-
PDF
- Split View
-
Views
-
Cite
Cite
Shu Zhang, Yong-Jie Zhang, Xing-Zhong Liu, Hong Zhang, Dian-Sheng Liu, On the reliability of DNA sequences of Ophiocordyceps sinensis in public databases, Journal of Industrial Microbiology and Biotechnology, Volume 40, Issue 3-4, 1 April 2013, Pages 365–378, https://doi.org/10.1007/s10295-012-1228-4
Close - Share Icon Share
Abstract
Some DNA sequences in the International Nucleotide Sequence Databases (INSD) are erroneously annotated, which has lead to misleading conclusions in publications. Ophiocordyceps sinensis (syn. Cordyceps sinensis) is a fungus endemic to the Tibetan Plateau, and more than 100 populations covering almost its distribution area have been examined by us over recent years. In this study, using the data from authentic materials, we have evaluated the reliability of nucleotide sequences annotated as O. sinensis in the INSD. As of October 15, 2012, the INSD contained 874 records annotated as O. sinensis, including 555 records representing nuclear ribosomal DNA (63.5 %), 197 representing protein-coding genes (22.5 %), 92 representing random markers with unknown functions (10.5 %), and 30 representing microsatellite loci (3.5 %). Our analysis indicated that 39 of the 397 internal transcribed spacer entries, 27 of the 105 small subunit entries, and five of the 53 large subunit entries were incorrectly annotated as belonging to O. sinensis. For protein-coding sequences, all records of serine protease genes, the mating-type gene MAT1-2-1, the DNA lyase gene, the two largest subunits of RNA polymerase II, and elongation factor-1α gene were correct, while 14 of the 73 β-tubulin entries were indeterminate. Genetic diversity analyses using those sequences correctly identified as O. sinensis revealed significant genetic differentiation in the fungus although the extent of genetic differentiation varied with the gene. The relationship between O. sinensis and some other related fungal taxa is also discussed.
Shu Zhang and Yong-Jie Zhang contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s10295-012-1228-4) contains supplementary material, which is available to authorized users.
Introduction
International Nucleotide Sequence Databases (INSD) such as GenBank, EMBL, and DDBJ are critical resources for molecular biology, evolutionary biology, and ecology. There has been dramatic increase in nucleotide records in these databases [1, 2], in part because most scientific journals require that relevant sequences be submitted to one of the databases before manuscript submission. Quality control of the raw data, however, often depends solely on the submitters. When reading scientific journals, one often finds papers with erroneous conclusions based on DNA sequence data obtained from organisms that were misidentified. Evaluations of fungal nuclear ribosomal DNA (nrDNA) sequences indicated that ~20 % of the sequences in public databases may be unreliable [5, 33]. Annotation errors of INSD sequences were also reported in many other groups of organisms [21, 28–30, 37]. The most common causes of errors include misidentification or mislabeling of original materials, contamination by other organisms, or technical faults (e.g., PCR-generated chimeric sequences) [44]. Error in nucleotide records is a serious problem that threatens the utility of the sequence databases.
The current report concerns the reliability of DNA sequences reported for the fungus Ophiocordyceps sinensis (syn. Cordyceps sinensis). O. sinensis parasitizes and mummifies underground caterpillars within the family Hepialidae, and both the fungus and its host insects are endemic to alpine regions on the Tibetan Plateau. The joint fungus–insect structure resulting from fungal parasitism of insect larvae has been termed the “natural O. sinensis specimen” and is commonly called “dong chong xia cao (冬虫夏草)” in Chinese; the recommended term in English is currently “Chinese cordyceps” [61]. Chinese cordyceps has been widely used in traditional Chinese medicine for the treatment of asthma, bronchial and lung inflammations, and other diseases [10, 68]. Because the demand for Chinese cordyceps has increased but its distribution in nature is limited and large-scale cultivation of its sexual fruiting body has not been successful, the harvest of Chinese cordyceps in recent years has been heavy and is depleting this natural resource [59]. This is unfortunate because O. sinensis acts as a flagship species for its ecosystem and has been nominated as the national fungus of China [6, 61]. O. sinensis has also been listed as an “endangered species for protection” in China [61].
Because O. sinensis is important ecologically, economically, and medicinally [61], recent research has been conducted on its morphology [50, 55], anamorph determination [18, 48], mycelial fermentation [12], sexual reproduction [58], genetic differentiation [11, 23, 63], rapid detection [19, 66], pharmacology [10], chemical components [14, 20], investigation of natural resources [22, 51], artificial cultivation [53], associated microbial community [65], and insect hosts [47]. As a result, many sequences annotated as O. sinensis are available in the INSD, but some records contain random as well as systematic errors [41]. During literature searches, we often came across papers with unexpected results. For example, some papers reported that O. sinensis was obtained from soils other than the Tibetan Plateau [3, 8, 31], from foxing spots on old paper artifacts [36], and from plants [25, 39]. Because O. sinensis is a specialized parasite of insects, we suspect that the fungi in these reports were misidentified. In addition, some sequences were obtained from Chinese cordyceps or from fungal isolates recovered from Chinese cordyceps, but they actually represent other fungi that were associated with the parasitized insects [13, 17]. The existence of significant genetic variation among O. sinensis isolates [63] makes the situation even more complex. Therefore, it is vital to verify the reliability of all sequences annotated as O. sinensis in the INSD.
