-
PDF
- Split View
-
Views
-
Cite
Cite
María Jesús Rodríguez-Palero, Jesús Fierro-Risco, Antonio C Codón, Tahía Benítez, Manuel J Valcárcel, Selection of an autochthonous Saccharomyces strain starter for alcoholic fermentation of Sherry base wines, Journal of Industrial Microbiology and Biotechnology, Volume 40, Issue 6, 1 June 2013, Pages 613–623, https://doi.org/10.1007/s10295-013-1251-0
Close - Share Icon Share
Abstract
Several indigenous Saccharomyces strains from musts were isolated in the Jerez de la Frontera region, at the end of spontaneous fermentation, in order to select the most suitable autochthonous yeast starter, during the 2007 vintage. Five strains were chosen for their oenological abilities and fermentative kinetics to elaborate a Sherry base wine. The selected autochthonous strains were characterized by molecular methods: electrophoretic karyotype and random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) and by physiological parameters: fermentative power, ethanol production, sugar consumption, acidity and volatile compound production, sensory quality, killer phenotype, desiccation, and sulphur dioxide tolerance. Laboratory- and pilot-scale fermentations were conducted with those autochthonous strains. One of them, named J4, was finally selected over all others for industrial fermentations. The J4 strain, which possesses exceptional fermentative properties and oenological qualities, prevails in industrial fermentations, and becomes the principal biological agent responsible for winemaking. Sherry base wine, industrially manufactured by means of the J4 strain, was analyzed, yielding, together with its sensory qualities, final average values of 0.9 g/l sugar content, 13.4 % (v/v) ethanol content and 0.26 g/l volatile acidity content; apart from a high acetaldehyde production, responsible for the distinctive aroma of “Fino”. This base wine was selected for “Fino” Sherry elaboration and so it was fortified; it is at present being subjected to biological aging by the so-called “flor” yeasts. The “flor” velum formed so far is very high quality. To the best of our knowledge, this is the first study covering from laboratory to industrial scale of characterization and selection of autochthonous starter intended for alcoholic fermentation in Sherry base wines. Since the 2010 vintage, the indigenous J4 strain is employed to industrially manufacture a homogeneous, exceptional Sherry base wine for “Fino” Sherry production.
Introduction
“Fino” wine is a fortified Sherry white wine manufactured in the Jerez de la Frontera region (Cádiz), southwestern Spain. Grapes, which are collected in September, used to be hand-picked, but mechanical harvesting methods are increasingly being employed now. The special production (supervised by the “Consejo Regulador de las Denominaciones de Origen Jerez-Xérès-Sherry, Manzanilla-Sanlúcar de Barrameda y Vinagre de Jerez”, http://www.sherry.org/) of such outstanding wine consists essentially of two different stages, each one conducted by means of two fully different microorganism populations. First, grape juice is fermented mainly by autochthonous fermentative yeasts to produce a low acidity white base wine. After fermentation, base wine is subjected to sensory classification by a panel of experts. According to such classification, the finest base wines are selected for “Fino” wine production and so are fortified to 15.5 % (v/v) ethanol content, intended to undergo biological aging under indigenous “flor” yeasts. The desirable Sherry base wine for “Fino” production is not only typically pale and low in volatile acidity content, but fruity and dry (under 1 g/l final sugar content) with over 11–12.5 % (v/v) ethanol content. Biological aging developed (for at least 3 years) under the oxidative metabolism of a population of “flor” yeasts forming a “flor” velum, a biofilm on the air–liquid interface of the wine. The final “Fino” wine is a straw-yellow, low-acidity dry wine (equal or under 0.25 g/l volatile acidity, equal or under 3.5 g/l total acidity) and high acetaldehyde content (up to 700–800 mg/l) possessing almond and yeast (“flor”) flavor [1, 5, 38].
The knowledge and literature on “flor” yeasts is increasing. Many different yeast populations have been identified [7, 11, 15, 22] and dynamics and successive “flor” yeast populations during biological aging have been described too [16, 18, 23, 27]. Velum formation by filmogenic “flor” yeasts and its metabolism at physiological and molecular level have been studied by various authors [2, 4, 14, 19, 25, 26, 45]. Additionally, biological aging conducted by selected “flor” yeast has been published [28, 31]. Despite the wide knowledge on “flor” yeast, the yeasts responsible for alcoholic fermentation in Sherry wine have not yet deserved a great deal of attention, and the literature gaps in this area are significant, for there are still very few studies [16].
The traditional Sherry winemaking method includes spontaneous fermentation that produces distinguished wines, but this practice involves certain well-known risks such as irreproducible or undesirable flavors and aromas, uncompleted sugar depletion, slow or stuck fermentations, etc. The inoculation of selected strains, such as commercial active dry yeast, has been a widespread winemaking practice over the last few decades as a means to conduct fermentation [34, 42]. Consequently, commercial active dry yeast is being used in some wineries at the Jerez de la Frontera region. Furthermore, there is a certain degree of controversy over this matter, since the use of commercial yeasts may diminish complexity and typicity. In addition, an indigenous starter adjusts to the ecological and technological features of a region all the more easily [21] preserving the wine’s typicity [20, 35, 43]. So, today, the goal is to select a suitable indigenous yeast capable of performing alcoholic fermentation [8, 12, 17, 33]. However, the literature about autochthonous fermentative strain selection, and/or its characterization, to obtain base wine that may be used in the Sherry system is almost non-existent. The goal of this study has been the isolation of such strains.
Autochthonous fermentative yeast starters, which are able to maintain typicity in Sherry wine, and can adjust to oenological practices and characteristics in this winemaking area, were isolated during the 2007 vintage. In the 2008–2010 vintages, pilot-scale and industrial-scale fermentations were carried out with some of those yeasts. One strain (named J4) was eventually chosen over the rest to routinely conduct alcoholic fermentations at a local winery within the first stage in “Fino” production. The isolation, selection, and characterization of yeast strains, covering from laboratory to industrial conditions, are described in this study.
Materials and methods
Laboratory and industrial media
Laboratory-, pilot-, and industrial-scale fermentations were performed in industrial musts from 2007 to 2010 vintages obtained from the Palomino “Fino” grape variety in the Jerez de la Frontera region. Those musts were kindly provided by Beam Global España S. L. winery, and typically contained 80–100 mg/l total added sulphur dioxide, Baumé grade (ºBé) from >10.5 to <14 and pH adjusted to 3.2–3.4. One ºBé equals 17 g/l fermentable sugar.
Laboratory media YPD and YPD agar (1 % yeast extract, 2 % glucose, 2 % peptone and 2 % agar) were used for propagation and maintenance of yeast.
Yeast strains
Reference strains for fermentation assays were: C1 (IFI 1693) wine yeast strain from the “Instituto de Fermentaciones Industriales” collection (Madrid, Spain) and C2 (Fermol® super16) commercial active dried yeast from AEB Group (Brescia, Italy). Sensitive killer (non-producer) laboratory strain 47G, K1 toxin producer killer strain 1101 and K2 toxin producer killer strain 1384 were kindly provided by Dr. R. Esteban (University of Salamanca, Spain).
Isolation and selection of yeasts from spontaneous industrial fermentations
Two spontaneous fermentations were carried out in 30,000-l stainless-steel vats, each containing 25,000 l of must, at a Jerez de la Frontera cellar during the 2007 vintage. Fermentation processes were checked by four daily temperature and sugar content (ºBé) measurements. ºBé was determined by means of a density meter according to the Office International de la Vigne et du Vin procedure (OIV) [30]. At the end of fermentations (typically ºBé reached 0 value), 50-ml samples were taken in sterile bottles from the middle zone in each fermenter. Samples were transported on ice to the laboratory for processing. One hundred Saccharomyces colonies, 50 from each fermenter, were randomly selected for study following procedures described [3].
At a first stage, yeasts were screened for the ability to ferment vigorously Palomino “Fino” grape musts at high temperature (30 °C) in sterile small-scale fermentations (10 ml in duplicate). At a second phase, volatile acidity was measured at the end of those small-scale fermentations, production of final volatile acidity showing a Gaussian distribution (mean value was 0.71 g/l and mode value was 0.42 g/l; standard deviation was 0.25, n = 100). The five strains that produced less volatile acidity and were also able to exhaust sugar content at 30 °C were then named J1, J2, J3, J4, and J5, and were chosen for further characterization.
