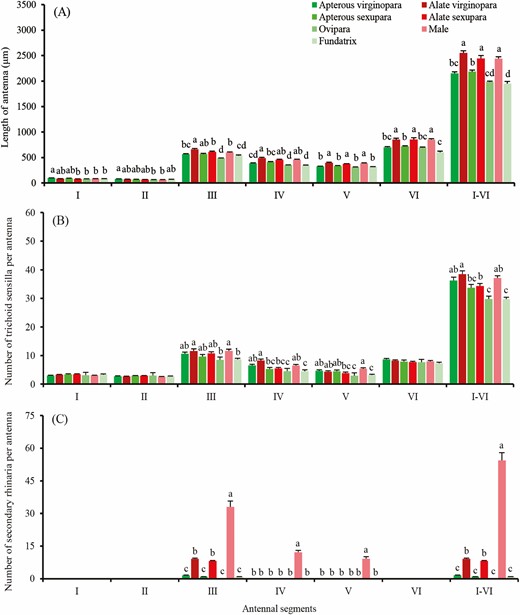
Length of antenna and number of antennal sensilla of different adult Sitobion avenae morphs. A, Length of antenna. B and C, Number of trichoid sensilla and small multiporous placoid sensillum (secondary rhinaria). I–VI, Antennal segments from I to VI. Different lowercase letters indicate significant differences (P < 0.05) between columns and absence of lowercase letters within a segment group indicates no significant differences (P > 0.05) were found (Tukey multiple test).
Sensilla on Antennae
Based on external morphology, four major sensilla types were identified on the antennae of S. avenae: trichoid sensilla, placoid sensilla, coeloconic sensilla, and campaniform sensilla (Fig. 2). Trichoid sensilla were further classified into type I on antennal segments I–VI (Fig. 2B) and type II at the tip of antennae (Fig. 2C1 and C2).
Trichoid Sensilla
Type I trichoid sensilla are distributed along each segment of the antenna, mostly on the flagellum, with only a few on the scape and pedicel in each morph. The percentage of type I trichoid sensilla on the flagellum were 76.0–83.0% at various morphs, with a greater level on antennal segment III in each morph. There were significant differences in the total number of trichoid sensilla among morphs (F = 13.59; df = 6, 119; P < 0.001). Fundatrices trichoid sensilla were the fewest in number and apterous virginoparae, apterous sexuparae, and oviparae had fewer trichoid sensilla than the alate virginoparae, alate sexuparae, and males (Fig. 3B).
Type I trichoid sensilla were significantly different in average length (µm) among morphs, with the shortest found in fundatrices (F = 10.62; df = 6, 125; P < 0.001; Fig. 4A), whereas no significant differences were found in the average length of type II trichoid sensilla (F = 2.15; df = 6, 121; P = 0.052; Fig. 4C). Both type I and type II trichoid sensilla showed significant differences in basal diameter among different morphs, with the smallest found in apterous sexuparae (F = 2.17; df = 6, 125; P = 0.017; Fig. 4B; F = 5.13; df = 6, 121; P < 0.001; Fig. 4D).
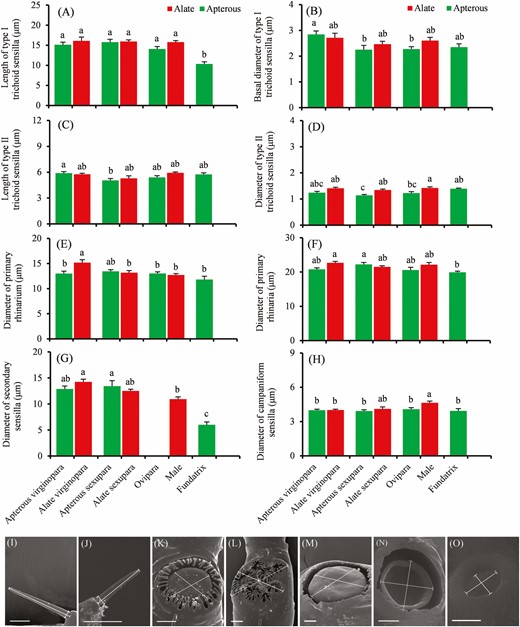
Length and diameter of antennal sensilla of different adult Sitobion avenae morphs. A and B, Length of type I and type II trichoid sensilla on antennal segment III, respectively. C, D, E, F, G, and H, Basal diameter of type I and type II trichoid sensilla on antennal segment III, big multiporous placoid sensillum (primary rhinarium) on antennal segment V, and a group of sensilla (primary rhinaria) on antennal segment VI, small multiporous placoid sensillum (secondary rhinaria) on antennal segment III, and campaniform sensilla, respectively. Different lowercase letters indicate significant differences (P < 0.05) between columns (Tukey multiple test: P < 0.05). I–O, Schematic drawing of sensilla measurements; (I and J) Length and basal diameter of type I and type II trichoid sensillum; K and L, Diameter of primary rhinarium and primary rhinaria; M and N, Diameter of secondary rhinarium; O, Diameter of campaniform sensillum. Scale bars: I, J, K, L, M, N, and O = 5 μm.