Over recent years, we have collected and evaluated the DNA sequences from more than 100 populations of O. sinensis, covering almost its entire distribution. These data represent a useful basis for evaluating the reliability of DNA sequences annotated as O. sinensis. The four aims of the work are: to summarize sequences annotated as O. sinensis in the INSD as of October 15, 2012; to determine the reliability of these INSD sequences; to explore the intraspecific genetic variations of O. sinensis based on correct INSD sequences; and finally to elucidate the relationship between O. sinensis and some other related fungal taxa.
Materials and methods
Sequence download and arrangement
Nucleotide sequences of O. sinensis in the INSD were obtained by searching with the keyword “O. sinensis” in GenBank (http://www.ncbi.nlm.nih.gov/). These sequences were recorded according to type of genes, submitter, country affiliation of submitters, submission year, publication status, etc. All sequences representing the same gene were compiled as an individual file in fasta format.
Amplification of O. sinensis genes from authentic cultures
Five Chinese cordyceps samples were collected from different, widely separated regions on the Tibetan Plateau, and one isolate tentatively identified as O. sinensis was obtained from each sample (Table 1). Based on morphological analysis and sequence analysis of the internal transcribed spacer (ITS) region of nrDNA, the identity of these isolates as O. sinensis was confirmed. These five isolates were cultivated as described previously [62], and fresh mycelia cultivated on cellophane papers were used for genomic DNA extraction with the cetyltrimethylammonium bromide-based approach [64].
Ophiocordyceps sinensis isolates used in this study and their GenBank accession numbers for various gene fragments
| Strain . | Origin . | Latitude (north) . | Longitude (east) . | GenBank accession no. . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS . | nrSSU . | nrLSU . | tub . | tef1 . | rpb1 . | rpb2 . | ||||
| QH09-201 | Gonghe, Hainan, Qinghai, China | 36.43 | 99.47 | JQ325080 | JX968024 | JX968029 | JX968019 | JX968014 | JX968004 | JX968009 |
| QH06-197 | Yushu, Yushu, Qinghai, China | 32.58 | 96.51 | FJ654228a | JX968025 | JX968030 | JX968020 | JX968015 | JX968005 | JX968010 |
| XZ06-44 | Gyalsa, Lhoka, Tibet, China | 29.29 | 92.72 | FJ654218a | JX968026 | JX968031 | JX968021 | JX968016 | JX968006 | JX968011 |
| YN07-8 | Deqin, Diqing, Yunnan, China | 28.34 | 99.07 | FJ654237a | JX968027 | JX968032 | JX968022 | JX968017 | JX968007 | JX968012 |
| YN09-64 | Lanping, Nujiang, Yunnan, China | 26.37 | 99.43 | JQ325141 | JX968028 | JX968033 | JX968023 | JX968018 | JX968008 | JX968013 |
| Strain . | Origin . | Latitude (north) . | Longitude (east) . | GenBank accession no. . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS . | nrSSU . | nrLSU . | tub . | tef1 . | rpb1 . | rpb2 . | ||||
| QH09-201 | Gonghe, Hainan, Qinghai, China | 36.43 | 99.47 | JQ325080 | JX968024 | JX968029 | JX968019 | JX968014 | JX968004 | JX968009 |
| QH06-197 | Yushu, Yushu, Qinghai, China | 32.58 | 96.51 | FJ654228a | JX968025 | JX968030 | JX968020 | JX968015 | JX968005 | JX968010 |
| XZ06-44 | Gyalsa, Lhoka, Tibet, China | 29.29 | 92.72 | FJ654218a | JX968026 | JX968031 | JX968021 | JX968016 | JX968006 | JX968011 |
| YN07-8 | Deqin, Diqing, Yunnan, China | 28.34 | 99.07 | FJ654237a | JX968027 | JX968032 | JX968022 | JX968017 | JX968007 | JX968012 |
| YN09-64 | Lanping, Nujiang, Yunnan, China | 26.37 | 99.43 | JQ325141 | JX968028 | JX968033 | JX968023 | JX968018 | JX968008 | JX968013 |
aThese accession numbers are sequences from Zhang et al. [63]. Other sequences were reported in this study
Ophiocordyceps sinensis isolates used in this study and their GenBank accession numbers for various gene fragments
| Strain . | Origin . | Latitude (north) . | Longitude (east) . | GenBank accession no. . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS . | nrSSU . | nrLSU . | tub . | tef1 . | rpb1 . | rpb2 . | ||||
