-
PDF
- Split View
-
Views
-
Cite
Cite
Anna E Ledin, John D Styrsky, Jennifer Nesbitt Styrsky, Friend or Foe? Orb-Weaver Spiders Inhabiting Ant–Acacias Capture Both Herbivorous Insects and Acacia Ant Alates, Journal of Insect Science, Volume 20, Issue 4, July 2020, 16, https://doi.org/10.1093/jisesa/ieaa076
Close - Share Icon Share
Abstract
The orb-weaver spiders Eustala oblonga (Chickering) and Eustala illicita (O. Picard-Cambridge) (Araneae: Araneidae) inhabit the ant-defended acacias Vachellia melanocerus (Beurling) and Vachellia collinsii (Safford) (Fabales: Fabaceae), respectively, in Panama. These spiders do not capture patrolling Pseudomyrmex ants but exploit their plant-protection services to escape predation. What effect the spiders have on the ant-acacia mutualisms is unknown. They may provide an additional layer of plant defense by capturing flying herbivorous insects in their webs. Alternatively, the spiders may disrupt the ant–acacia mutualisms by capturing alate acacia ants during nuptial flights. We evaluated these two hypotheses by sampling insects flying through acacia foliage and by identifying prey remains in webs. The proportions of insects captured on sticky card traps and in webs varied with taxonomic order and ecological role. Herbivorous insects greatly outnumbered other groups captured on sticky cards and were captured in spiders’ webs in both acacia species but made up a minority of prey remains in webs. Instead, insect predators and parasitoids made up the majority of prey remains and were comprised primarily by alate ant mutualists of the host acacias. These results provide indirect support for both hypotheses and suggest that the spiders potentially both benefit and harm their host ant-acacia mutualisms. The net effect of spider exploitation, however, is unclear and is likely based on both the effectiveness of plant protection from herbivory provided by the spiders relative to that provided by acacia ants, as well as the overall proportion of the ant reproductive caste the spiders actually capture.
Although ecologists define mutualism generally as a pairwise, reciprocal exchange of resources between individuals of two species that affords net benefits to both, mutualistic interactions hardly exist in isolation (Schwartz and Hoeksema 1998, Bronstein 2001, Yu 2001, Palmer et al. 2015). Other, third-party species interact with mutualisms in multiple ways. Some capitalize directly from mutualisms by parasitizing or preying on either mutualist species (e.g., West and Herre 1994, Schatz et al. 2006, Proffit et al. 2009, Borges 2015, Shi 2019). Third-party species are also attracted to the resources exchanged by mutualists, however, rendering mutualisms vulnerable to exploitation (i.e., consumption of resources without reciprocation [Bronstein 2001]) (e.g., Clement et al. 2008, Jones et al. 2009, Meehan et al. 2009, Sachs 2015). Depending on their magnitude, such negative effects of third-party species might destabilize mutualisms, possibly even threatening their persistence evolutionarily (Sachs and Simms 2006, but see Frederickson 2017). In contrast, third-party species might actually strengthen mutualisms. Ants that patrol the surface of figs, for example, attack parasitoid wasps that parasitize obligate fig-wasp pollinators of fig trees, thereby indirectly protecting fig wasp – fig tree mutualisms (Compton and Robertson 1988, Schatz et al. 2006). Just how important third-party species are to the structure and functioning of mutualisms, and by what mechanisms and under what conditions they influence mutualisms remain incompletely answered questions.
In central Panama, two ant-defended acacia species are inhabited by two species of Eustala orb-weaver spiders (Araneae: Araneidae; Hesselberg and Triana 2010, Garcia and Styrsky 2013). Eustala oblonga (Chickering) and Eustala illicita (O. Picard-Cambridge) construct and occupy their webs at night among the branches of Vachellia melanocerus (Beurling) (Fabales: Fabaceae) and Vachellia collinsii (Safford), respectively (Hesselberg and Triana 2010; H. Wolfe and J. D. Styrsky, unpubl. data). During the day, however, these spiders spend most of their time crouched against acacia leaves, stems, and thorns despite the vigorous patrolling activity of two species of obligate ant (Hymenoptera: Formicidae) mutualists (Pseudomyrmex satanicus [Wheeler] on V. melanocerus and Pseudomyrmex spinicola [Emery] on V. collinsii; Hesselberg and Triana 2010, Garcia and Styrsky 2013).
Previous field experiments indicate that the spider E. oblonga is adapted to avoid acacia ant aggression behaviorally by limiting movement on the plant surface in general and refraining from moving specifically when encountered by patrolling ants (Garcia and Styrsky 2013). Additional field experiments in which entire colonies of acacia ants were removed from their host acacias suggest that E. oblonga exploits the ant–acacia mutualism for enemy-free space (Styrsky 2014). Spider densities decreased significantly over time on acacias without ants relative to control acacias, whereas the densities of potential spider predators (arthropods, anoles, and birds) concomitantly increased, suggesting that by inhabiting ant-defended acacias, the spiders are protected from their own natural enemies (Styrsky 2014).
Whereas the spiders apparently gain from inhabiting ant-defended acacias, whether either E. oblonga or E. illicita provide any service to or inflict any harm on their respective host ant–acacia mutualism has not been investigated. As orb-weavers, both spider species capture flying insects in their webs but do not feed on either the patrolling ant guards or the nutritional rewards provided by the acacias (J. D. Styrsky, personal observations). Spider interception and predation of herbivorous insects, therefore, could provide an additional layer of defense for the acacias (plant defense hypothesis). The spiders may also capture, however, alate individuals of the reproductive caste of acacia ants as they disperse from acacia thorns on nuptial flights (alate predation hypothesis). In this latter scenario, the potential cost to both acacia ants and their host acacias in terms of colony foundation could be significant because high spider densities result in a profusion of webs amongst acacia foliage (J. D. Styrsky, unpubl. data). We evaluated the plant defense and the alate predation hypotheses by sampling insects flying through acacia foliage and by analyzing prey remains in spiders’ webs.