Placoid Sensillum and Coeloconic Sensilla
In each morph, there was a single big multiporous placoid sensillum (primary rhinarium) on antennal segment V and there was a group of sensilla (primary rhinaria) on antennal segment VI, typically consisting of one big placoid sensillum, two smaller ones (accessory rhinaria), and four coeloconic sensilla (accessory rhinaria) (Fig. 2D–G). The diameter of primary rhinarium on antennal segment V (F = 4.77; df = 6, 117; P < 0.001; Fig. 4E) and primary rhinaria on antennal segment VI (F = 3.59; df = 6, 70; P = 0.004; Fig. 4F) were different among morphs, with the smallest diameter of both types being found in the fundatrices.
Small multiporous placoid sensillum (secondary rhinaria) in S. avenae showed obvious differences among adult morphs. This polyphenism of secondary rhinaria was represented mainly in the distribution, number, size, and external morphological characteristics. Secondary rhinaria of the fundatrices, alate and apterous virginoparae, and alate and apterous sexuparae were found on antennal segment III (Fig. 3C). In males they could be seen on antennal segments III–V (Fig. 2 H1–H3), whereas no secondary rhinaria were observed anywhere on the antennae of the oviparae (Fig. 3C). Additionally, the total number of secondary rhinaria were different among adult S. avenae morphs (F = 220.70; df = 6, 114; P < 0.001), with the males exhibiting the greatest number, with an average of 54.38 ± 3.59 rhinaria on each antenna. In males, antennal segment III had considerably more sensilla, with an average proportion of 62.64% of the total number of secondary rhinaria, while antennal segment IV and V accounted for 21.43% and 15.93% of the total number of the secondary rhinaria, respectively. Other alate morphs (virginoparae and sexuparae) had from six to fourteen secondary sensilla on each antenna; apterous morphs (fundatrices, virginoparae, and sexuparae) only had one or two secondary sensilla on each antenna, and secondary sensilla had disappeared completely in oviparae (Fig. 3C).
Secondary rhinaria of S. avenae, lying in cuticle cavity, were found exhibiting two different external morphological characteristics. One was convex shaped and the other was concave shaped in the center of rhinaria (Fig. 2 H4, H5, and I). The fundatrices and the alate and apterous virginoparae had a convex form (Fig. 5A, A1, B, B1, C, and C1), whereas alate and apterous sexuparae both had a concave form (Fig. D, D1, E, and E1). Oviparae had no secondary rhinaria present on the antennal segment III (Fig. 5G), whereas in males both convex and concave forms were present (Fig. 5F, F1, and F2).
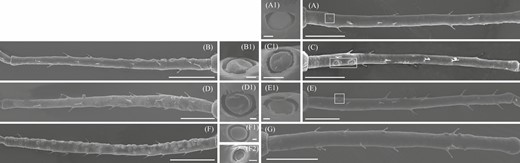
Comparison of scanning electron microscopy images of the central morphology of the cavity of secondary rhinaria (small multiporous placoid sensillum) found in different adult Sitobion avenae morphs. A, B, C, D, E, F, and G, Antennal segment III of fundatrices, alate and apterous virginoparae, alate and apterous sexuparae, males, and oviparae, respectively. A1, B1, C1, D1, and E1, Secondary rhinaria morphology of fundatrices, alate and apterous virginoparae, alate and apterous sexuparae, respectively. F1 and F2, Convex and concave forms of secondary rhinaria of males, respectively. Scale bars: A, B, C, D, E, F, and G = 100 μm; A1, B1, C1, D1, E1, F1, and F2 = 2.5 μm.
The percentages of convex form of secondary rhinaria on antennal segment III, IV, and V were 52.52%, 66.21%, and 53.36% of the total number of secondary rhinaria on antennal segment III, IV, and V in males of S. avenae, respectively. There were differences in the number of both forms on antennal segment IV (t = 2.28; df = 30; P = 0.03), whereas no differences were found for antennal segment III (t = 0.38; df = 30; P = 0.79) and V (t = 1.08; df = 30; P = 0.29) (Fig 6A). Among different morphs, secondary rhinaria showed differences in the diameter (F = 21.48; df = 5, 127; P < 0.001), with the smallest diameter found in fundatrices, and the greatest in alate virginoparae (Fig 4G). In addition, differences for males were found in the diameter of convex and concave forms of secondary rhinaria on antennal segment III (t = -3.76; df = 58; P < 0.001), whereas no significant differences were found for antennal segment IV (t = -1.62; df = 47; P = 0.11) and V (t = 0.04; df = 54; P = 0.97) (Fig 6B).