| QH09-201 | Gonghe, Hainan, Qinghai, China | 36.43 | 99.47 | JQ325080 | JX968024 | JX968029 | JX968019 | JX968014 | JX968004 | JX968009 |
| QH06-197 | Yushu, Yushu, Qinghai, China | 32.58 | 96.51 | FJ654228a | JX968025 | JX968030 | JX968020 | JX968015 | JX968005 | JX968010 |
| XZ06-44 | Gyalsa, Lhoka, Tibet, China | 29.29 | 92.72 | FJ654218a | JX968026 | JX968031 | JX968021 | JX968016 | JX968006 | JX968011 |
| YN07-8 | Deqin, Diqing, Yunnan, China | 28.34 | 99.07 | FJ654237a | JX968027 | JX968032 | JX968022 | JX968017 | JX968007 | JX968012 |
| YN09-64 | Lanping, Nujiang, Yunnan, China | 26.37 | 99.43 | JQ325141 | JX968028 | JX968033 | JX968023 | JX968018 | JX968008 | JX968013 |
| Strain . | Origin . | Latitude (north) . | Longitude (east) . | GenBank accession no. . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS . | nrSSU . | nrLSU . | tub . | tef1 . | rpb1 . | rpb2 . | ||||
| QH09-201 | Gonghe, Hainan, Qinghai, China | 36.43 | 99.47 | JQ325080 | JX968024 | JX968029 | JX968019 | JX968014 | JX968004 | JX968009 |
| QH06-197 | Yushu, Yushu, Qinghai, China | 32.58 | 96.51 | FJ654228a | JX968025 | JX968030 | JX968020 | JX968015 | JX968005 | JX968010 |
| XZ06-44 | Gyalsa, Lhoka, Tibet, China | 29.29 | 92.72 | FJ654218a | JX968026 | JX968031 | JX968021 | JX968016 | JX968006 | JX968011 |
| YN07-8 | Deqin, Diqing, Yunnan, China | 28.34 | 99.07 | FJ654237a | JX968027 | JX968032 | JX968022 | JX968017 | JX968007 | JX968012 |
| YN09-64 | Lanping, Nujiang, Yunnan, China | 26.37 | 99.43 | JQ325141 | JX968028 | JX968033 | JX968023 | JX968018 | JX968008 | JX968013 |
aThese accession numbers are sequences from Zhang et al. [63]. Other sequences were reported in this study
DNA fragments of nrDNA ITS, the large and small subunits (nrLSU and nrSSU) of nrDNA, two largest subunits of RNA polymerase II (rpb1 and rpb2), elongation factor-1α (tef1), and β-tubulin (tub) were amplified from the five O. sinensis isolates (Table 1). Primers and annealing temperatures used in this study are listed in Table 2. PCR was performed in a TGRADIENT thermocycler (Biometra, Göttingen, Germany). After confirmation of the PCR products by agarose gel electrophoresis, the fragments were cleaned with the 3S Spin PCR Product Purification Kit (Biocolor Bioscience & Technology Company, China). The purified PCR products of nrDNA ITS, nrSSU, nrLSU, and tub were directly sequenced with PCR primers using an ABI 3730 XL DNA sequencer with BigDye 3.1 Terminators (Applied Biosystems, Carlsbad, CA, USA). PCR products of rpb1, rpb2, and tef1 were inserted into the plasmid pMD18-T (TaKaRa, Japan) and then transformed into Escherichia coli DH5α before being sequenced using the primers M13-47 (5′-CGCCAGGGTTTTCCCAGTCACGAC-3′) and/or RV-M (5′-GAGCGGATAACAATTTCACACAGG-3′). The obtained sequences were used as “reference sequences” to verify the reliability of INSD sequences as described in the next subsection.
Primers for amplification of O. sinensis genes
| Gene fragment . | Primer name . | Primer sequence (5′–3′) . | Directiona . | Annealing temperature (°C)b . | Expected size of amplified fragments (bp) . | Reference . |
|---|---|---|---|---|---|---|
| nrDNA ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | F | 54 | ~550 | [45] |
| ITS4 | TCCTCCGCTTATTGATATGC | R | ||||
| nrSSU | NS1 | GTAGTCATATGCTTGTCTC | F | 50 | ~1,100 | [45] |
| NS4 | CTTCCGTCAATTCCTTTAAG | R | ||||
| SR9R | YAGAGGTGAAATTCT | F | 42 | ~850 | Vilgalys labc | |
| SR6 | TGTTACGACTTTTACTT | R | ||||
| nrLSU | LR0R | ACCCGCTGAACTTAAGC | F | 51 | ~1,100 | Vilgalys lab |
| LR6 | CGCCAGTTCTGCTTACC | R | ||||
| LR17R | TAACCTATTCTCAAACTT | F | 51 → 42 | ~1,100 | Vilgalys lab | |
| LR9 | AGAGCACTGGGCAGAAA | R | ||||
| tub | T1 | AACATGCGTGAGATTGTAAGT | F | 55 → 51 | ~1,600 | [34] |
| T22 | TCTGGATGTTGTTGGGAATCC | R | ||||
| tef1 | 983F | GCYCCYGGHCAYCGTGAYTTYAT | F | 65 → 57 | ~1,100 | [42] |
| 2218R | ATGACACCRACRGCRACRGTYTG | R | ||||
| rpb1 | CRPB1 | CCWGGYTTYATCAAGAARGT | F | 58 → 52 | ~700 | [7] |
| RPB1Cr | CCNGCDATNTCRTTRTCCATRTA | R | ||||
| rpb2 | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | F | 58 → 52 | ~1,200 | [26] |
| fRPB2-7cR | CCCATRGCTTGTYYRCCCAT | R |