Electrophoretic karyotype
The basic procedure followed for chromosomal DNA preparation was that of Codón et al. [10]. The system used was a CHEF-DRII® gel electrophoresis apparatus from Bio-Rad Laboratories (Hercules, CA, USA). Electrophoresis was carried out at 14 °C and 200 V for 16 h with a 70 s switching time and then for 12 h with 120 s switching time.
Random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR)
DNA extractions were performed with the help of MasterPure™ Yeast DNA Purification Kit (Epicentre Biotechnologies, Madison, WI, USA) following the manufacturers’ instructions. RAPD profiles were generated using decamer primers from “Operon Technologies Primer Kit”. The nucleotide sequences of primers used were the following: OPA-1, 5′-CAGGCCCTTC-3′; OPA-10, 5′-GTGATCGCAG-3′. Amplifications were carried out in a TC-512 thermocycler (Techne Inc., Burlington, USA) with Illustra puReTaq Ready-To-Go PCR Beads (GE Healthcare, Hertfordshire, UK). Condition reactions were as described in Fernandez-Espinar et al. [41]. RAPD-PCR profiles were analyzed by agarose gel (1 %) electrophoresis; image was captured under UV light in UVP Imaging System.
Killer assays
Production, sensitivity, and/or resistance to killer toxins K1 or K2 were determined on YPD (pH 4.2) blue plates as described [37].
Microvinification assays
Pure microvinification assays were conducted in sterile flasks containing 800 ml of must, without shaking, at 28 °C. Pre-cultured strains were inoculated at 0.1 optical density, measured at 660 nm (O.D.660), the industrial must was previously sterilized by filtration (0.45-μm pore-size membrane filters). Samples were taken daily under sterile conditions in order to monitor fermentations; reduced sugar content was measured as described by Somogyi [36] and modified by Nelson [29].
Oenological parameters
Final sugar concentration and volatile acidity were determined by injection flux in an Autoanalyzer AIII (Bran + Luebbe GmbH, Norderstedt, Germany) according to the manufacturers’ indications, and were expressed as g/l. Ethanol content was determined by distillation, following the official OIV method (OIV) [30].
Volatile compounds were determined by gas chromatography in a Hewlett-Packard (Palo Alto, CA, USA) 5890 Series II chromatograph equipped with capillary injector (250 °C), flame ionization detector (250 °C), capillary column (CP-WAX-57CB from Agilent Technologies) (45–200 °C, with 3 °C/min ramp), and spilt injection; nitrogen was the carrier gas. Acetaldehyde was determined by gas chromatography in a Hewlett-Packard (Palo Alto, CA, USA) 5890 Series II chromatograph equipped with packed columns injector flame ionization detector, and a column containing 0.5 % Carbowax 1500 on Carbopack. Temperatures of injector and detector were 175–250 °C, respectively; nitrogen was the carrier gas (30 ml/min). Glycerine was determined by HPLC with a Waters chromatograph equipped with a refractive index detector, four 15-cm Fast-Fruit-Juice column from Waters. Temperatures of the column and detector were 55 and 38 °C, respectively; mobile phase was phosphoric acid at 0.25 ml/l in Milli-Q quality water.
Sensory evaluation
Sensory evaluations were conducted according to the procedure previously employed for other base wines, as described in Torrens et al. [42]. Fermented musts were sensory evaluated by five to seven wine-taster experts per test, according to availability. Those experts were prequalified and trained and were those who usually evaluate stocks from the winery. Four independent tests were carried out. Must fermented by means of each strain was present in 2–4 tests. Fermented musts were presented in clear glass bottles, and aliquot samples were served in wine-taster glasses. Evaluations were conducted at room temperature. The tasters were asked to rate global sensory quality of samples from 1 (worst) to 6 (best) in order to evaluate acceptability and potential use of base wine intended for Sherry production, following the standard procedure of the winery. The final scores were normalized on a scale from 1 to 10.
Sulphur dioxide (SO2) tolerance test
SO2 content was adjusted by adding sulphating agent “Winy” from Enartis (Novara, Italy), that contains SO2 in the potassium metabisulphite form, to the grape juice. Strains, pre-cultured in sterile Palomino must (without SO2), were inoculated at 0.01 optical density, measured at 620 nm (O.D.620), in sterile must containing different amounts of total SO2 (0, 50, 100, 125, 150, 175, 200, 250, 300 ppm) in accordance with winery criteria. Cultures were aliquoted in 96-well microplates: each microplate was prepared in quadruplicate and was incubated for 24 h; two of them at 27 °C, and the other two at 34 °C. O.D.620 was measured from 10 to 24 h in a microplate reader (Labsystems iEMS Reader MF, Ramat-Gan, Israel). Alternatively, tolerance tests were carried out for longer periods of time and greater volumes; pre-cultured strains were inoculated at 0.1 (O.D.660) in 150 ml of must containing a total of 300 ppm SO2; sugar content was measured daily.
Desiccation tolerance test
A desiccation tolerance test was performed as described by Takagi et al. [40] with some modifications: Samples at stationary phase were taken from YPD cultures, washed in sterile distilled water, filtered, and dried at 30 °C until constant dried weight was reached. After that, samples from the dried biomass were rehydrated in sterile distilled water at 37 °C for 30 min.
Viability was determined by a flow-cytometric assay (BD FACSCalibur flow cytometer, NJ, USA) as described by Boid et al. [6] using fluorescence dye oxonol, which stains dead cells bright green.
Pilot-scale vinifications
Pilot-scale vinifications, during the 2008 vintage, were carried out in 30,000-l stainless-steel vats, each containing 25,000 l of must. The temperature was kept below 28 °C. Alcoholic fermentation behavior was evaluated daily by ºBé measurement and by counting cells. For scaling-up, selected yeast strains were cultured at the laboratory in sterile flasks containing 20 l of YPD until the stationary phase was reached. Each culture was aliquoted in several sterile bottles and biomass was then collected by centrifugation. The pellets were resuspended in sterile distilled water, collected, and transferred together to a sole sterile flask; they were washed twice in sterile distilled water. Moist pellets were transported to a cellar. Those moist pellets were divided into two halves to inoculate two non-sterile vats containing 20 l of Palomino “Fino” musts. When must reached vigorous fermentation (after 24–48 h, cell density was ca. 108 cells/ml, and ºBé ca. 6–7) it was used to inoculate bigger volumes of non-sterile must. Cell number was estimated by microscopy using a Bürker chamber. Typically, inoculum size represents 5–7 % the next scaling-up volume. At the end of scaling-up, 5,000 l of must in vigorous fermentation was used to inoculate 25,000 l of must in each vat; at this stage, prefermented must represented 20 % final volume. That is, four vats containing 25,000 l were fermented at the winery from an initial 20 l of YPD culture from the laboratory.
Industrial-scale vinifications
Industrial vinifications were carried out in 38,500-l stainless-steel vats, each containing 30,000 l of must. The temperature was kept below 28 °C. At the end of scaling-up, 2,500 l of must in vigorous fermentation was used to inoculate 30,000 l of must; at this point, prefermented must represented 8.3 % final volume. All fermentations were followed by four daily temperature and sugar content (ºBé) measurements. In addition, 50-ml samples were taken from the middle zone in each fermenter in the course of fermentation process, and were carried to the laboratory. Cell number was estimated by microscopy using a Bürker chamber. Serial dilutions were carried out, and samples were plated on YPD and incubated for 3 days at 28 °C; isolated colonies were randomly selected and used to perform RAPD-PCRs intended to test presence of J4 strain.
Biological aging
The musts, fermented in pilot-scale vinifications, in 30,000-l stainless-steel vats as described above, were further fortified to 15.5 % (v/v) ethanol content. For each aging test, 249 butts from the same cellar stocks, each one containing 12 “arrobas” (one “arroba” equals 16.66 l) wine aging under “flor” yeasts, were filled with 15 “arrobas” of the aforementioned fortified wine to be tested. The mixed wine was allowed to age under the “flor” velum naturally occurring on the wine surface. Samples from the middle section in each butt were taken and pooled; glycerine, ethanol, acetaldehyde and volatile acidity contents in mixed wine (the most important metabolites of Sherry wine [26]) were measured as described above. The quality of “flor” velum was evaluated “de visu” according to three parameters, i.e., thickness (thicker as higher quality, vs. thinner), color (whiter as higher quality, vs. yellow brown) and covered area (continuous as higher quality, vs. broken or isolated) [25].