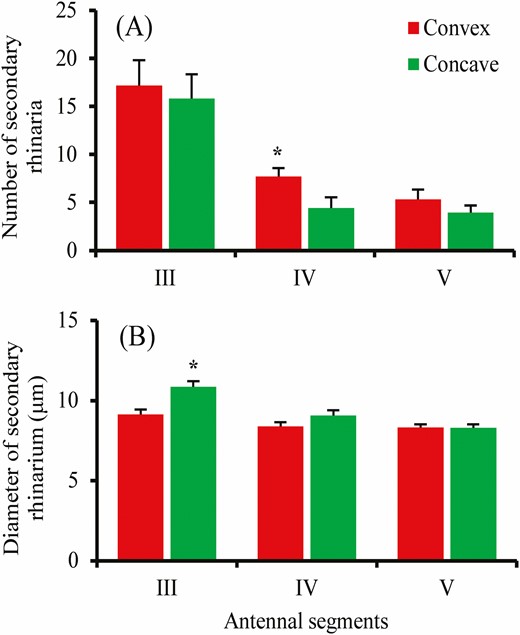
Number (A) and diameter (B) of convex and concave form of small multiporous placoid sensillum (secondary rhinarium) on antennal segments in male Sitobion avenae. Bars with an asterisk indicates significant differences between forms (Independent sample t-tests: *P < 0.05, differences without an asterisk are not significant at P > 0.05).
Campaniform Sensilla
Campaniform sensillum was found to have a button-like convex formation in the center, surrounded by a thickened edge (Fig. 2J). Generally, only one sensillum was recorded on each antenna of each morph, located on the dorsal pedicel. In males, the diameter of the campaniform sensilla was larger than in other morphs (F = 2.94; df = 6, 130; P < 0.001; Fig. 4H).
Discussion
The spatial distribution of the trichoid sensilla, big multiporous placoid sensilla (primary rhinaria), a group of sensilla (primary rhinaria), and campaniform sensillum were similar among morphs, whereas small multiporous placoid sensilla (secondary rhinaria) exhibited markable polyphenism (Figs. 3–6).
Trichoid sensilla are generally distributed on insect antennae (Zhao and Ban 2011, Pezzi et al. 2017, Bari et al. 2019). This study confirmed the presence of numerous type I trichoid sensilla along the antenna and four type II trichoid sensilla at the antennal tip in each morph of S. avenae. This is similar to the morphs of other Aphididae species, including Myzus persicae and Acyrthosiphon pisum (Zhang and Zhang 2000, Kanturski et al. 2020). However, five type II trichoid sensilla were found at the antennal tip of Aphididae species, including Nippolachnus micromeli and Pseudessigella brachychaeta (Kanturski et al. 2017b, Kanturski et al. 2018a). Furthermore, in alate morphs trichoid sensilla are greater in number than in the corresponding apterous morphs of S. avenae (Fig. 3B).
Big multiporous placoid sensilla (primary rhinarium) and a group of sensilla (primary rhinaria) share similar distributions and numbers among different morphs of S. avenae, consistent with previous observations in other Aphididae species, including Ac. pisum, Aphis glycines, Aphis fabae, My. persicae, Rhopalosiphum padi, Macrosiphoniella davazhamci, and Rhinariaphis tuberculata. (Du et al. 1995, Park et al. 2000, Park and Hardie 2004, Kanturski and Barjadze 2018, Kanturski and Stekolshchikov 2018, Kanturski et al. 2020). However, among the different aphid species, primary rhinarium and primary rhinaria can be found in different numbers and locations. Oviparae and apterous males of P. brachychaeta had five-segment antenna, their primary rhinarium and primary rhinaria were on antennal segment IV and V (Kanturski et al. 2017a, b). There are slight differences in primary rhinaria, whose number of placoid sensilla were found to range from one to three (Bromley et al. 1979, Zhao and Ban 2011, Zhong et al. 2019). Studies on aphids have suggested that the primary rhinarium on antennal segment V are involved in plant odor detection and primary rhinaria on antennal segment VI are plant odor and alarm pheromone detectors (Hardie et al. 1994, Du et al. 1995, Park et al. 2000, Park and Hardie 2004). Furthermore, primary rhinaria responded to nepetalactol and nepetalactone in four morphs in Ap. fabae and Rho. padi (Park and Hardie 2004). It is thus possible that primary rhinaria perform a similar chemosensory function in each morph of S. avenae.