| Gene fragment . | Primer name . | Primer sequence (5′–3′) . | Directiona . | Annealing temperature (°C)b . | Expected size of amplified fragments (bp) . | Reference . |
|---|---|---|---|---|---|---|
| nrDNA ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | F | 54 | ~550 | [45] |
| ITS4 | TCCTCCGCTTATTGATATGC | R | ||||
| nrSSU | NS1 | GTAGTCATATGCTTGTCTC | F | 50 | ~1,100 | [45] |
| NS4 | CTTCCGTCAATTCCTTTAAG | R | ||||
| SR9R | YAGAGGTGAAATTCT | F | 42 | ~850 | Vilgalys labc | |
| SR6 | TGTTACGACTTTTACTT | R | ||||
| nrLSU | LR0R | ACCCGCTGAACTTAAGC | F | 51 | ~1,100 | Vilgalys lab |
| LR6 | CGCCAGTTCTGCTTACC | R | ||||
| LR17R | TAACCTATTCTCAAACTT | F | 51 → 42 | ~1,100 | Vilgalys lab | |
| LR9 | AGAGCACTGGGCAGAAA | R | ||||
| tub | T1 | AACATGCGTGAGATTGTAAGT | F | 55 → 51 | ~1,600 | [34] |
| T22 | TCTGGATGTTGTTGGGAATCC | R | ||||
| tef1 | 983F | GCYCCYGGHCAYCGTGAYTTYAT | F | 65 → 57 | ~1,100 | [42] |
| 2218R | ATGACACCRACRGCRACRGTYTG | R | ||||
| rpb1 | CRPB1 | CCWGGYTTYATCAAGAARGT | F | 58 → 52 | ~700 | [7] |
| RPB1Cr | CCNGCDATNTCRTTRTCCATRTA | R | ||||
| rpb2 | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | F | 58 → 52 | ~1,200 | [26] |
| fRPB2-7cR | CCCATRGCTTGTYYRCCCAT | R |
a F forward, R reverse
bFor fragments with two separate annealing temperatures indicated by a right-directed arrow, PCR was first performed at a higher annealing temperature for five cycles, then at a lower annealing temperature for the remaining 30 cycles. For other fragments, PCR was performed at a constant annealing temperature for 35 cycles
cVilgalys R Conserved primer sequences for PCR amplification and sequencing from nuclear ribosomal RNA. http://www.biology.duke.edu/fungi/mycolab/primers.htm
Primers for amplification of O. sinensis genes
| Gene fragment . | Primer name . | Primer sequence (5′–3′) . | Directiona . | Annealing temperature (°C)b . | Expected size of amplified fragments (bp) . | Reference . |
|---|---|---|---|---|---|---|
| nrDNA ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | F | 54 | ~550 | [45] |
| ITS4 | TCCTCCGCTTATTGATATGC | R | ||||
| nrSSU | NS1 | GTAGTCATATGCTTGTCTC | F | 50 | ~1,100 | [45] |
| NS4 | CTTCCGTCAATTCCTTTAAG | R | ||||
| SR9R | YAGAGGTGAAATTCT | F | 42 | ~850 | Vilgalys labc | |
| SR6 | TGTTACGACTTTTACTT | R | ||||
| nrLSU | LR0R | ACCCGCTGAACTTAAGC | F | 51 | ~1,100 | Vilgalys lab |
| LR6 | CGCCAGTTCTGCTTACC | R | ||||
| LR17R | TAACCTATTCTCAAACTT | F | 51 → 42 | ~1,100 | Vilgalys lab | |
| LR9 | AGAGCACTGGGCAGAAA | R | ||||
| tub | T1 | AACATGCGTGAGATTGTAAGT | F | 55 → 51 | ~1,600 | [34] |
| T22 | TCTGGATGTTGTTGGGAATCC | R | ||||
| tef1 | 983F | GCYCCYGGHCAYCGTGAYTTYAT | F | 65 → 57 | ~1,100 | [42] |
| 2218R | ATGACACCRACRGCRACRGTYTG | R | ||||
| rpb1 | CRPB1 | CCWGGYTTYATCAAGAARGT | F | 58 → 52 | ~700 | [7] |
| RPB1Cr | CCNGCDATNTCRTTRTCCATRTA | R | ||||
| rpb2 | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | F | 58 → 52 | ~1,200 | [26] |
| fRPB2-7cR | CCCATRGCTTGTYYRCCCAT | R |
| Gene fragment . | Primer name . | Primer sequence (5′–3′) . | Directiona . | Annealing temperature (°C)b . | Expected size of amplified fragments (bp) . | Reference . |
|---|---|---|---|---|---|---|
| nrDNA ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | F | 54 | ~550 | [45] |
| ITS4 | TCCTCCGCTTATTGATATGC | R | ||||
| nrSSU | NS1 | GTAGTCATATGCTTGTCTC | F | 50 | ~1,100 | [45] |
| NS4 | CTTCCGTCAATTCCTTTAAG | R | ||||
| SR9R | YAGAGGTGAAATTCT | F | 42 | ~850 | Vilgalys labc | |
| SR6 | TGTTACGACTTTTACTT | R | ||||
| nrLSU | LR0R | ACCCGCTGAACTTAAGC | F | 51 | ~1,100 | Vilgalys lab |
| LR6 | CGCCAGTTCTGCTTACC | R | ||||
| LR17R | TAACCTATTCTCAAACTT | F | 51 → 42 | ~1,100 | Vilgalys lab | |
| LR9 | AGAGCACTGGGCAGAAA | R | ||||
| tub | T1 | AACATGCGTGAGATTGTAAGT | F | 55 → 51 | ~1,600 | [34] |
| T22 | TCTGGATGTTGTTGGGAATCC | R | ||||
| tef1 | 983F | GCYCCYGGHCAYCGTGAYTTYAT | F | 65 → 57 | ~1,100 | [42] |
| 2218R | ATGACACCRACRGCRACRGTYTG | R | ||||
| rpb1 | CRPB1 | CCWGGYTTYATCAAGAARGT | F | 58 → 52 | ~700 | [7] |
| RPB1Cr | CCNGCDATNTCRTTRTCCATRTA | R | ||||
| rpb2 | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | F | 58 → 52 | ~1,200 | [26] |