Results
Molecular characterization and oenological parameters of J1, J2, J3, J4, and J5 strains
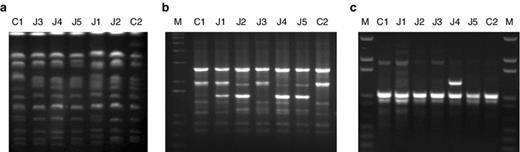
a Chromosomal patterns of C1 and C2 reference strains, and J3, J4, J5, J1, and J2 isolated strains. b Examples of RAPD-PCR profiles from the reference and the isolated strains established for primer OPA-1 and c for primer OPA-10. M corresponds to DNA marker ladder
To evaluate fermentation behavior and characterize oenological parameters in selected strains, microvinifications were performed. All candidates reached volatile acidity production values under wine reference C1 strain, J4 being the lowest producer (Table 1). All five isolated strains showed appropriate fermentative kinetics comparable to those obtained for reference C1 and C2 strains. The isolates displayed similar sugar content consumption slopes, reaching an ethanol grade equal to that of reference strains and flocculating at the end of fermentation, leaving a clear white wine (data not shown). After fermentations were completed, base wines were subjected to a sensory test by a panel of experts from the winery in order to evaluate potential use intended for Sherry production. Their fresh and fruity character and the absence of off-flavor, together with well-balanced acidity, all of them desirable properties in this base wine, were evaluated; J4 strain obtained the highest score (Table 1). The killer phenotype (K1 and K2 biotype) of selected strains was evaluated as well (Table 1).
Oenological parameters for J1, J2, J3, J4, J5, and reference strains under laboratory conditions
| Strain . | Volatile acidity production (g/l)a . | Killer K1 biotype . | Killer K2 biotype . | Killer-sensitive biotype . | Average score tastingf . | Viability after desiccation (%)g . |
|---|---|---|---|---|---|---|
| J1 | 0.43 ± 0.07 | r−b | R+c | k−d | 7.09 | n.d.h |
| J2 | 0.37 ± 0.08 | r− | R+ | K+e | 6.00 | n.d. |
| J3 | 0.29 ± 0.06 | r− | R+ | K+ | 6.27 | n.d. |
| J4 | 0.18 ± 0.02 | r− | R+ | K+ | 10.00 | 63.56 ± 7.62 |
| J5 | 0.31 ± 0.01 | r− | R+ | K+ | 8.91 | 57.94 ± 7.50 |
| C1 | 0.69 ± 0.20 | r− | r− | k− | 9.27 | 75.03 ± 11.41 |
| C2 | 0.41 ± 0.04 | r− | r− | k− | 3.73 | 62.32 ± 20.40 |
| Strain . | Volatile acidity production (g/l)a . | Killer K1 biotype . | Killer K2 biotype . | Killer-sensitive biotype . | Average score tastingf . | Viability after desiccation (%)g . |
|---|---|---|---|---|---|---|
| J1 | 0.43 ± 0.07 | r−b | R+c | k−d | 7.09 | n.d.h |
| J2 | 0.37 ± 0.08 | r− | R+ | K+e | 6.00 | n.d. |
| J3 | 0.29 ± 0.06 | r− | R+ | K+ | 6.27 | n.d. |
| J4 | 0.18 ± 0.02 | r− | R+ | K+ | 10.00 | 63.56 ± 7.62 |
| J5 | 0.31 ± 0.01 | r− | R+ | K+ | 8.91 | 57.94 ± 7.50 |
| C1 | 0.69 ± 0.20 | r− | r− | k− | 9.27 | 75.03 ± 11.41 |
| C2 | 0.41 ± 0.04 | r− | r− | k− | 3.73 | 62.32 ± 20.40 |
aResults are average, and standard deviation of two to five experiments
br− indicates absence of growth in the presence of killer strain
cR+ indicates capacity of growth in the presence of killer strain
dk− indicates inability to inhibit growth of killer sensitive strain
eK+ indicates capacity to inhibit growth of killer sensitive strain
fArbitrary units, on a scale from 1 (worst) to 10 (best). Values are average ranging from two to four tasting tests
gResults are average, and standard deviation of three experiments in duplicate
hn.d. means not determined
Oenological parameters for J1, J2, J3, J4, J5, and reference strains under laboratory conditions
| Strain . | Volatile acidity production (g/l)a . | Killer K1 biotype . | Killer K2 biotype . | Killer-sensitive biotype . | Average score tastingf . | Viability after desiccation (%)g . |
|---|---|---|---|---|---|---|
| J1 | 0.43 ± 0.07 | r−b | R+c | k−d | 7.09 | n.d.h |
| J2 | 0.37 ± 0.08 | r− | R+ | K+e | 6.00 | n.d. |
| J3 | 0.29 ± 0.06 | r− | R+ | K+ | 6.27 | n.d. |
| J4 | 0.18 ± 0.02 | r− | R+ | K+ | 10.00 | 63.56 ± 7.62 |
| J5 | 0.31 ± 0.01 | r− | R+ | K+ | 8.91 | 57.94 ± 7.50 |
| C1 | 0.69 ± 0.20 | r− | r− | k− | 9.27 | 75.03 ± 11.41 |
| C2 | 0.41 ± 0.04 | r− | r− | k− | 3.73 | 62.32 ± 20.40 |
| Strain . | Volatile acidity production (g/l)a . | Killer K1 biotype . | Killer K2 biotype . | Killer-sensitive biotype . | Average score tastingf . | Viability after desiccation (%)g . |
|---|---|---|---|---|---|---|
| J1 | 0.43 ± 0.07 | r−b | R+c | k−d | 7.09 | n.d.h |
| J2 | 0.37 ± 0.08 | r− | R+ | K+e | 6.00 | n.d. |
| J3 | 0.29 ± 0.06 | r− | R+ | K+ | 6.27 | n.d. |
| J4 | 0.18 ± 0.02 | r− | R+ | K+ | 10.00 | 63.56 ± 7.62 |
| J5 | 0.31 ± 0.01 | r− | R+ | K+ | 8.91 | 57.94 ± 7.50 |
| C1 | 0.69 ± 0.20 | r− | r− | k− | 9.27 | 75.03 ± 11.41 |
| C2 | 0.41 ± 0.04 | r− | r− | k− | 3.73 | 62.32 ± 20.40 |
aResults are average, and standard deviation of two to five experiments
br− indicates absence of growth in the presence of killer strain
cR+ indicates capacity of growth in the presence of killer strain
dk− indicates inability to inhibit growth of killer sensitive strain
eK+ indicates capacity to inhibit growth of killer sensitive strain
fArbitrary units, on a scale from 1 (worst) to 10 (best). Values are average ranging from two to four tasting tests
gResults are average, and standard deviation of three experiments in duplicate
hn.d. means not determined
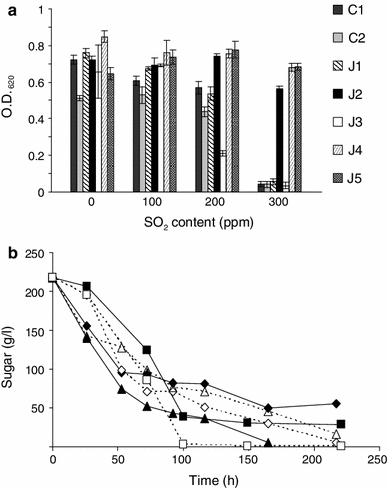
a Ability to grow (O.D. 620) of C1, C2 reference strains and J1, J2, J3, J4, and J5 isolated strains in must in the presence of increasing amounts (0, 100, 200, 300 ppm) of SO2. Assays carried out at 27 °C in 96-well microplates. Results are average, and standard deviations of two experiments in duplicate. b Ability to ferment (sugar consumption) must containing 300 ppm of SO2 at 25 °C (empty symbol) or at 34 °C (filled symbol) of reference strain C2 (squares), J4 strain (diamonds) and J5 strain (triangles). Results are average of two measurements
New microvinification assays were also conducted with J4 and J5 strains and for C1 and C2 reference strains. At the end of fermentation, these Sherry base wines were stored at 4 °C and, after discarding deposited sediments, some physicochemical parameters and volatile compounds were determined (Table 2). Data show a representative example: different must batches could yield quantitative differences between assays, but qualitative differences among strains were always repetitive. Although the C1 reference strain showed the highest acetaldehyde concentration, the J4 strain always rendered high acetaldehyde, together with lowest volatile acid final content.