The biggest differences in aphid sensilla were observed in small multiporous placoid sensillum (secondary rhinaria). Secondary rhinaria are found in alate morphs but not in apterous morphs in Nippolachnus piri, Ap. glycines, Ap. fabae, My. persicae, Rho. padi, and Eulachnus agilis (Du et al. 1995, Zhang and Zhang 2000, Park and Hardie 2004, Kanturski et al. 2015, Kanturski et al. 2018b). In addition, they are also present in both alate and apterous morphs in Ac. pisum, Therioaphis trifolii, Ma. davazhamci, and Rhi. tuberculata (Kanturski and Barjadze 2018, Kanturski and Stekolshchikov 2018, Kanturski et al. 2020, Song et al. 2020). In this study, fundatrices, apterous virginoparae, and apterous sexuparae had one or two secondary rhinaria, whereas oviparae had no secondary rhinarium in S. avenae. A great abundance of secondary rhinaria were observed in alate morphs, with the greatest number in males (Fig. 3C), Previous studies had shown numerous secondary rhinaria on antennal segments III–V both in alate males Ap. glycines, My. persicae, Ap. fabae, and Rho. padi (Du et al. 1995, Zhang and Zhang 2000, Park and Hardie 2002) and in apterous males P. brachychaeta (Kanturski et al. 2017b). This suggests that, regardless of alate or apterous morphs, increased secondary rhinaria is a general characteristic of the male, when compared to other morphs. In addition to distribution and number, we found that the external morphology displayed two forms in seven morphs of S. avenae (Fig. 6). There exist both convex and concave forms of secondary rhinaria in their central part, whereas sexuparae have the concave form of secondary rhinaria. This partly suggests that males might differ from gynoparae in the functioning and response of their secondary rhinaria to sex pheromone components (Park and Hardie 2004). Sex pheromones are composed of nepetalactone and nepetalol in different ratios in aphid species (Dunn 1978, Hardie et al. 1990). Further research is needed to determine if the form of the secondary rhinaria might be related to the response to pheromone in aphid morphs.
Previous work on aphid sensilla has shown that there is one campaniform sensillum on the pedicel in other Aphididae virginoparae of N. piri, Ac. pisum, My. persicae, Macrosiphonella sanborni, Brevicoryne brassicae, Nasonovia ribisnigri (Bromley and Anderson 1982, Zhang and Zhang 2000, Kanturski et al. 2018a, Zhong et al. 2019, Kanturski et al. 2020). In oviparae and males, one campaniform sensillum also was visible on the pedicel of Ac. pisum and Ma. davazhamci (Kanturski and Barjadze 2018, Kanturski et al. 2020). This study confirmed the presence of a single campaniform sensillum on the dorsal pedicel of the antenna in each morph of S. avenae. Furthermore, males have a significantly larger diameter compared to those of other morphs (Fig. 4H). It is speculated that the most probable function of the campaniform sensillum is coordination between the scape and pedicel movement to ensure that antennae chemoreceptors are in the correct position to function (Bromley et al. 1980).
Antennal sensilla play an important role in aphid chemical ecology (Dawson et al. 1990, Pickett et al. 1992, Lilley and Hardie 1996, Ban et al. 2015, De Biasio et al. 2015, Fan et al. 2015, Bari et al. 2019). Across the life history, each adult S. avenae morph exhibits a distinct spatial distribution of sensilla on their antenna. In general, the alate morphs possess a greater abundance of secondary rhinaria. Polyphenism in secondary rhinaria likely has great impact on the life cycle strategy, allowing alate morphs to migrate and locate host plants, allowing males to search for mates more efficiently, assisted by sex pheromones released by oviparae (Pickett et al. 1992, Hardie et al. 1994, Powell and Hardie 2001, Park and Hardie 2002, 2004).
Conclusions
This is the first report of polyphenism in antennal sensilla morphology in seven adult morphs of S. avenae. The secondary rhinaria showed markable polyphenism among morphs. In addition to distribution and number, polyphenism of secondary rhinaria in the external morphology was found in seven morphs. This work will be useful for further behavioral and physiological studies on S. avenae and other aphid species, and may provide a basis for revealing the adaptive evolution of the antennal sensilla during the switching of reproductive modes across the life cycle.
Acknowledgments
This work was supported by the Chinese Universities Scientific Fund (2452018108) and the National Natural Science Foundation of China (31101433).
Author Contributions
Y.W.: investigation; data acquisition; data analysis; writing and editing original draft. M.W.: investigation; data acquisition; Z.H.: investigation. X.H.: review. X.X.: conceptualization; funding acquisition; data analysis; writing and editing original draft; review.