Materials and Methods
We conducted the majority of fieldwork during the wet-season months of July and August 2014 at two sites in central Panama: 1) the Rio Limbo basin in Parque Nacional Soberania (9°9′35″N, 79°44′36″W), and 2) a forested area at Madden Dam adjacent to Parque Nacional Chagres (9°12′47.1″N, 79°37′00.5″W). We conducted additional fieldwork in the dry season month of March 2020 at these same two sites and a third site, Parque Natural Metropolitano (8°59′07.6″N, 79°32′48.4″W). The Rio Limbo and Madden Dam sites are separated by only 18 km but the ranges of the two focal acacia species do not overlap. Vachellia melanocerus is adapted to the understory of lowland tropical moist forest that characterizes the Caribbean slope of central Panama including the Rio Limbo basin (Robinson et al. 2000). This species occurs in low densities and is the only Vachellia species found in its limited range (Janzen 1974). Vachellia collinsii is distributed more widely across Central America. In Panama, this species occurs generally along the western half of the Pacific side of the isthmus, in areas characterized by meteorological and geological conditions that support tropical dry forest, such as those found at Madden Dam and Parque Natural Metropolitano (Janzen 1974).
We utilized both indirect and direct observational methods to investigate potential interactions between the Eustala spiders as predators and the ant–acacia mutualisms they exploit. First, we used 7.62 × 12.7-cm yellow ‘sticky card’ traps to identify and estimate the relative proportions of different taxonomic groups of insects flying through acacia foliage that could be caught in the spiders’ webs (2014 only). Sticky cards are covered with a strong adhesive designed by IPM practitioners to trap and monitor pest insects that alight on or collide with the cards. Insects groups are differentially attracted to various colors of sticky cards, but yellow is most often used in ecological studies to estimate diversity because it attracts the widest range of insect taxa and functional groups (e.g., Hoback et al. 1999, Rodriguez-Saona et al. 2012, Larsen et al. 2014, O’Brien et al. 2017). Patrolling Pseudomyrmex ants were prevented from crawling onto the sticky cards by suspending each card from a branch using a partially unfolded paper clip slathered in petroleum jelly. In total, 64 and 79 sticky cards were placed in 32 V. melanocerus plants and 40 V. collinsii plants, respectively, over four weeks. Each sticky card was left in place 48 h.
We also collected silk-wrapped prey remains from webs of E. oblonga and E. illicita to assess directly what the spiders captured and consumed (2014 and 2020). Although we surveyed spider webs almost daily, it was somewhat serendipitous to find captured prey. Prey remains are infrequently encountered in the webs of either Eustala species because the spiders consume their typically very small prey within 24–48 h and then discard the remains (J. D. Styrsky, personal observations). We removed prey remains from webs by enclosing the section of the web with the prey in a small plastic vial so as not to damage the remains and make their identification more difficult. We collected prey remains from webs during the same 4-wk period that we deployed the sticky cards in 2014, but did not use both sampling procedures at the same time in the same acacias. Importantly, this same 4-wk period also fell within the time of year (middle rainy season) that both Pseudomyrmex ant species are known to produce alates (J. D. Styrsky, personal observations), although nuptial flights probably occur throughout the year (cf. Janzen 1967). We also searched for prey remains in webs for one week in the dry season in March 2020 to investigate potential seasonality in the effects of the spiders.
We analyzed the insects captured on sticky cards and the prey remains collected from webs using a dissecting microscope in a laboratory. Each individual insect was identified to order and family, and then categorized by ecological role based on what they consume as adults (herbivore, predator/parasitoid, scavenger, or other/unknown [e.g., insects that do not feed as adults or occasionally feed on nectar]). We noted specifically any remains identified as acacia ant mutualists P. satanicus or P. spinicola.
We used χ 2 goodness-of-fit tests to determine whether the percentages of insects captured on sticky cards and in webs varied with taxonomic order and ecological role. These tests were conducted for each of the two spider–ant–acacia interactions separately. We analyzed our data using the SAS/STAT package of SAS software (SAS Institute Inc. 2019).
Results
Insect Captures by Taxonomic Order
Sticky cards captured 541 insects in seven orders and 23 identifiable families in the acacia V. melanocerus, and 1,158 insects in 8 orders and 31 identifiable families in V. collinsii (Table 1). The proportion of insects captured on sticky cards in both acacia species varied with taxonomic order (V. melanocerus: χ26 = 409.0, P < 0.0001; V. collinsii: χ27 = 2044.5, P < 0.0001; Fig. 1a). The top three orders of insects on sticky cards in V. melanocerus were flies (Diptera, 35.3%), beetles (Coleoptera, 22%), and Hemiptera (21.8%, primarily Cicadellidae). Alate ants and wasps (Hymenoptera) comprised 17.2% of trapped insects (Fig. 1a) but did not include alates of the mutualist acacia ant, P. satanicus. Similarly, the top three orders of insects on sticky cards in V. collinsii were Hemiptera (40.8%, again, primarily Cicadellidae), beetles (38.3%), and flies (17.9%). Only 2.4% of trapped insects, however, were in the order Hymenoptera (Fig. 1a), and, again, none were alates of the mutualist acacia ant, P. spinicola.