| fRPB2-7cR | CCCATRGCTTGTYYRCCCAT | R |
a F forward, R reverse
bFor fragments with two separate annealing temperatures indicated by a right-directed arrow, PCR was first performed at a higher annealing temperature for five cycles, then at a lower annealing temperature for the remaining 30 cycles. For other fragments, PCR was performed at a constant annealing temperature for 35 cycles
cVilgalys R Conserved primer sequences for PCR amplification and sequencing from nuclear ribosomal RNA. http://www.biology.duke.edu/fungi/mycolab/primers.htm
Reliability analysis of nucleotide records in the INSD
The reliability of O. sinensis sequences in the INSD was examined by two approaches. In the first and most important approach, genetic distances were compared after they were calculated with the Kimura 2-parameter (K2P) model as implemented in MEGA version 5 [43]. In this approach, the K2P distances among the five “reference sequences” for each of the seven genes were first calculated, and the criteria that would be used to evaluate the reliability of INSD sequences were determined; these criteria were generally the maximum value for the K2P distances for each gene (Table 3). Then, for each of the seven genes, the K2P distances between INSD sequences and the five “reference sequences” were calculated and compared with the criteria values. Those INSD entries with distance values smaller than or equal to the criteria values with at least three of the “reference sequences” for each gene were directly considered as correct O. sinensis sequences. Otherwise, the INSD sequences were subjected to further analyses by the second approach. In the second approach, BLAST analyses were performed against the nucleotide database or against the non-redundant protein sequence database at NCBI. If the BLAST result for an INSD entry reported strong hits with non-O. sinensis fungi, that entry was regarded as incorrect. If the BLAST result for an INSD entry reported strong hits with correctly identified O. sinensis sequences, that entry was generally regarded as indeterminate. One exception was for ITS sequences; an ITS entry was also regarded as incorrect if the identity values with correct sequences were below 90 %, an identity threshold largely beyond a fungal species in most cases [32]. In addition, if different parts (>100 bp for each part) of an INSD entry had significant hits with different fungal taxa, that entry was regarded as chimeric.
Nucleotide variations among reference sequences from Ophiocordyceps sinensis and the criteria used for verifying the INSD sequences
| Gene . | No. isolates . | Length (bp) . | No. variable sites . | No. indel sites . | Mean K2P . | Max. K2P . | Criterion . |
|---|---|---|---|---|---|---|---|
| nrDNA ITS | 5 | 538 | 21 | 3 | 0.0186 | 0.0306 | 0.0430a |
| nrSSU | 5 | 1,666 | 11 | 0 | 0.0028 | 0.0048 | 0.0048 |
| nrLSU | 5 | 2,050 | 11 | 0 | 0.0026 | 0.0039 | 0.0039 |
| tub | 5 | 1,339 | 22 | 11 | 0.0074 | 0.0129 | 0.0129 |
| tef1 | 5 | 988 | 22 | 0 | 0.0095 | 0.0185 | 0.0185 |
| rpb1 | 5 | 717 | 15 | 0 | 0.0085 | 0.0199 | 0.0199 |
| rpb2 | 5 | 1,194 | 15 | 0 | 0.0052 | 0.0093 | 0.0093 |
| Gene . | No. isolates . | Length (bp) . | No. variable sites . | No. indel sites . | Mean K2P . | Max. K2P . | Criterion . |
|---|---|---|---|---|---|---|---|
| nrDNA ITS | 5 | 538 | 21 | 3 | 0.0186 | 0.0306 | 0.0430a |
| nrSSU | 5 | 1,666 | 11 | 0 | 0.0028 | 0.0048 | 0.0048 |
| nrLSU | 5 | 2,050 | 11 | 0 | 0.0026 | 0.0039 | 0.0039 |
| tub | 5 | 1,339 | 22 | 11 | 0.0074 | 0.0129 | 0.0129 |
| tef1 | 5 | 988 | 22 | 0 | 0.0095 | 0.0185 | 0.0185 |
| rpb1 | 5 | 717 | 15 | 0 | 0.0085 | 0.0199 | 0.0199 |
| rpb2 | 5 | 1,194 | 15 | 0 | 0.0052 | 0.0093 | 0.0093 |
aThe criterion for nrDNA ITS is based on Zhang et al. [63]; that paper reported a K2P value of 0.043 based on ITS sequences from 56 O. sinensis isolates. This value is also correct when more than 100 O. sinensis isolates from different areas were evaluated in our laboratory
Nucleotide variations among reference sequences from Ophiocordyceps sinensis and the criteria used for verifying the INSD sequences