Final parameters measured after fermentations by J4, J5, and reference strains in sterile musts (pure microvinifications)
| Parametersa . | C1 . | C2 . | J4 . | J5 . |
|---|---|---|---|---|
| Sugar content (g/l) | 1.08 | 0.41 | 1.13 | 1.07 |
| Ethanol content (%) | 12.17 | 12.35 | 12.18 | 12.28 |
| Glycerol (g/l) | 7.01 | 6.35 | 6.46 | 6.68 |
| Acetaldehyde (mg/l) | 60.32 | 35.84 | 43.65 | 28.91 |
| Volatile acidity (g/l) | 0.44 | 0.23 | 0.17 | 0.24 |
| Total acidity (g/l) | 5.43 | 5.43 | 5.07 | 5.48 |
| pH | 3 | 3 | 3 | 3 |
| Methanol (mg/l) | 66.35 | 66.78 | 64.66 | 69.79 |
| Propanol (mg/l) | 24.56 | 19.99 | 19.33 | 18.61 |
| Ethyl acetate (mg/l) | 26.62 | 23.52 | 18.78 | 21.35 |
| I-butanol (mg/l) | 54.31 | 45.64 | 33.44 | 33.97 |
| 2-Methyl-1-butanol (mg/l) | 20.18 | 24.93 | 26.3 | 29.94 |
| 3-Methyl-1-butanol (mg/l) | 84.15 | 105.89 | 130.19 | 142.53 |
| C6 (mg/l) | 0.3 | 0.2 | 0.4 | 0.3 |
| Ethyl lactate (mg/l) | 2.9 | 2.4 | 3.3 | 4.4 |
| Hexanol (mg/l) | 1.4 | 1.1 | 1.3 | 1.4 |
| C8 (mg/l) | 0.9 | 0.3 | 0.4 | 0.3 |
| C10 (mg/l) | 1.3 | 1.4 | 2.4 | 1.7 |
| Diethyl succinate (mg/l) | 0.3 | 0.3 | 0.3 | 0.4 |
| C12 (mg/l) | 0.2 | 0.2 | 0.3 | 0.2 |
| 2-phenylethanol (mg/l) | 3.1 | 5.5 | 7.4 | 7.5 |
| Parametersa . | C1 . | C2 . | J4 . | J5 . |
|---|---|---|---|---|
| Sugar content (g/l) | 1.08 | 0.41 | 1.13 | 1.07 |
| Ethanol content (%) | 12.17 | 12.35 | 12.18 | 12.28 |
| Glycerol (g/l) | 7.01 | 6.35 | 6.46 | 6.68 |
| Acetaldehyde (mg/l) | 60.32 | 35.84 | 43.65 | 28.91 |
| Volatile acidity (g/l) | 0.44 | 0.23 | 0.17 | 0.24 |
| Total acidity (g/l) | 5.43 | 5.43 | 5.07 | 5.48 |
| pH | 3 | 3 | 3 | 3 |
| Methanol (mg/l) | 66.35 | 66.78 | 64.66 | 69.79 |
| Propanol (mg/l) | 24.56 | 19.99 | 19.33 | 18.61 |
| Ethyl acetate (mg/l) | 26.62 | 23.52 | 18.78 | 21.35 |
| I-butanol (mg/l) | 54.31 | 45.64 | 33.44 | 33.97 |
| 2-Methyl-1-butanol (mg/l) | 20.18 | 24.93 | 26.3 | 29.94 |
| 3-Methyl-1-butanol (mg/l) | 84.15 | 105.89 | 130.19 | 142.53 |
| C6 (mg/l) | 0.3 | 0.2 | 0.4 | 0.3 |
| Ethyl lactate (mg/l) | 2.9 | 2.4 | 3.3 | 4.4 |
| Hexanol (mg/l) | 1.4 | 1.1 | 1.3 | 1.4 |
| C8 (mg/l) | 0.9 | 0.3 | 0.4 | 0.3 |
| C10 (mg/l) | 1.3 | 1.4 | 2.4 | 1.7 |
| Diethyl succinate (mg/l) | 0.3 | 0.3 | 0.3 | 0.4 |
| C12 (mg/l) | 0.2 | 0.2 | 0.3 | 0.2 |
| 2-phenylethanol (mg/l) | 3.1 | 5.5 | 7.4 | 7.5 |
aDifferent batches give rise to quantitative differences in absolute values of parameters indicated above, but qualitative differences were maintained. For this reason, a representative example is shown
Final parameters measured after fermentations by J4, J5, and reference strains in sterile musts (pure microvinifications)
| Parametersa . | C1 . | C2 . | J4 . | J5 . |
|---|---|---|---|---|
| Sugar content (g/l) | 1.08 | 0.41 | 1.13 | 1.07 |
| Ethanol content (%) | 12.17 | 12.35 | 12.18 | 12.28 |
| Glycerol (g/l) | 7.01 | 6.35 | 6.46 | 6.68 |
| Acetaldehyde (mg/l) | 60.32 | 35.84 | 43.65 | 28.91 |
| Volatile acidity (g/l) | 0.44 | 0.23 | 0.17 | 0.24 |
| Total acidity (g/l) | 5.43 | 5.43 | 5.07 | 5.48 |
| pH | 3 | 3 | 3 | 3 |
| Methanol (mg/l) | 66.35 | 66.78 | 64.66 | 69.79 |
| Propanol (mg/l) | 24.56 | 19.99 | 19.33 | 18.61 |
| Ethyl acetate (mg/l) | 26.62 | 23.52 | 18.78 | 21.35 |
| I-butanol (mg/l) | 54.31 | 45.64 | 33.44 | 33.97 |
| 2-Methyl-1-butanol (mg/l) | 20.18 | 24.93 | 26.3 | 29.94 |
| 3-Methyl-1-butanol (mg/l) | 84.15 | 105.89 | 130.19 | 142.53 |
| C6 (mg/l) | 0.3 | 0.2 | 0.4 | 0.3 |
| Ethyl lactate (mg/l) | 2.9 | 2.4 | 3.3 | 4.4 |
| Hexanol (mg/l) | 1.4 | 1.1 | 1.3 | 1.4 |
| C8 (mg/l) | 0.9 | 0.3 | 0.4 | 0.3 |
| C10 (mg/l) | 1.3 | 1.4 | 2.4 | 1.7 |
| Diethyl succinate (mg/l) | 0.3 | 0.3 | 0.3 | 0.4 |
| C12 (mg/l) | 0.2 | 0.2 | 0.3 | 0.2 |
| 2-phenylethanol (mg/l) | 3.1 | 5.5 | 7.4 | 7.5 |
| Parametersa . | C1 . | C2 . | J4 . | J5 . |
|---|---|---|---|---|
| Sugar content (g/l) | 1.08 | 0.41 | 1.13 | 1.07 |
| Ethanol content (%) | 12.17 | 12.35 | 12.18 | 12.28 |
| Glycerol (g/l) | 7.01 | 6.35 | 6.46 | 6.68 |
| Acetaldehyde (mg/l) | 60.32 | 35.84 | 43.65 | 28.91 |
| Volatile acidity (g/l) | 0.44 | 0.23 | 0.17 | 0.24 |
| Total acidity (g/l) | 5.43 | 5.43 | 5.07 | 5.48 |
| pH | 3 | 3 | 3 | 3 |
| Methanol (mg/l) | 66.35 | 66.78 | 64.66 | 69.79 |
| Propanol (mg/l) | 24.56 | 19.99 | 19.33 | 18.61 |
| Ethyl acetate (mg/l) | 26.62 | 23.52 | 18.78 | 21.35 |
| I-butanol (mg/l) | 54.31 | 45.64 | 33.44 | 33.97 |
| 2-Methyl-1-butanol (mg/l) | 20.18 | 24.93 | 26.3 | 29.94 |
| 3-Methyl-1-butanol (mg/l) | 84.15 | 105.89 | 130.19 | 142.53 |
| C6 (mg/l) | 0.3 | 0.2 | 0.4 | 0.3 |
| Ethyl lactate (mg/l) | 2.9 | 2.4 | 3.3 | 4.4 |
| Hexanol (mg/l) | 1.4 | 1.1 | 1.3 | 1.4 |
| C8 (mg/l) | 0.9 | 0.3 | 0.4 | 0.3 |
| C10 (mg/l) | 1.3 | 1.4 | 2.4 | 1.7 |
| Diethyl succinate (mg/l) | 0.3 | 0.3 | 0.3 | 0.4 |
| C12 (mg/l) | 0.2 | 0.2 | 0.3 | 0.2 |
| 2-phenylethanol (mg/l) | 3.1 | 5.5 | 7.4 | 7.5 |
aDifferent batches give rise to quantitative differences in absolute values of parameters indicated above, but qualitative differences were maintained. For this reason, a representative example is shown
Subsequently, desiccation tolerance tests of J4 and J5 strains were carried out in order to try their potential use as active dried yeast, since J4 and J5 isolates may be used as fermentation starters. Strains C1 and C2 were used as reference strains. C2 strain is routinely commercialized as active dried yeast. The recovery percentage of viable cells from J4 and J5 strains after desiccation and rehydration moved in the same range as that of C2 strain (Table 1).