Number of insects and percentage of total number captured on sticky card traps in two species of ant-acacia in Panama during the 2014 wet season by taxonomic order, family, and ecological role as adults
| ORDER/family . | Ecological rolea . | No. captured in Vachellia melanocerus . | Percent of total no. captured . | No. captured in Vachellia collinsii . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Blattaria | |||||
| Blatellidae | S | 1 | 0.18 | – | – |
| Coleoptera | |||||
| Bostrichidae | H | – | – | 1 | 0.09 |
| Buprestidae | H | – | – | 60 | 5.18 |
| Chrysomelidae | H | 69 | 12.8 | 336 | 29.02 |
| Cleridae | P | – | – | 1 | 0.09 |
| Coccinellidae | P | 9 | 1.66 | 9 | 0.78 |
| Curculionidae | H | 23 | 4.25 | 18 | 1.55 |
| Elateridae | H | 1 | 0.18 | 3 | 0.26 |
| Histeridae | H | – | – | 1 | 0.09 |
| Lampyridae | P | – | – | 1 | 0.09 |
| Lycidae | H | – | – | 1 | 0.09 |
| Nitidulidae | H | – | – | 1 | 0.09 |
| Phengodidae | O | – | – | 1 | 0.09 |
| Staphylinidae | P | 9 | 1.66 | 3 | 0.26 |
| Unknown | O | 8 | 1.48 | 7 | 0.60 |
| Diptera | |||||
| Asilidae | P | – | – | 1 | 0.09 |
| Chironomidae | O | 7 | 1.29 | 15 | 1.30 |
| Culicidae | P | 6 | 1.11 | - | - |
| Mycetophilidae | H | 35 | 6.47 | 1 | 0.09 |
| Psychodidae | O | 81 | 14.97 | – | – |
| Stratiomyidae | O | – | – | 1 | 0.09 |
| Unknown | O | 57 | 10.54 | 180 | 15.54 |
| Hemiptera | |||||
| Anthocoridae | P | – | – | 1 | 0.09 |
| Aphididae | H | 3 | 0.55 | – | – |
| Aradidae | H | – | – | 1 | 0.09 |
| Berytidae | H | – | – | 1 | 0.09 |
| Cercopidae | H | 4 | 0.74 | 3 | 0.26 |
| Cicadellidae | H | 97 | 17.93 | 408 | 35.23 |
| Cixiidae | H | – | – | 1 | 0.09 |
| Cydnidae | H | 1 | 0.18 | 1 | 0.09 |
| Derbidae | H | – | – | 1 | 0.09 |
| Membracidae | H | 2 | 0.37 | 4 | 0.35 |
| Miridae | H | 2 | 0.37 | 48 | 4.15 |
| Tingidae | H | 2 | 0.37 | 1 | 0.09 |
| Unknown | O | 7 | 1.29 | 2 | 0.17 |
| Hymenoptera | |||||
| Braconidae | P | 1 | 0.18 | – | – |
| Evaniidae | P | 1 | 0.18 | – | – |
| Formicidaeb | P | 56 | 10.35 | 1 | 0.09 |
| Ichneumonidae | P | 2 | 0.37 | 2 | 0.17 |
| Sphecidae | P | – | – | 1 | 0.09 |
| Unknown | P | 33 | 6.10 | 24 | 2.07 |
| Lepidoptera | |||||
| Unknown | O | – | – | 2 | 0.17 |
| Orthoptera | |||||
| Gryllidae | H | 13 | 2.40 | 3 | 0.26 |
| Psocoptera | |||||
| Unknown | H | 6 | 1.11 | 1 | 0.09 |
| Trichoptera | |||||
| Unknown | O | – | – | 1 | 0.09 |
| Total | 541 | 100.00 | 1158 | 100.00 |
| ORDER/family . | Ecological rolea . | No. captured in Vachellia melanocerus . | Percent of total no. captured . | No. captured in Vachellia collinsii . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Blattaria | |||||
| Blatellidae | S | 1 | 0.18 | – | – |
| Coleoptera | |||||
| Bostrichidae | H | – | – | 1 | 0.09 |
| Buprestidae | H | – | – | 60 | 5.18 |
| Chrysomelidae | H | 69 | 12.8 | 336 | 29.02 |
| Cleridae | P | – | – | 1 | 0.09 |
| Coccinellidae | P | 9 | 1.66 | 9 | 0.78 |
| Curculionidae | H | 23 | 4.25 | 18 | 1.55 |
| Elateridae | H | 1 | 0.18 | 3 | 0.26 |
| Histeridae | H | – | – | 1 | 0.09 |
| Lampyridae | P | – | – | 1 | 0.09 |
| Lycidae | H | – | – | 1 | 0.09 |
| Nitidulidae | H | – | – | 1 | 0.09 |
| Phengodidae | O | – | – | 1 | 0.09 |
| Staphylinidae | P | 9 | 1.66 | 3 | 0.26 |
| Unknown | O | 8 | 1.48 | 7 | 0.60 |
| Diptera | |||||
| Asilidae | P | – | – | 1 | 0.09 |
| Chironomidae | O | 7 | 1.29 | 15 | 1.30 |
| Culicidae | P | 6 | 1.11 | - | - |
| Mycetophilidae | H | 35 | 6.47 | 1 | 0.09 |
| Psychodidae | O | 81 | 14.97 | – | – |
| Stratiomyidae | O | – | – | 1 | 0.09 |
| Unknown | O | 57 | 10.54 | 180 | 15.54 |
| Hemiptera | |||||
| Anthocoridae | P | – | – | 1 | 0.09 |
| Aphididae | H | 3 | 0.55 | – | – |
| Aradidae | H | – | – | 1 | 0.09 |
| Berytidae | H | – | – | 1 | 0.09 |
| Cercopidae | H | 4 | 0.74 | 3 | 0.26 |
| Cicadellidae | H | 97 | 17.93 | 408 | 35.23 |
| Cixiidae | H | – | – | 1 | 0.09 |
| Cydnidae | H | 1 | 0.18 | 1 | 0.09 |
| Derbidae | H | – | – | 1 | 0.09 |
| Membracidae | H | 2 | 0.37 | 4 | 0.35 |
| Miridae | H | 2 | 0.37 | 48 | 4.15 |
| Tingidae | H | 2 | 0.37 | 1 | 0.09 |
| Unknown | O | 7 | 1.29 | 2 | 0.17 |
| Hymenoptera | |||||
| Braconidae | P | 1 | 0.18 | – | – |
| Evaniidae | P | 1 | 0.18 | – | – |
| Formicidaeb | P | 56 | 10.35 | 1 | 0.09 |
| Ichneumonidae | P | 2 | 0.37 | 2 | 0.17 |
| Sphecidae | P | – | – | 1 | 0.09 |
| Unknown | P | 33 | 6.10 | 24 | 2.07 |
| Lepidoptera | |||||
| Unknown | O | – | – | 2 | 0.17 |
| Orthoptera | |||||
| Gryllidae | H | 13 | 2.40 | 3 | 0.26 |
| Psocoptera | |||||
| Unknown | H | 6 | 1.11 | 1 | 0.09 |
| Trichoptera | |||||
| Unknown | O | – | – | 1 | 0.09 |
| Total | 541 | 100.00 | 1158 | 100.00 |
–, Absent.
aH, herbivore; O, other/unknown; P, predator/parasitoid; S, scavenger.
bAlates of several species of ants, but neither of the two species of acacia ant mutualists, Pseudomyrmex satanicus and P. spinicola.