| Gene . | No. isolates . | Length (bp) . | No. variable sites . | No. indel sites . | Mean K2P . | Max. K2P . | Criterion . |
|---|---|---|---|---|---|---|---|
| nrDNA ITS | 5 | 538 | 21 | 3 | 0.0186 | 0.0306 | 0.0430a |
| nrSSU | 5 | 1,666 | 11 | 0 | 0.0028 | 0.0048 | 0.0048 |
| nrLSU | 5 | 2,050 | 11 | 0 | 0.0026 | 0.0039 | 0.0039 |
| tub | 5 | 1,339 | 22 | 11 | 0.0074 | 0.0129 | 0.0129 |
| tef1 | 5 | 988 | 22 | 0 | 0.0095 | 0.0185 | 0.0185 |
| rpb1 | 5 | 717 | 15 | 0 | 0.0085 | 0.0199 | 0.0199 |
| rpb2 | 5 | 1,194 | 15 | 0 | 0.0052 | 0.0093 | 0.0093 |
| Gene . | No. isolates . | Length (bp) . | No. variable sites . | No. indel sites . | Mean K2P . | Max. K2P . | Criterion . |
|---|---|---|---|---|---|---|---|
| nrDNA ITS | 5 | 538 | 21 | 3 | 0.0186 | 0.0306 | 0.0430a |
| nrSSU | 5 | 1,666 | 11 | 0 | 0.0028 | 0.0048 | 0.0048 |
| nrLSU | 5 | 2,050 | 11 | 0 | 0.0026 | 0.0039 | 0.0039 |
| tub | 5 | 1,339 | 22 | 11 | 0.0074 | 0.0129 | 0.0129 |
| tef1 | 5 | 988 | 22 | 0 | 0.0095 | 0.0185 | 0.0185 |
| rpb1 | 5 | 717 | 15 | 0 | 0.0085 | 0.0199 | 0.0199 |
| rpb2 | 5 | 1,194 | 15 | 0 | 0.0052 | 0.0093 | 0.0093 |
aThe criterion for nrDNA ITS is based on Zhang et al. [63]; that paper reported a K2P value of 0.043 based on ITS sequences from 56 O. sinensis isolates. This value is also correct when more than 100 O. sinensis isolates from different areas were evaluated in our laboratory
Genetic diversity in O. sinensis
Intraspecific genetic diversity of O. sinensis was investigated based on the sequences of each of the seven genes in the INSD that were herein determined to be correctly identified as belonging to O. sinensis (see section “Results”). Numbers of variable nucleotide sites and alleles were calculated with DnaSP version 5.10 [24]. K2P distance values were calculated as described above.
Genetic difference between O. sinensis and related fungi
Nucleotide sequences of O. sinensis-related taxa were downloaded from the INSD. These included O. robertsii, O. nepalensis, O. multiaxialis, O. crassispora, O. gansuensis, and groups B and C identified by Stensrud et al. [41]. K2P values between O. sinensis reference sequences and these related taxa were calculated as described above.
Phylogenetic analysis
Two different sequence alignments were constructed based on (1) the ITS1-5.8S-ITS2 region (521 characters), and (2) the 5.8S coding region alone (153 characters). Each sequence alignment included 61 ITS sequences of O. sinensis submitted by our research group and 33 (for the former alignment) or 37 (for the latter alignment) INSD entries that were erroneously accessioned. Minimum evolutionary phylogenetic analyses were conducted with the K2P model using MEGA version 5 [43]. The validity of the phylogenetic relationships was assessed by bootstrap tests with 1,000 resamplings.
Nucleotide sequence accession numbers
The nucleotide sequence data reported in this paper were deposited in GenBank under accession numbers JX968004 to JX968033 (Table 1).
Results
Statistics of INSD records
With “O. sinensis” as the keyword query, 874 DNA entries were retrieved from the INSD on October 15, 2012. In addition, there were 254 genome survey sequences of the fungus in GenBank submitted by our research group; they all originated from a shotgun genomic library constructed using the O. sinensis isolate YN07-8 [60] and were not analyzed in the following sections. The 874 DNA entries include sequences of nrDNA (ITS, nrLSU, and nrSSU), the mating-type gene MAT1-2-1, the DNA lyase gene, serine protease genes (csp1 and csp2), rpb1, rpb2, tef1, tub, random marker sequences (OSRCs) with unknown functions, and microsatellite loci. Sequences of nrDNA (555 entries) accounted for 63.5 % of the entries, and ITS sequences (397 entries) accounted for 45.4 % of the entries or 71.5 % of nrDNA entries (Table 4). For protein-coding genes, MAT1-2-1 and tub had the largest number of entries (>60 entries) while csp1, csp2, and rpb2 had only 1–2 entries (Table 4). The lengths of sequences of each individual gene in the INSD varied greatly, especially those of ITS, nrSSU, nrLSU, and tub (Table 4).