Pilot-scale fermentations
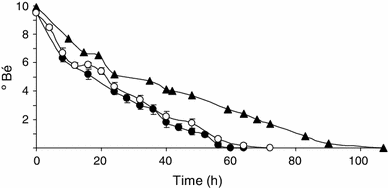
Sugar consumption (ºBé) in pilot-scale fermentations. Results are average, and standard deviations of four fermentations conducted by J4 strain (filled circles) and four fermentations conducted by J5 strain (empty circles), compared with one representative fermentation conducted by C2 reference strain (triangles)
Final parameters for J4, J5, and reference strains measured at the end of non-sterile pilot-scale fermentations
| Strain . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . |
|---|---|---|---|---|---|
| J4 | 13.26 ± 0.15 | 0.29 ± 0.01 | 0.28 ± 0.06 | 3.15 ± 0.01 | 5.68 ± 0.07 |
| J5 | 12.81 ± 0.04 | 0.30 ± 0.01 | 0.28 ± 0.04 | 3.16 ± 0.01 | 5.69 ± 0.04 |
| Sa | 12.79 | 0.32 | 0.45 | 3.18 | 5.47 |
| C2 | 12.96 | 0.31 | 2.54 | 3.22 | 7.36 |
| Strain . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . |
|---|---|---|---|---|---|
| J4 | 13.26 ± 0.15 | 0.29 ± 0.01 | 0.28 ± 0.06 | 3.15 ± 0.01 | 5.68 ± 0.07 |
| J5 | 12.81 ± 0.04 | 0.30 ± 0.01 | 0.28 ± 0.04 | 3.16 ± 0.01 | 5.69 ± 0.04 |
| Sa | 12.79 | 0.32 | 0.45 | 3.18 | 5.47 |
| C2 | 12.96 | 0.31 | 2.54 | 3.22 | 7.36 |
aS indicates spontaneous fermentation
J4 and J5 results are average, and standard deviation of four pilot fermentations in duplicate
S and C2 are representative fermentations
Final parameters for J4, J5, and reference strains measured at the end of non-sterile pilot-scale fermentations
| Strain . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . |
|---|---|---|---|---|---|
| J4 | 13.26 ± 0.15 | 0.29 ± 0.01 | 0.28 ± 0.06 | 3.15 ± 0.01 | 5.68 ± 0.07 |
| J5 | 12.81 ± 0.04 | 0.30 ± 0.01 | 0.28 ± 0.04 | 3.16 ± 0.01 | 5.69 ± 0.04 |
| Sa | 12.79 | 0.32 | 0.45 | 3.18 | 5.47 |
| C2 | 12.96 | 0.31 | 2.54 | 3.22 | 7.36 |
| Strain . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . |
|---|---|---|---|---|---|
| J4 | 13.26 ± 0.15 | 0.29 ± 0.01 | 0.28 ± 0.06 | 3.15 ± 0.01 | 5.68 ± 0.07 |
| J5 | 12.81 ± 0.04 | 0.30 ± 0.01 | 0.28 ± 0.04 | 3.16 ± 0.01 | 5.69 ± 0.04 |
| Sa | 12.79 | 0.32 | 0.45 | 3.18 | 5.47 |
| C2 | 12.96 | 0.31 | 2.54 | 3.22 | 7.36 |
aS indicates spontaneous fermentation
J4 and J5 results are average, and standard deviation of four pilot fermentations in duplicate
S and C2 are representative fermentations
Behavior under biological aging
These Sherry base wines fermented in pilot scales during the 2008 vintage by J4, J5, and C2 strains were further fortified (15.5 % v/v ethanol content) and subjected to normal biological aging process for Sherry wines, according to the “solera” system [5]. The wines were then subjected for 18 months to biological aging under the naturally developed “flor” velum. Wine samples from butts were taken; some oenological parameters were determined, and the dynamic development of “flor” velum (types and frequencies) was evaluated “de visu” (Table 4). Three types of “flor” velum qualities (A, B and C) were observed: continuous, white and thick “flor” velum (type A, highest quality); continuous, white but thinner “flor” velum (type B, medium quality); and broken or absent “flor” velum (type C, poorest quality). Type A, which is less common after summer, was predominant in all cases. Such seasonal “flor” velum behavior is common in this winegrowing area. After 18 months, as a result of “flor” yeast metabolism, wines reduced glycerine content, volatile acidity content and ethanol content, as expected. In addition, acetaldehyde content, responsible for the distinctive aroma of “Fino”, increased (Table 4). All those parameters indicate correct “flor” velum formation (in spite of J4 and J5 strains being k2) and excellent organoleptic characteristics under biological aging in these Sherry base wines.