Number of insects and percentage of total number captured on sticky card traps in two species of ant-acacia in Panama during the 2014 wet season by taxonomic order, family, and ecological role as adults
| ORDER/family . | Ecological rolea . | No. captured in Vachellia melanocerus . | Percent of total no. captured . | No. captured in Vachellia collinsii . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Blattaria | |||||
| Blatellidae | S | 1 | 0.18 | – | – |
| Coleoptera | |||||
| Bostrichidae | H | – | – | 1 | 0.09 |
| Buprestidae | H | – | – | 60 | 5.18 |
| Chrysomelidae | H | 69 | 12.8 | 336 | 29.02 |
| Cleridae | P | – | – | 1 | 0.09 |
| Coccinellidae | P | 9 | 1.66 | 9 | 0.78 |
| Curculionidae | H | 23 | 4.25 | 18 | 1.55 |
| Elateridae | H | 1 | 0.18 | 3 | 0.26 |
| Histeridae | H | – | – | 1 | 0.09 |
| Lampyridae | P | – | – | 1 | 0.09 |
| Lycidae | H | – | – | 1 | 0.09 |
| Nitidulidae | H | – | – | 1 | 0.09 |
| Phengodidae | O | – | – | 1 | 0.09 |
| Staphylinidae | P | 9 | 1.66 | 3 | 0.26 |
| Unknown | O | 8 | 1.48 | 7 | 0.60 |
| Diptera | |||||
| Asilidae | P | – | – | 1 | 0.09 |
| Chironomidae | O | 7 | 1.29 | 15 | 1.30 |
| Culicidae | P | 6 | 1.11 | - | - |
| Mycetophilidae | H | 35 | 6.47 | 1 | 0.09 |
| Psychodidae | O | 81 | 14.97 | – | – |
| Stratiomyidae | O | – | – | 1 | 0.09 |
| Unknown | O | 57 | 10.54 | 180 | 15.54 |
| Hemiptera | |||||
| Anthocoridae | P | – | – | 1 | 0.09 |
| Aphididae | H | 3 | 0.55 | – | – |
| Aradidae | H | – | – | 1 | 0.09 |
| Berytidae | H | – | – | 1 | 0.09 |
| Cercopidae | H | 4 | 0.74 | 3 | 0.26 |
| Cicadellidae | H | 97 | 17.93 | 408 | 35.23 |
| Cixiidae | H | – | – | 1 | 0.09 |
| Cydnidae | H | 1 | 0.18 | 1 | 0.09 |
| Derbidae | H | – | – | 1 | 0.09 |
| Membracidae | H | 2 | 0.37 | 4 | 0.35 |
| Miridae | H | 2 | 0.37 | 48 | 4.15 |
| Tingidae | H | 2 | 0.37 | 1 | 0.09 |
| Unknown | O | 7 | 1.29 | 2 | 0.17 |
| Hymenoptera | |||||
| Braconidae | P | 1 | 0.18 | – | – |
| Evaniidae | P | 1 | 0.18 | – | – |
| Formicidaeb | P | 56 | 10.35 | 1 | 0.09 |
| Ichneumonidae | P | 2 | 0.37 | 2 | 0.17 |
| Sphecidae | P | – | – | 1 | 0.09 |
| Unknown | P | 33 | 6.10 | 24 | 2.07 |
| Lepidoptera | |||||
| Unknown | O | – | – | 2 | 0.17 |
| Orthoptera | |||||
| Gryllidae | H | 13 | 2.40 | 3 | 0.26 |
| Psocoptera | |||||
| Unknown | H | 6 | 1.11 | 1 | 0.09 |
| Trichoptera | |||||
| Unknown | O | – | – | 1 | 0.09 |
| Total | 541 | 100.00 | 1158 | 100.00 |
| ORDER/family . | Ecological rolea . | No. captured in Vachellia melanocerus . | Percent of total no. captured . | No. captured in Vachellia collinsii . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Blattaria | |||||
| Blatellidae | S | 1 | 0.18 | – | – |
| Coleoptera | |||||
| Bostrichidae | H | – | – | 1 | 0.09 |
| Buprestidae | H | – | – | 60 | 5.18 |
| Chrysomelidae | H | 69 | 12.8 | 336 | 29.02 |
| Cleridae | P | – | – | 1 | 0.09 |
| Coccinellidae | P | 9 | 1.66 | 9 | 0.78 |
| Curculionidae | H | 23 | 4.25 | 18 | 1.55 |
| Elateridae | H | 1 | 0.18 | 3 | 0.26 |
| Histeridae | H | – | – | 1 | 0.09 |
| Lampyridae | P | – | – | 1 | 0.09 |
| Lycidae | H | – | – | 1 | 0.09 |
| Nitidulidae | H | – | – | 1 | 0.09 |
| Phengodidae | O | – | – | 1 | 0.09 |
| Staphylinidae | P | 9 | 1.66 | 3 | 0.26 |
| Unknown | O | 8 | 1.48 | 7 | 0.60 |
| Diptera | |||||
| Asilidae | P | – | – | 1 | 0.09 |
| Chironomidae | O | 7 | 1.29 | 15 | 1.30 |
| Culicidae | P | 6 | 1.11 | - | - |
| Mycetophilidae | H | 35 | 6.47 | 1 | 0.09 |
| Psychodidae | O | 81 | 14.97 | – | – |
| Stratiomyidae | O | – | – | 1 | 0.09 |
| Unknown | O | 57 | 10.54 | 180 | 15.54 |
| Hemiptera | |||||
| Anthocoridae | P | – | – | 1 | 0.09 |
| Aphididae | H | 3 | 0.55 | – | – |
| Aradidae | H | – | – | 1 | 0.09 |
| Berytidae | H | – | – | 1 | 0.09 |
| Cercopidae | H | 4 | 0.74 | 3 | 0.26 |
| Cicadellidae | H | 97 | 17.93 | 408 | 35.23 |
| Cixiidae | H | – | – | 1 | 0.09 |
| Cydnidae | H | 1 | 0.18 | 1 | 0.09 |
| Derbidae | H | – | – | 1 | 0.09 |
| Membracidae | H | 2 | 0.37 | 4 | 0.35 |
| Miridae | H | 2 | 0.37 | 48 | 4.15 |
| Tingidae | H | 2 | 0.37 | 1 | 0.09 |
| Unknown | O | 7 | 1.29 | 2 | 0.17 |
| Hymenoptera | |||||
| Braconidae | P | 1 | 0.18 | – | – |
| Evaniidae | P | 1 | 0.18 | – | – |
| Formicidaeb | P | 56 | 10.35 | 1 | 0.09 |
| Ichneumonidae | P | 2 | 0.37 | 2 | 0.17 |
| Sphecidae | P | – | – | 1 | 0.09 |
| Unknown | P | 33 | 6.10 | 24 | 2.07 |
| Lepidoptera | |||||
| Unknown | O | – | – | 2 | 0.17 |
| Orthoptera | |||||
| Gryllidae | H | 13 | 2.40 | 3 | 0.26 |
| Psocoptera | |||||
| Unknown | H | 6 | 1.11 | 1 | 0.09 |
| Trichoptera | |||||
| Unknown | O | – | – | 1 | 0.09 |
| Total | 541 | 100.00 | 1158 | 100.00 |
–, Absent.