Statistics of genes annotated as O. sinensis in the INSD
| Gene . | No. INSD entries . | Percentage (%) . | Accession no., author, and year of the first entry in INSD . | Length (bp) . |
|---|---|---|---|---|
| nrDNA ITS | 397 | 45.4 | AF056582, Kang (1998) (AF122027, Chao and Li 1999)a | 261–687b |
| nrSSU | 105 | 12.0 | D86053, Ito and Hirano (1996) (AJ007566, Chen and Hseu 1997)a | 260–1,791 |
| nrLSU | 53 | 6.1 | AB067704, Kinjo (2001) | 461–3,315 |
| tub | 73 | 8.4 | HM804956, Pandey et al. (2010) | 433–1,201 |
| tef1 | 31 | 3.5 | EF468767, Sung et al. (2007) | 533–674 |
| rpb1 | 29 | 3.3 | EF468874, Sung et al. (2007) | 521–703 |
| rpb2 | 2 | 0.2 | EF468924, Sung et al. (2007) | 890–1,040 |
| MAT1-2-1 | 60 | 6.9 | FJ654150, Zhang et al. (2009) | 877–882 (7,996)c |
| DNA lyase gene | 1d | – | HM212637, Zhang et al. (2010) | 898 |
| csp1 | 1 | 0.1 | EU282382, Zhang et al. (2007) | 2,143 |
| csp2 | 1 | 0.1 | EU282383, Zhang et al. (2007) | 2,566 |
| OSRC1 | 4 | 0.5 | JQ277357, Zhang et al. (2012) | 604 |
| OSRC2 | 4 | 0.5 | JQ277361, Zhang et al. (2012) | 665 |
| OSRC3 | 4 | 0.5 | JQ277365, Zhang et al. (2012) | 570–573 |
| OSRC4 | 4 | 0.5 | JQ277369, Zhang et al. (2012) | 717 |
| OSRC7 | 4 | 0.5 | JQ277373, Zhang et al. (2012) | 461–464 |
| OSRC9 | 4 | 0.5 | JQ277377, Zhang et al. (2012) | 377 |
| OSRC11 | 4 | 0.5 | JQ277381, Zhang et al. (2012) | 471–478 |
| OSRC13 | 4 | 0.5 | JQ277385, Zhang et al. (2012) | 584–588 |
| OSRC14 | 4 | 0.5 | JQ277389, Zhang et al. (2012) | 577–590 |
| OSRC16 | 4 | 0.5 | JQ277393, Zhang et al. (2012) | 513 |
| OSRC17 | 4 | 0.5 | JQ277397, Zhang et al. (2012) | 480 |
| OSRC18 | 4 | 0.5 | JQ277401, Zhang et al. (2012) | 378 |
| OSRC19 | 4 | 0.5 | JQ277405, Zhang et al. (2012) | 525 |
| OSRC21 | 4 | 0.5 | JQ277409, Zhang et al. (2012) | 455 |
| OSRC22 | 4 | 0.5 | JQ277413, Zhang et al. (2012) | 485 |
| OSRC23 | 4 | 0.5 | JQ277417, Zhang et al. (2012) | 716–721 |
| OSRC24 | 4 | 0.5 | JQ277421, Zhang et al. (2012) | 534 |
| OSRC25 | 4 | 0.5 | JQ277425, Zhang et al. (2012) | 563–564 |
| OSRC26 | 4 | 0.5 | JQ277429, Zhang et al. (2012) | 559 |
| OSRC27 | 4 | 0.5 | JQ277433, Zhang et al. (2012) | 572–575 |
| OSRC28 | 4 | 0.5 | JQ277437, Zhang et al. (2012) | 723 |
| OSRC31 | 4 | 0.5 | JQ277441, Zhang et al. (2012) | 400 |
| OSRC32 | 4 | 0.5 | JQ277445, Zhang et al. (2012) | 614–638 |
| Microsatellites | 30 | 3.4 | JF781128, Wang et al. (2012) | 56–204 |
| Total | 874 |
| Gene . | No. INSD entries . | Percentage (%) . | Accession no., author, and year of the first entry in INSD . | Length (bp) . |
|---|---|---|---|---|
| nrDNA ITS | 397 | 45.4 | AF056582, Kang (1998) (AF122027, Chao and Li 1999)a | 261–687b |
| nrSSU | 105 | 12.0 | D86053, Ito and Hirano (1996) (AJ007566, Chen and Hseu 1997)a | 260–1,791 |
| nrLSU | 53 | 6.1 | AB067704, Kinjo (2001) | 461–3,315 |
| tub | 73 | 8.4 | HM804956, Pandey et al. (2010) | 433–1,201 |
| tef1 | 31 | 3.5 | EF468767, Sung et al. (2007) | 533–674 |
| rpb1 | 29 | 3.3 | EF468874, Sung et al. (2007) | 521–703 |
| rpb2 | 2 | 0.2 | EF468924, Sung et al. (2007) | 890–1,040 |
| MAT1-2-1 | 60 | 6.9 | FJ654150, Zhang et al. (2009) | 877–882 (7,996)c |
| DNA lyase gene | 1d | – | HM212637, Zhang et al. (2010) | 898 |
| csp1 | 1 | 0.1 | EU282382, Zhang et al. (2007) | 2,143 |
| csp2 | 1 | 0.1 | EU282383, Zhang et al. (2007) | 2,566 |
| OSRC1 | 4 | 0.5 | JQ277357, Zhang et al. (2012) | 604 |
| OSRC2 | 4 | 0.5 | JQ277361, Zhang et al. (2012) | 665 |
| OSRC3 | 4 | 0.5 | JQ277365, Zhang et al. (2012) | 570–573 |
| OSRC4 | 4 | 0.5 | JQ277369, Zhang et al. (2012) | 717 |
| OSRC7 | 4 | 0.5 | JQ277373, Zhang et al. (2012) | 461–464 |
| OSRC9 | 4 | 0.5 | JQ277377, Zhang et al. (2012) | 377 |