Dynamic development and metabolism of the “flor” velum naturally occurring on Sherry wines, previously fermented by either C2, J4, or J5 strains, fortified and subjected to biological aging for up to 18 months
| Percentage of butts . | Total production (+) or consumption (−) after 18 months of agingc . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fermenting strain . | Flor velum typea . | Time of aging (months) . | ||||||||
| April 2009 (t = 1) . | September 2009 (t = 6) . | January 2010 (t = 10) . | May 2010 (t = 14) . | September 2010 (t = 18) . | Ethanol (%) . | Volatile acidity (g/l) . | Glycerine (g/l) . | Acetaldehyde (mg/l) . | ||
| J4b | A | 73 | 71 | 85 | 85 | 68 | −1.3 | −0.09 | −1.8 | +48 |
| B | 15 | 18 | 12 | 11 | 17 | |||||
| C | 12 | 11 | 3 | 4 | 15 | |||||
| J5b | A | 72 | 67 | 81 | 83 | 69 | −1.4 | −0.10 | −1.8 | +40 |
| B | 22 | 24 | 11 | 8 | 21 | |||||
| C | 6 | 9 | 8 | 9 | 10 | |||||
| C2b | A | 83 | 69 | 88 | 83 | 59 | −1.2 | −0.13 | −1.9 | +47 |
| B | 16 | 22 | 5 | 12 | 21 | |||||
| C | 1 | 9 | 7 | 5 | 20 | |||||
| Percentage of butts . | Total production (+) or consumption (−) after 18 months of agingc . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fermenting strain . | Flor velum typea . | Time of aging (months) . | ||||||||
| April 2009 (t = 1) . | September 2009 (t = 6) . | January 2010 (t = 10) . | May 2010 (t = 14) . | September 2010 (t = 18) . | Ethanol (%) . | Volatile acidity (g/l) . | Glycerine (g/l) . | Acetaldehyde (mg/l) . | ||
| J4b | A | 73 | 71 | 85 | 85 | 68 | −1.3 | −0.09 | −1.8 | +48 |
| B | 15 | 18 | 12 | 11 | 17 | |||||
| C | 12 | 11 | 3 | 4 | 15 | |||||
| J5b | A | 72 | 67 | 81 | 83 | 69 | −1.4 | −0.10 | −1.8 | +40 |
| B | 22 | 24 | 11 | 8 | 21 | |||||
| C | 6 | 9 | 8 | 9 | 10 | |||||
| C2b | A | 83 | 69 | 88 | 83 | 59 | −1.2 | −0.13 | −1.9 | +47 |
| B | 16 | 22 | 5 | 12 | 21 | |||||
| C | 1 | 9 | 7 | 5 | 20 | |||||
aA highest, B medium, C poorest quality
bTotal number of butts: 249
cData show averages
Dynamic development and metabolism of the “flor” velum naturally occurring on Sherry wines, previously fermented by either C2, J4, or J5 strains, fortified and subjected to biological aging for up to 18 months
| Percentage of butts . | Total production (+) or consumption (−) after 18 months of agingc . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fermenting strain . | Flor velum typea . | Time of aging (months) . | ||||||||
| April 2009 (t = 1) . | September 2009 (t = 6) . | January 2010 (t = 10) . | May 2010 (t = 14) . | September 2010 (t = 18) . | Ethanol (%) . | Volatile acidity (g/l) . | Glycerine (g/l) . | Acetaldehyde (mg/l) . | ||
| J4b | A | 73 | 71 | 85 | 85 | 68 | −1.3 | −0.09 | −1.8 | +48 |
| B | 15 | 18 | 12 | 11 | 17 | |||||
| C | 12 | 11 | 3 | 4 | 15 | |||||
| J5b | A | 72 | 67 | 81 | 83 | 69 | −1.4 | −0.10 | −1.8 | +40 |
| B | 22 | 24 | 11 | 8 | 21 | |||||
| C | 6 | 9 | 8 | 9 | 10 | |||||
| C2b | A | 83 | 69 | 88 | 83 | 59 | −1.2 | −0.13 | −1.9 | +47 |
| B | 16 | 22 | 5 | 12 | 21 | |||||
| C | 1 | 9 | 7 | 5 | 20 | |||||
| Percentage of butts . | Total production (+) or consumption (−) after 18 months of agingc . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fermenting strain . | Flor velum typea . | Time of aging (months) . | ||||||||
| April 2009 (t = 1) . | September 2009 (t = 6) . | January 2010 (t = 10) . | May 2010 (t = 14) . | September 2010 (t = 18) . | Ethanol (%) . | Volatile acidity (g/l) . | Glycerine (g/l) . | Acetaldehyde (mg/l) . | ||
| J4b | A | 73 | 71 | 85 | 85 | 68 | −1.3 | −0.09 | −1.8 | +48 |
| B | 15 | 18 | 12 | 11 | 17 | |||||
| C | 12 | 11 | 3 | 4 | 15 | |||||
| J5b | A | 72 | 67 | 81 | 83 | 69 | −1.4 | −0.10 | −1.8 | +40 |
| B | 22 | 24 | 11 | 8 | 21 | |||||
| C | 6 | 9 | 8 | 9 | 10 | |||||
| C2b | A | 83 | 69 | 88 | 83 | 59 | −1.2 | −0.13 | −1.9 | +47 |
| B | 16 | 22 | 5 | 12 | 21 | |||||
| C | 1 | 9 | 7 | 5 | 20 | |||||
aA highest, B medium, C poorest quality
bTotal number of butts: 249
cData show averages
Industrial-scale vinifications
As a result, during the 2009–2010 vintages, strain J4 was selected to perform industrial vinifications in cellar with vats of similar capacity to those of pilot-scale vinifications, but increasing considerably vat number. In 2009, the best-quality non-sterile musts were fermented with J4 candidate strain and with C2 reference strain. A total of 350,000 l of wine was produced with the C2 strain, and 950,000 l with the J4 strain. Analytical parameters measured at the end of fermentation are summarized in Table 5. Those Sherry base wines were further tested by a panel of testers from the winery. Wines elaborated with J4 were better evaluated by testers than those obtained with commercial C2 strain. As a result, since 2010 vintage, the J4 strain was selected to routinely ferment the best-quality musts at the cellar. Analytical parameters measured at the end of fermentation in 2010 vintage are summarized in Table 5, as well. The base wines obtained were excellent and rather similar to those from 2009 vintage.
Features of musts before fermentation and oenological parameters of wine obtained after non-sterile industrial scale fermentation carried out by either C2 or J4
| . | Density (°Bé) . | Total acidity (g/l) . | pH . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . | SO2 (ppm) . |
|---|---|---|---|---|---|---|---|---|---|
| Strain | Musta (Vintage 2009) | Base wine | |||||||
| C2 | 12.5 | 3.1 | 3.7 | 12.8 | 0.26 | 0.7 | 3.2 | 5.1 | 59 |
| 12.6 | 3.4 | 3.9 | 12.9 | 0.27 | 1.1 | 3.2 | 5.1 | 64 | |
| 12.7 | 3.8 | 4.0 | 13.1 | 0.29 | 1.2 | 3.2 | 5.2 | 69 | |
| J4 | 12.4 | 2.7 | 3.7 | 12.8 | 0.23 | 0.1 | 3.1 | 4.9 | 59 |
| 13.0 | 3.4 | 3.9 | 13.4 | 0.26 | 0.9 | 3.2 | 5.4 | 62 | |
| 13.5 | 3.5 | 4.1 | 13.9 | 0.31 | 2.2 | 3.3 | 5.9 | 68 | |
| Strain | Musta (Vintage 2010) | Base wine | |||||||
| J4 | 11.2 | 2.9 | 3.6 | 11.4 | 0.18 | 0.4 | 3.1 | 4.8 | 48 |
| 11.9 | 3.5 | 4.0 | 12.5 | 0.24 | 1.0 | 3.2 | 5.3 | 68 | |
| 12.8 | 3.8 | 4.2 | 13.0 | 0.34 | 2.7 | 3.3 | 6.1 | 79 | |
| . | Density (°Bé) . | Total acidity (g/l) . | pH . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . | SO2 (ppm) . |
|---|---|---|---|---|---|---|---|---|---|
| Strain | Musta (Vintage 2009) | Base wine | |||||||
| C2 | 12.5 | 3.1 | 3.7 | 12.8 | 0.26 | 0.7 | 3.2 | 5.1 | 59 |
| 12.6 | 3.4 | 3.9 | 12.9 | 0.27 | 1.1 | 3.2 | 5.1 | 64 | |
| 12.7 | 3.8 | 4.0 | 13.1 | 0.29 | 1.2 | 3.2 | 5.2 | 69 | |
| J4 | 12.4 | 2.7 | 3.7 | 12.8 | 0.23 | 0.1 | 3.1 | 4.9 | 59 |
| 13.0 | 3.4 | 3.9 | 13.4 | 0.26 | 0.9 | 3.2 | 5.4 | 62 | |
| 13.5 | 3.5 | 4.1 | 13.9 | 0.31 | 2.2 | 3.3 | 5.9 | 68 | |
| Strain | Musta (Vintage 2010) | Base wine | |||||||
| J4 | 11.2 | 2.9 | 3.6 | 11.4 | 0.18 | 0.4 | 3.1 | 4.8 | 48 |
| 11.9 | 3.5 | 4.0 | 12.5 | 0.24 | 1.0 | 3.2 | 5.3 | 68 | |
| 12.8 | 3.8 | 4.2 | 13.0 | 0.34 | 2.7 | 3.3 | 6.1 | 79 | |
Data show minimum, average, and maximum values
aData before fermentation and pH and SO2 adjustment
Features of musts before fermentation and oenological parameters of wine obtained after non-sterile industrial scale fermentation carried out by either C2 or J4