aH, herbivore; O, other/unknown; P, predator/parasitoid; S, scavenger.
bAlates of several species of ants, but neither of the two species of acacia ant mutualists, Pseudomyrmex satanicus and P. spinicola.
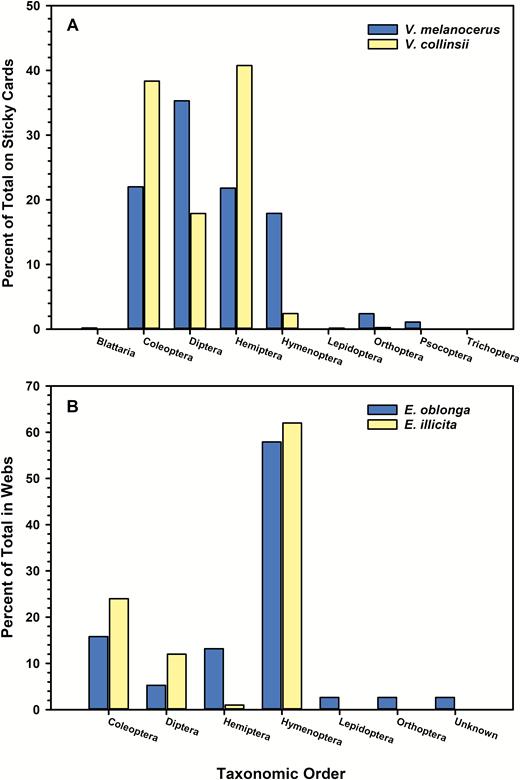
Percent of total insects captured by taxonomic order. A) Insects captured on sticky card traps in ant-acacias Vachellia melanocerus and Vachellia collinsii. B) Insect prey remains collected from webs of spiders Eustala oblonga on V. melanocerus and Eustala illicita on V. collinsii.
During the 2014 wet season, we collected 38 prey remains (from seven orders and eight identifiable families) from the webs of E. oblonga on the acacia V. melanocerus, and 50 prey remains (from four orders and eight identifiable families) from the webs of E. illicita on the acacia V. collinsii (Table 2). Although cicadellid leafhoppers, beetles (mostly chrysomelids), and flies were the groups most frequently captured on sticky cards in both acacia species, hymenopterans made up the majority of prey remains (57.9 and 62%, respectively) collected from the webs of E. oblonga (χ26 = 63.7, P < 0.0001) and E. illicita (χ23 = 41.4, P < 0.0001) (Fig. 1b). Hymenopteran prey remains consisted mostly of alates of several ant species (Formicidae), including both species of acacia ant mutualists (Table 2). Beetles (mostly chrysomelids) were the next most frequently captured prey by E. oblonga (15.8%) and E. illicita (24%) (Fig. 1b).
Insects identified from silk-wrapped prey remains in the webs of Eustala oblonga in the ant-acacia Vachellia melanocerus and in the webs of Eustala illicita in the ant-acacia Vachellia collinsii in Panama during the 2014 wet season by taxonomic order, family, and ecological role as adults
| Order/family . | Ecological rolea . | No. captured by E. oblonga . | Percent of total no. captured . | No. captured by E. illicita . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Coleoptera | |||||
| Buprestidae | H | – | – | 4 | 8.00 |
| Chrysomelidae | H | 4 | 10.53 | 5 | 10.00 |
| Curculionidae | H | 1 | 2.63 | – | – |
| Staphylinidae | P | – | – | 2 | 4.00 |
| Unknown | O | 1 | 2.63 | – | – |
| Diptera | |||||
| Chironomidae | O | – | – | 1 | 2.00 |
| Lampyridae | P | – | – | 1 | 2.00 |
| Mycetophilidae | S | 1 | 2.63 | – | – |
| Unknown | O | 1 | 2.63 | 5 | 10.00 |
| Hemiptera | |||||
| Cercopidae | H | 2 | 5.26 | – | – |
| Cicadellidae | H | 1 | 2.63 | 1 | 2.00 |
| Reduviidae | P | 1 | 2.63 | – | – |
| Unknownb | H | 1 | 2.63 | – | – |
| Hymenoptera | |||||
| Bethylidae | P | – | – | 1 | 2.00 |
| Chalcidae | P | 1 | 2.63 | - | - |
| Formicidaec | P | 10 | 26.32 | 2 | 4.00 |
| Formicidaed | M | 9 | 23.68 | 25 | 50.00 |
| Unknown bee | O | 1 | 2.63 | 1 | 2.00 |
| Unknown wasp | P | 2 | 5.26 | 2 | 4.00 |
| Lepidoptera | |||||
| Unknown | O | 1 | 2.63 | – | – |
| Orthoptera | |||||
| Gryllidae | H | 1 | 2.63 | – | – |
| Total | 38 | 100.00 | 50 | 100.00 |
| Order/family . | Ecological rolea . | No. captured by E. oblonga . | Percent of total no. captured . | No. captured by E. illicita . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Coleoptera | |||||
| Buprestidae | H | – | – | 4 | 8.00 |
| Chrysomelidae | H | 4 | 10.53 | 5 | 10.00 |
| Curculionidae | H | 1 | 2.63 | – | – |
| Staphylinidae | P | – | – | 2 | 4.00 |
| Unknown | O | 1 | 2.63 | – | – |
| Diptera | |||||
| Chironomidae | O | – | – | 1 | 2.00 |
| Lampyridae | P | – | – | 1 | 2.00 |
| Mycetophilidae | S | 1 | 2.63 | – | – |
| Unknown | O | 1 | 2.63 | 5 | 10.00 |
| Hemiptera | |||||
| Cercopidae | H | 2 | 5.26 | – | – |
| Cicadellidae | H | 1 | 2.63 | 1 | 2.00 |
| Reduviidae | P | 1 | 2.63 | – | – |
| Unknownb | H | 1 | 2.63 | – | – |
| Hymenoptera | |||||
| Bethylidae | P | – | – | 1 | 2.00 |
| Chalcidae | P | 1 | 2.63 | - | - |
| Formicidaec | P | 10 | 26.32 | 2 | 4.00 |
| Formicidaed | M | 9 | 23.68 | 25 | 50.00 |
| Unknown bee | O | 1 | 2.63 | 1 | 2.00 |
| Unknown wasp | P | 2 | 5.26 | 2 | 4.00 |
| Lepidoptera | |||||
| Unknown | O | 1 | 2.63 | – | – |
| Orthoptera | |||||
| Gryllidae | H | 1 | 2.63 | – | – |
| Total | 38 | 100.00 | 50 | 100.00 |
–, Absent.
aH, herbivore; M, mutualist; O, other/unknown; P, predator/parasitoid; S, scavenger.
bSuborder Auchenorrhyncha.
cAlates of several species of ants, but neither of the two species of acacia ant mutualists, Pseudomyrmex satanicus and P. spinicola.
dAlates of acacia ant mutualists, P. satanicus and P. spinicola.
Insects identified from silk-wrapped prey remains in the webs of Eustala oblonga in the ant-acacia Vachellia melanocerus and in the webs of Eustala illicita in the ant-acacia Vachellia collinsii in Panama during the 2014 wet season by taxonomic order, family, and ecological role as adults
| Order/family . | Ecological rolea . | No. captured by E. oblonga . | Percent of total no. captured . | No. captured by E. illicita . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Coleoptera | |||||
| Buprestidae | H | – | – | 4 | 8.00 |
| Chrysomelidae | H | 4 | 10.53 | 5 | 10.00 |
| Curculionidae | H | 1 | 2.63 | – | – |
| Staphylinidae | P | – | – | 2 | 4.00 |
| Unknown | O | 1 | 2.63 | – | – |
| Diptera | |||||
| Chironomidae | O | – | – | 1 | 2.00 |
| Lampyridae | P | – | – | 1 | 2.00 |
| Mycetophilidae | S | 1 | 2.63 | – | – |
| Unknown | O | 1 | 2.63 | 5 | 10.00 |
| Hemiptera | |||||
| Cercopidae | H | 2 | 5.26 | – | – |
| Cicadellidae | H | 1 | 2.63 | 1 | 2.00 |
| Reduviidae | P | 1 | 2.63 | – | – |
| Unknownb | H | 1 | 2.63 | – | – |
| Hymenoptera | |||||
| Bethylidae | P | – | – | 1 | 2.00 |
| Chalcidae | P | 1 | 2.63 | - | - |
| Formicidaec | P | 10 | 26.32 | 2 | 4.00 |
| Formicidaed | M | 9 | 23.68 | 25 | 50.00 |
| Unknown bee | O | 1 | 2.63 | 1 | 2.00 |
| Unknown wasp | P | 2 | 5.26 | 2 | 4.00 |
| Lepidoptera | |||||
| Unknown | O | 1 | 2.63 | – | – |
| Orthoptera | |||||
| Gryllidae | H | 1 | 2.63 | – | – |
| Total | 38 | 100.00 | 50 | 100.00 |
| Order/family . | Ecological rolea . | No. captured by E. oblonga . | Percent of total no. captured . | No. captured by E. illicita . | Percent of total no. captured . |
|---|---|---|---|---|---|
| Coleoptera | |||||
| Buprestidae | H | – | – | 4 | 8.00 |
| Chrysomelidae | H | 4 | 10.53 | 5 | 10.00 |
| Curculionidae | H | 1 | 2.63 | – | – |
| Staphylinidae | P | – | – | 2 | 4.00 |
| Unknown | O | 1 | 2.63 | – | – |
| Diptera | |||||
| Chironomidae | O | – | – | 1 | 2.00 |
| Lampyridae | P | – | – | 1 | 2.00 |
| Mycetophilidae | S | 1 | 2.63 | – | – |
| Unknown | O | 1 | 2.63 | 5 | 10.00 |
| Hemiptera | |||||
| Cercopidae | H | 2 | 5.26 | – | – |
| Cicadellidae | H | 1 | 2.63 | 1 | 2.00 |
| Reduviidae | P | 1 | 2.63 | – | – |
| Unknownb | H | 1 | 2.63 | – | – |
| Hymenoptera | |||||
| Bethylidae | P | – | – | 1 | 2.00 |
| Chalcidae | P | 1 | 2.63 | - | - |
| Formicidaec | P | 10 | 26.32 | 2 | 4.00 |
| Formicidaed | M | 9 | 23.68 | 25 | 50.00 |
| Unknown bee | O | 1 | 2.63 | 1 | 2.00 |
| Unknown wasp | P | 2 | 5.26 | 2 | 4.00 |
| Lepidoptera | |||||
| Unknown | O | 1 | 2.63 | – | – |
| Orthoptera | |||||
| Gryllidae | H | 1 | 2.63 | – | – |
| Total | 38 | 100.00 | 50 | 100.00 |
–, Absent.
aH, herbivore; M, mutualist; O, other/unknown; P, predator/parasitoid; S, scavenger.
bSuborder Auchenorrhyncha.
cAlates of several species of ants, but neither of the two species of acacia ant mutualists, Pseudomyrmex satanicus and P. spinicola.
dAlates of acacia ant mutualists, P. satanicus and P. spinicola.