| . | Density (°Bé) . | Total acidity (g/l) . | pH . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . | SO2 (ppm) . |
|---|---|---|---|---|---|---|---|---|---|
| Strain | Musta (Vintage 2009) | Base wine | |||||||
| C2 | 12.5 | 3.1 | 3.7 | 12.8 | 0.26 | 0.7 | 3.2 | 5.1 | 59 |
| 12.6 | 3.4 | 3.9 | 12.9 | 0.27 | 1.1 | 3.2 | 5.1 | 64 | |
| 12.7 | 3.8 | 4.0 | 13.1 | 0.29 | 1.2 | 3.2 | 5.2 | 69 | |
| J4 | 12.4 | 2.7 | 3.7 | 12.8 | 0.23 | 0.1 | 3.1 | 4.9 | 59 |
| 13.0 | 3.4 | 3.9 | 13.4 | 0.26 | 0.9 | 3.2 | 5.4 | 62 | |
| 13.5 | 3.5 | 4.1 | 13.9 | 0.31 | 2.2 | 3.3 | 5.9 | 68 | |
| Strain | Musta (Vintage 2010) | Base wine | |||||||
| J4 | 11.2 | 2.9 | 3.6 | 11.4 | 0.18 | 0.4 | 3.1 | 4.8 | 48 |
| 11.9 | 3.5 | 4.0 | 12.5 | 0.24 | 1.0 | 3.2 | 5.3 | 68 | |
| 12.8 | 3.8 | 4.2 | 13.0 | 0.34 | 2.7 | 3.3 | 6.1 | 79 | |
| . | Density (°Bé) . | Total acidity (g/l) . | pH . | Ethanol content (%) . | Volatile acidity (g/l) . | Sugars content (g/l) . | pH . | Total acidity (g/l) . | SO2 (ppm) . |
|---|---|---|---|---|---|---|---|---|---|
| Strain | Musta (Vintage 2009) | Base wine | |||||||
| C2 | 12.5 | 3.1 | 3.7 | 12.8 | 0.26 | 0.7 | 3.2 | 5.1 | 59 |
| 12.6 | 3.4 | 3.9 | 12.9 | 0.27 | 1.1 | 3.2 | 5.1 | 64 | |
| 12.7 | 3.8 | 4.0 | 13.1 | 0.29 | 1.2 | 3.2 | 5.2 | 69 | |
| J4 | 12.4 | 2.7 | 3.7 | 12.8 | 0.23 | 0.1 | 3.1 | 4.9 | 59 |
| 13.0 | 3.4 | 3.9 | 13.4 | 0.26 | 0.9 | 3.2 | 5.4 | 62 | |
| 13.5 | 3.5 | 4.1 | 13.9 | 0.31 | 2.2 | 3.3 | 5.9 | 68 | |
| Strain | Musta (Vintage 2010) | Base wine | |||||||
| J4 | 11.2 | 2.9 | 3.6 | 11.4 | 0.18 | 0.4 | 3.1 | 4.8 | 48 |
| 11.9 | 3.5 | 4.0 | 12.5 | 0.24 | 1.0 | 3.2 | 5.3 | 68 | |
| 12.8 | 3.8 | 4.2 | 13.0 | 0.34 | 2.7 | 3.3 | 6.1 | 79 | |
Data show minimum, average, and maximum values
aData before fermentation and pH and SO2 adjustment
Prevalence of J4 strain under industrial fermentation
To prove the prevalence of inoculated J4 strain under industrial conditions, samples from industrial fermentation, conducted with J4 strain as starter, were taken and compared with spontaneous, non-inoculated fermentation. Alcoholic fermentation behavior was evaluated daily by ºBé measurement. The presence of J4 inoculated strain was evaluated from samples taken all through the fermentation process. For this purpose, RAPD-PCRs analyses with OPA-10 primer were carried out; DNA was extracted from 50 colonies randomly selected from each sample (Table 6). J4 was the predominant strain during vigorous fermentation in the inoculated vat. Some physicochemical parameters and volatile compounds were also determined at the end of fermentations (Table 6). Data show a representative example: different must batches could render quantitative differences among fermentations, carried out spontaneously or by the different strains, but qualitative differences were repetitive. Similar values for mainly oenological parameters were reached. In addition, acetaldehyde content in the inoculated vats reached a considerable high value.
Detection (%) of J4 strain during fermentation process and final parameters measured at the end of non-sterile industrial scale fermentation
| . | Inoculated strain . | |||
|---|---|---|---|---|
| Time (h) . | J4 . | Sa . | ||
| . | °Bé . | % J4 strain . | °Bé . | % J4 strain . |
| 48 | 7.3 | 56 | 8 | 10 |
| 72 | 4.1 | 68 | 3.9 | 6 |
| 144 | 0.9 | 30 | 0.9 | 8 |
| 192 | 0.9 | 18 | 0.9 | 16 |
| Final parametersb | ||||
| Ethanol content (%) | 12.43 | 12.57 | ||
| Acetaldehyde (mg/l) | 82.24 | 56.62 | ||
| Volatile acidity (g/l) | 0.22 | 0.19 | ||
| Total acidity (g/l) | 7.92 | 8.23 | ||
| pH | 3.15 | 3.13 | ||
| Methanol (mg/l) | 67.37 | 64.03 | ||
| Propanol (mg/l) | 18.77 | 17.88 | ||
| Ethyl acetate (mg/l) | 19.03 | 19.53 | ||
| I-butanol (mg/l) | 43.79 | 60.11 | ||
| 2-Methyl-1-butanol (mg/l) | 62.79 | 52.65 | ||
| 3-Methyl-1-butanol (mg/l) | 243.41 | 226.07 | ||
| C6 (mg/l) | 0.2 | 0.2 | ||
| Ethyl lactate (mg/l) | 6.0 | 5.5 | ||
| Hexanol (mg/l) | 1.9 | 1.8 | ||
| C8 (mg/l) | 0.8 | 0.3 | ||
| C10 (mg/l) | 1.5 | 1.5 | ||
| Diethyl succinate (mg/l) | 0.3 | 0.3 | ||
| C12 (mg/l) | 0.2 | 0.2 | ||
| 2-phenylethanol (mg/l) | 9.0 | 13.7 | ||
| . | Inoculated strain . | |||
|---|---|---|---|---|
| Time (h) . | J4 . | Sa . | ||
| . | °Bé . | % J4 strain . | °Bé . | % J4 strain . |
| 48 | 7.3 | 56 | 8 | 10 |
| 72 | 4.1 | 68 | 3.9 | 6 |
| 144 | 0.9 | 30 | 0.9 | 8 |
| 192 | 0.9 | 18 | 0.9 | 16 |
| Final parametersb | ||||
| Ethanol content (%) | 12.43 | 12.57 | ||
| Acetaldehyde (mg/l) | 82.24 | 56.62 | ||
| Volatile acidity (g/l) | 0.22 | 0.19 | ||
| Total acidity (g/l) | 7.92 | 8.23 | ||
| pH | 3.15 | 3.13 | ||
| Methanol (mg/l) | 67.37 | 64.03 | ||
| Propanol (mg/l) | 18.77 | 17.88 | ||
| Ethyl acetate (mg/l) | 19.03 | 19.53 | ||
| I-butanol (mg/l) | 43.79 | 60.11 | ||
| 2-Methyl-1-butanol (mg/l) | 62.79 | 52.65 | ||
| 3-Methyl-1-butanol (mg/l) | 243.41 | 226.07 | ||
| C6 (mg/l) | 0.2 | 0.2 | ||
| Ethyl lactate (mg/l) | 6.0 | 5.5 | ||
| Hexanol (mg/l) | 1.9 | 1.8 | ||
| C8 (mg/l) | 0.8 | 0.3 | ||
| C10 (mg/l) | 1.5 | 1.5 | ||
| Diethyl succinate (mg/l) | 0.3 | 0.3 | ||
| C12 (mg/l) | 0.2 | 0.2 | ||
| 2-phenylethanol (mg/l) | 9.0 | 13.7 | ||
aS indicates spontaneous fermentation
bDifferent batches give rise to quantitative differences in absolute values of parameters indicated above, but qualitative differences were maintained. For this reason, a representative example is shown
Detection (%) of J4 strain during fermentation process and final parameters measured at the end of non-sterile industrial scale fermentation
| . | Inoculated strain . | |||
|---|---|---|---|---|
| Time (h) . | J4 . | Sa . | ||
| . | °Bé . | % J4 strain . | °Bé . | % J4 strain . |
| 48 | 7.3 | 56 | 8 | 10 |
| 72 | 4.1 | 68 | 3.9 | 6 |
| 144 | 0.9 | 30 | 0.9 | 8 |
| 192 | 0.9 | 18 | 0.9 | 16 |
| Final parametersb | ||||
| Ethanol content (%) | 12.43 | 12.57 | ||
| Acetaldehyde (mg/l) | 82.24 | 56.62 | ||
| Volatile acidity (g/l) | 0.22 | 0.19 | ||
| Total acidity (g/l) | 7.92 | 8.23 | ||
| pH | 3.15 | 3.13 | ||
| Methanol (mg/l) | 67.37 | 64.03 | ||
| Propanol (mg/l) | 18.77 | 17.88 | ||
| Ethyl acetate (mg/l) | 19.03 | 19.53 | ||
| I-butanol (mg/l) | 43.79 | 60.11 | ||
| 2-Methyl-1-butanol (mg/l) | 62.79 | 52.65 | ||
| 3-Methyl-1-butanol (mg/l) | 243.41 | 226.07 | ||
| C6 (mg/l) | 0.2 | 0.2 | ||
| Ethyl lactate (mg/l) | 6.0 | 5.5 | ||
| Hexanol (mg/l) | 1.9 | 1.8 | ||
| C8 (mg/l) | 0.8 | 0.3 | ||
| C10 (mg/l) | 1.5 | 1.5 | ||
| Diethyl succinate (mg/l) | 0.3 | 0.3 | ||
| C12 (mg/l) | 0.2 | 0.2 | ||
| 2-phenylethanol (mg/l) | 9.0 | 13.7 | ||
| . | Inoculated strain . | |||
|---|---|---|---|---|
| Time (h) . | J4 . | Sa . | ||
| . | °Bé . | % J4 strain . | °Bé . | % J4 strain . |
| 48 | 7.3 | 56 | 8 | 10 |
| 72 | 4.1 | 68 | 3.9 | 6 |
| 144 | 0.9 | 30 | 0.9 | 8 |
| 192 | 0.9 | 18 | 0.9 | 16 |
| Final parametersb | ||||
| Ethanol content (%) | 12.43 | 12.57 | ||
| Acetaldehyde (mg/l) | 82.24 | 56.62 | ||
| Volatile acidity (g/l) | 0.22 | 0.19 | ||
| Total acidity (g/l) | 7.92 | 8.23 | ||
| pH | 3.15 | 3.13 | ||
| Methanol (mg/l) | 67.37 | 64.03 | ||
| Propanol (mg/l) | 18.77 | 17.88 | ||