During the 2020 dry season, we collected only one prey remain from webs of each spider species: a nonmutualist worker ant (subfamily Ponerinae) in an E. oblonga web and a beetle (Cleridae) in an E. illicita web.
Insect Captures by Ecological Role
Herbivores made up the majority of insects captured on sticky cards in the acacia V. melanocerus (χ23 = 253.7, P < 0.0001) and in the acacia V. collinsii (χ23 = 1779.8, P < 0.0001) (Table 1, Fig. 2a). Herbivores made up proportionally more of the insects on sticky cards in V. collinsii (77.2%), however, than on sticky cards in V. melanocerus (47.7%) (Fig. 2a). Conversely, of the insects categorized as predators/parasitoids, proportionally more were trapped in V. melanocerus (21.6%) than in V. collinsii (3.8%) (Fig. 2a). No alates of either species of mutualist acacia ant were trapped on sticky cards.
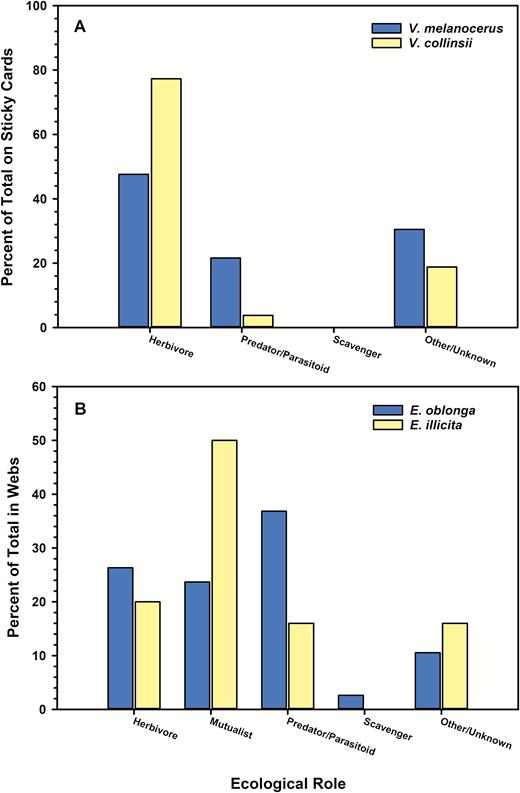
Percent of total insects captured by ecological role. A) Insects captured on sticky card traps in ant-acacias Vachellia melanocerus and Vachellia collinsii. B) Insect prey remains collected from webs of spiders Eustala oblonga on V. melanocerus and Eustala illicita on V. collinsii.
The proportion of insects collected from the webs of each spider species in 2014 also varied with ecological role (E. oblonga: χ24 = 13.8, P = 0.008; E. illicita: χ23 = 17.0, P = 0.0007; Fig. 2b). In contrast to the sticky cards, the majority or insects captured in webs of E. illicita were not herbivores. Alates of P. spinicola (the mutualist acacia ant of V. collinsii) made up the majority (50.0%) of prey remains, followed by insects categorized as herbivores (20.0%) (Fig. 2b). Similarly, E. oblonga captured alates of P. satanicus (the mutualist acacia ant of V. melanocerus), but the alates made up only 23.7% of prey remains (Fig. 2b). Insects categorized as herbivores and predators/parasitoids made up similar but slightly higher proportions of E. oblonga prey remains (26.3 and 36.8%, respectively) (Fig. 2b).
Both the ponerine ant collected from the web of E. oblonga and the clerid beetle collected from the web of E. illicita in 2020 were species of predatory insects.
Discussion
Proportions of potential prey captured on sticky cards and actual prey in spiders’ webs varied significantly among taxonomic groups and ecological roles. Herbivorous insects, primarily chrysomelid beetles and several families of hoppers (Hemiptera: Auchenorrhyncha), comprised large percentages (V. melanocerus, 47.7% and V. collinsii, 77.2%) of insects flying through acacia foliage. Further, herbivorous insects comprised 26.3 and 20.0% of prey remains in the webs of E. oblonga and E. illicita, respectively. Many of the insect herbivore groups captured on the traps or in the webs, including sap-feeding leafhoppers (Cicadellidae), treehoppers (Membracidae), and plant bugs (Miridae), foliage-browsing chrysomelid beetles, and stem-boring buprestid beetles have been documented feeding on another Central American ant–acacia, V. cornigera, when acacia ant colonies were experimentally removed (Janzen 1967). The color yellow is particularly attractive to some groups of Auchenorrhyncha, particularly Cicadellidae (e.g., Alverson et al. 1977, Lessio and Alma 2004, Rodriguez-Saona et al. 2012), thereby potentially inflating both the number of Hemiptera and the number of insects categorized as herbivores in the analyses. Even if Cicadellidae was removed from consideration, however, the data would still show that herbivores made up a large percentage of potential spider prey (i.e., insects captured on sticky cards). Further, given that only one cicadellid was sampled in the webs of each spider species, the percentage of actual spider prey that were herbivores would not appreciably change. These results, therefore, provide indirect support for the plant defense hypothesis that the acacia-inhabiting spiders can benefit their host ant–acacia mutualisms by defending them against acacia herbivory.
Taxa categorized as predators or parasitoids (the vast majority of which were Hymenopterans) comprised much smaller percentages of insects captured on sticky cards (V. melanocerus, 21.6% and V. collinsii, 3.8%) than did herbivorous taxa, but greatly outnumbered each other ecological group captured in spiders’ webs (E. oblonga, 60.5% and E. illicita, 60.0%). This pattern was strongly influenced by the fact that many of the Hymenoptera prey remains in webs were alates of the acacia ant mutualists of the spiders’ respective host acacias. These results, therefore, also provide indirect support for the alate predation hypothesis that the acacia-inhabiting spiders can harm their host ant–acacia mutualisms by preying on the reproductive caste of the mutualist ants.
The percentage of prey remains that were alate ant mutualists differed markedly, however, between the two spider species. Whereas alate acacia ants comprised 23.7% of prey recovered from the webs of E. oblonga (third in rank behind other predators/parasitoids and herbivores), alate acacia ants comprised the majority (50%) of the prey of E. illicita. This difference may be attributable to dissimilar densities of the two acacia species. The acacia V. melanocerus (the host of E. oblonga) occurs at an approximate density of five individuals per hectare at the 100-ha Rio Limbo site, whereas the density of V. collinsii (the host of E. illicita) at the 1.2-ha Madden Dam site is ~40 individuals per hectare (J.D. Styrsky and J.N. Styrsky, unpubl. data). So many individual acacias in close proximity at the Madden Dam site could have increased the frequency at which acacia ant alates were captured by E. illicita. Further, any effect of acacia density could have been amplified by differences between the two acacia ant species in mating behavior. Alates of monogynous Pseudomyrmex species, such as P. spinicola, engage in two-way traffic around their host acacias as males both disperse from and fly to acacias in response to sex pheromones produced by virgin queens (Janzen 1973), thereby potentially doubling their chances of being captured in E. illicita webs. Both male and female alates of polygynous Pseudomyrmex species such as P. satanicus, however, disperse from their host acacias to mate elsewhere (Janzen 1973, 1974), potentially resulting in less activity around E. oblonga webs.
Although our data provide correlative support for both the plant defense and alate predation hypotheses, the overall consequences of the Eustala spiders to the ant–acacia mutualisms they exploit remain uncertain. Because Vachellia-inhabiting acacia ants provide such an effective defense against herbivory (Janzen 1966, 1967), any additional layer of defense provided by spider interception of insect herbivores could be moot. There are, however, some insects (e.g., a noctuid moth, a gelechiid moth, and a coreid bug) that have evolved specializations to evade patrolling acacia ants and feed unimpeded on Vachellia acacias (Janzen 1967, Eubanks et al. 1997, Whitehead et al. 2014, Coronado-Rivera et al. 2020, respectively). Eustala spiders could provide a level of protection against such specialist herbivores that the ants cannot, but we did not observe this on either V. melanocerus or V. collinsii in our study areas.
In contrast, Eustala spider predation of acacia ant alates could have a stronger effect on the ant–acacia mutualisms than spider predation of acacia herbivores, but the strength of this effect likely depends on the ratio of the number of alates captured to the number of alates produced by a particular colony. Quantifying either term in this ratio would be quite difficult. Janzen (1967, 1973, 1974) reported alate production year-round in several Pseudomyrmex species and estimated that mature colonies of monogynous species, such as P. spinicola, produce 20 alates (10 male and 10 female) per day, whereas mature colonies of polygynous species, such as P. satanicus, produce far more (fewer per queen, but by multiple queens). If only a small fraction of alates are ever captured in webs, then the effects of the spiders on the ant–acacia mutualisms are probably inconsequential. Foundation of new acacia ant colonies may be restricted far more by the limited number of acacias available to colonize (Janzen 1966, 1974).
Our interpretations thus far are based entirely on data collected during the rainy season. Practically nothing is known of the natural history of either species of Eustala spider during the dry season. Compared with spider densities in the rainy seasons of 2008 (mean ± 1 SE = 19.1 ± 2.9 E. oblonga/acacia [n = 19 acacias]) and 2009 (19.4 ± 2.1 E. oblonga/acacia [n = 30]; 15.5 ± 1.9 E. illicita/acacia [n = 22]) (J. D. Styrsky, unpubl. data), spider densities in the 2020 dry season were much lower (2.7 ± 0.5 E. oblonga/acacia [n = 29]; 2.9 ± 0.4 E. illicita/acacia [n = 31]). Similarly, Hesselberg and Triana (2010) noted lower densities of adult E. illicita on the acacia V. collinsii in the dry season versus the wet season in 2009, and that fewer adult spiders kept their webs up during the day in the dry season (31%) than in the wet season (95%). The overall lower densities of spiders in the 2020 dry season as compared with previous wet seasons, coupled with fewer spiders keeping their webs up during the day, undoubtedly limited our ability to sample spider prey. We have little information, therefore, to assert any meaningful inference about seasonality in the effects of the spiders on the ant–acacia mutualisms except that the magnitude of any effects (positive or negative) may vary with seasonal variation in spider abundance. Because investigation of direct and indirect effects of third-party species on mutualisms suggest that outcomes are labile and context-dependent (Chamberlain et al. 2014, Palmer et al. 2015), we have included here our very limited data from the 2020 dry season simply to highlight the potential for seasonal effects and to urge further investigation.
The overall importance of third-party exploiters in the outcomes of mutualisms varies widely and remains unclear. Third-party exploiters typically take advantage of a reward offered in a mutualism, thereby potentially imposing a cost (Bronstein 2001). The spiders E. oblonga and E. illicita, however, take advantage of protection services offered to acacias by Psuedomyrmex ants, presumably without a cost (Styrsky 2014). The work described here suggests that the net effects of these spiders on the two ant–acacia mutualisms they exploit may vary with the natural histories of both the ants and the acacias, but be negligible overall. In this example of third-party exploitation, the exploiter species may be more strongly influenced evolutionarily by the mutualism than the mutualism is by the exploiters.
Acknowledgments
We thank MiAmbiente for granting permission to conduct this research in the Republic of Panama and Autoridad del Canal de Panamá for allowing access to the Rio Limbo and Madden Dam study sites. The Smithsonian Tropical Research Institute provided critical logistical support. We are grateful to Daniel Clement for assistance with fieldwork and to Loriann Garcia for reviewing a draft of this manuscript. This research was supported by a grant (#J-894) from the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust, a Maurice L. Mednick Fellowship from the Virginia Foundation for Independent Colleges, and a Schewel Student-Faculty Research Award, Percy Wootton Student-Faculty Research Award, and Faculty Summer Research Grants provided by University of Lynchburg.