| Ethyl acetate (mg/l) | 19.03 | 19.53 | ||
| I-butanol (mg/l) | 43.79 | 60.11 | ||
| 2-Methyl-1-butanol (mg/l) | 62.79 | 52.65 | ||
| 3-Methyl-1-butanol (mg/l) | 243.41 | 226.07 | ||
| C6 (mg/l) | 0.2 | 0.2 | ||
| Ethyl lactate (mg/l) | 6.0 | 5.5 | ||
| Hexanol (mg/l) | 1.9 | 1.8 | ||
| C8 (mg/l) | 0.8 | 0.3 | ||
| C10 (mg/l) | 1.5 | 1.5 | ||
| Diethyl succinate (mg/l) | 0.3 | 0.3 | ||
| C12 (mg/l) | 0.2 | 0.2 | ||
| 2-phenylethanol (mg/l) | 9.0 | 13.7 | ||
aS indicates spontaneous fermentation
bDifferent batches give rise to quantitative differences in absolute values of parameters indicated above, but qualitative differences were maintained. For this reason, a representative example is shown
From data obtained and in accordance with results, J4 strain seems to be the best candidate to conduct alcoholic fermentations and produce Sherry base wine; it was chosen by the wine company to produce base Sherry white wine for the elaboration of commercial Sherry wine since 2010 vintage.
Discussion
In the last few decades, the tendency to apply commercial active dried yeasts in non-Sherry producing wineries has become widespread [17, 42, 44]. In addition, in recent times, the efforts are directed to select an appropriate indigenous yeast to perform alcoholic fermentation. Some exploratory studies in other different fortified wines are emerging [35], but to the best of our knowledge, none of those studies have been carried out in Sherry-producing cellars, although some Sherry-producing companies are investigating the use of selected strains of S. cerevisiae to conduct alcoholic fermentation. In “Fino” production, pale fermented and low acidity musts exhibit the best qualities. The mechanical harvest method applied over the last few decades partially favors the development of non-Saccharomyces yeasts that increase final acidity content, hence, the need to select a yeast starter capable of tolerating a fairly high concentration of sulphite, and in addition producing low volatile acidity final content and high acetaldehyde.
Five Saccharomyces strains from those isolated at the end of spontaneous fermentations in the Sherry winemaking area were selected and characterized in this study. The selection of yeasts at the end of spontaneous fermentations brings about isolate local strains that present high fermentative power and tolerance, well adjusted to the specific oenological practices of a region or a particular cellar and winemaking area [32]. The fermentative behavior in laboratory conditions of the isolates was appropriate, comparable to those of the reference strains. The J4 strain, capable of tolerating a fairly high concentration of sulphite, showed the lowest volatile acidity together with high acetaldehyde content, but since laboratory-scale experiences do not always mimic industrial scale, pilot and industrial fermentations were also performed. In fact, highly clarified must fermentation kinetics and metabolite synthesis are strongly affected by the experiment scale [9]. Although the Sherry must intended for “Fino” production is a highly clarified must, the candidates tested behaved as expected in non-sterile pilot fermentations and the results were promising.
The indigenous J4 strain was selected over the rest and was tested under industrial conditions; the inoculated strain was the most abundant yeast in the course of the vigorous fermentation (56–68 %), where metabolic activity is maximal, and so it became the principal alcoholic fermentation agent; flocculation may account for the low percentage of J4 at the end of fermentation. At the cellar where the study was conducted, musts can reach high temperatures (under 30 °C) before and during transfer to fermentation vats, thus allowing musts to partially ferment. For this reason, some natural microorganisms grow, and the inoculated strain has to coexist with other species. The development of Saccharomyces and non-Saccharomyces species at the beginning of fermentation could be desirable, as it provides complexity to the resulting wine [13, 39]. However, as data show, the J4 strain prevailed during vigorous fermentation process. Inoculum size and must state, together with strain competence abilities, can be essential to determine which species are predominant. Variations in inoculum protocol could modulate propagation of starter strains, as has been suggested [33]. In our experience, the volume of inoculum size was 8.3 % final volume in industrial fermentation, allowing the J4 strain to conduct alcoholic fermentation, apart from coexisting with natural microbiota, thus rendering a low volatile acidity and high acetaldehyde final content base wine. A similar percentage of J4 at the end of inoculated and spontaneous fermentations may be due to the fact that J4 is a resident strain of the cellar. This suggestion is based on two facts, namely its isolation from local spontaneous fermentation and its prevalence throughout several vintage fermentations.
Nevertheless, the ultimate goal for a Sherry base wine produced by Sherry wine manufacturers is biological aging, which is carried out under a natural “flor” velum formed on the wine surface by the so-called “flor” yeasts [24, 26]. All fermentative autochthonous yeasts tested in this work were tolerant to K2 toxin, and all but one were producers. Furthermore, viability of the J4 strain was 11–12 % after fortifying the wine to 15.5 % ethanol prior to aging (data not shown). The Sherry base wine elaborated with the selected strain is further subjected to the regular biological aging of Sherry wines. Stocks are under biological aging and results point to an excellent “flor” velum formation. Therefore, the killer phenotype in the selected fermentative strain does not interfere either with subsequent indigenous “flor” yeast development or with “flor” velum formation, which occurs at low temperature and pH, optimal for the toxin to be active, probably because some “flor” yeasts are K2 resistant [22] or because the toxin may not be active at such ethanol concentrations.
Desiccation tolerance of J4 and J5 indigenous strains has been tested too, and it has proved to be similar to that of commercial C2 strain. This fact opens the possibility to store and use indigenous strains as commercial dried yeasts.
The J4 strain is currently being used routinely to ferment Palomino musts with a commercial purpose.
Acknowledgments
This research was supported by CICYT projects AGL2006-03947, TRACE PET2008_0283, and Junta de Andalucía (PAI CVI-107 PO6-CVI-01546). There was financial support from Beam Global-Jerez de la Frontera (OG-127/06, OG-185/07). The authors thank the cellar and laboratory staff at Beam Global España S. L. for their kind help; they also thank A. M. Rincón for useful criticism.
References
Benítez T, Rincón AM, Codón AC (2011) Yeasts used in biologically aged wines. In: Carrascosa AV, Munoz D, González R (eds) Molecular wine microbiology. Elsevier, San Diego p 51–84
OIV (1990) Recueil des Methodes Internationales d’Analysis des Vins et des Mouts. Office Internationale Vigne Vin, Paris