| OSRC11 | 4 | 0.5 | JQ277381, Zhang et al. (2012) | 471–478 |
| OSRC13 | 4 | 0.5 | JQ277385, Zhang et al. (2012) | 584–588 |
| OSRC14 | 4 | 0.5 | JQ277389, Zhang et al. (2012) | 577–590 |
| OSRC16 | 4 | 0.5 | JQ277393, Zhang et al. (2012) | 513 |
| OSRC17 | 4 | 0.5 | JQ277397, Zhang et al. (2012) | 480 |
| OSRC18 | 4 | 0.5 | JQ277401, Zhang et al. (2012) | 378 |
| OSRC19 | 4 | 0.5 | JQ277405, Zhang et al. (2012) | 525 |
| OSRC21 | 4 | 0.5 | JQ277409, Zhang et al. (2012) | 455 |
| OSRC22 | 4 | 0.5 | JQ277413, Zhang et al. (2012) | 485 |
| OSRC23 | 4 | 0.5 | JQ277417, Zhang et al. (2012) | 716–721 |
| OSRC24 | 4 | 0.5 | JQ277421, Zhang et al. (2012) | 534 |
| OSRC25 | 4 | 0.5 | JQ277425, Zhang et al. (2012) | 563–564 |
| OSRC26 | 4 | 0.5 | JQ277429, Zhang et al. (2012) | 559 |
| OSRC27 | 4 | 0.5 | JQ277433, Zhang et al. (2012) | 572–575 |
| OSRC28 | 4 | 0.5 | JQ277437, Zhang et al. (2012) | 723 |
| OSRC31 | 4 | 0.5 | JQ277441, Zhang et al. (2012) | 400 |
| OSRC32 | 4 | 0.5 | JQ277445, Zhang et al. (2012) | 614–638 |
| Microsatellites | 30 | 3.4 | JF781128, Wang et al. (2012) | 56–204 |
| Total | 874 |
aThe first records of nrDNA ITS and nrSSU in the INSD were erroneously annotated. The earliest correct ones are in parentheses
bPartial sequences of nrLSU and nrSSU were included in some nrDNA ITS entries. The actual length of the ITS region (including ITS1, 5.8S, and ITS2) was ~500 bp
cFifty-nine of MAT1-2-1 entries were 877–882 bp long except for HM212637, which was 6,996 bp long because both flanking sequences were included in this entry
dThe sequence of the DNA lyase gene was embedded in a MAT1-2-1 entry, and they share the accession no. of HM212637
Statistics of genes annotated as O. sinensis in the INSD
| Gene . | No. INSD entries . | Percentage (%) . | Accession no., author, and year of the first entry in INSD . | Length (bp) . |
|---|---|---|---|---|
| nrDNA ITS | 397 | 45.4 | AF056582, Kang (1998) (AF122027, Chao and Li 1999)a | 261–687b |
| nrSSU | 105 | 12.0 | D86053, Ito and Hirano (1996) (AJ007566, Chen and Hseu 1997)a | 260–1,791 |
| nrLSU | 53 | 6.1 | AB067704, Kinjo (2001) | 461–3,315 |
| tub | 73 | 8.4 | HM804956, Pandey et al. (2010) | 433–1,201 |
| tef1 | 31 | 3.5 | EF468767, Sung et al. (2007) | 533–674 |
| rpb1 | 29 | 3.3 | EF468874, Sung et al. (2007) | 521–703 |
| rpb2 | 2 | 0.2 | EF468924, Sung et al. (2007) | 890–1,040 |
| MAT1-2-1 | 60 | 6.9 | FJ654150, Zhang et al. (2009) | 877–882 (7,996)c |
| DNA lyase gene | 1d | – | HM212637, Zhang et al. (2010) | 898 |
| csp1 | 1 | 0.1 | EU282382, Zhang et al. (2007) | 2,143 |
| csp2 | 1 | 0.1 | EU282383, Zhang et al. (2007) | 2,566 |
| OSRC1 | 4 | 0.5 | JQ277357, Zhang et al. (2012) | 604 |
| OSRC2 | 4 | 0.5 | JQ277361, Zhang et al. (2012) | 665 |
| OSRC3 | 4 | 0.5 | JQ277365, Zhang et al. (2012) | 570–573 |
| OSRC4 | 4 | 0.5 | JQ277369, Zhang et al. (2012) | 717 |
| OSRC7 | 4 | 0.5 | JQ277373, Zhang et al. (2012) | 461–464 |
| OSRC9 | 4 | 0.5 | JQ277377, Zhang et al. (2012) | 377 |
| OSRC11 | 4 | 0.5 | JQ277381, Zhang et al. (2012) | 471–478 |
| OSRC13 | 4 | 0.5 | JQ277385, Zhang et al. (2012) | 584–588 |
| OSRC14 | 4 | 0.5 | JQ277389, Zhang et al. (2012) | 577–590 |
| OSRC16 | 4 | 0.5 | JQ277393, Zhang et al. (2012) | 513 |
| OSRC17 | 4 | 0.5 | JQ277397, Zhang et al. (2012) | 480 |
| OSRC18 | 4 | 0.5 | JQ277401, Zhang et al. (2012) | 378 |
| OSRC19 | 4 | 0.5 | JQ277405, Zhang et al. (2012) | 525 |
| OSRC21 | 4 | 0.5 | JQ277409, Zhang et al. (2012) | 455 |
| OSRC22 | 4 | 0.5 | JQ277413, Zhang et al. (2012) | 485 |
| OSRC23 | 4 | 0.5 | JQ277417, Zhang et al. (2012) | 716–721 |
| OSRC24 | 4 | 0.5 | JQ277421, Zhang et al. (2012) | 534 |
| OSRC25 | 4 | 0.5 | JQ277425, Zhang et al. (2012) | 563–564 |
| OSRC26 | 4 | 0.5 | JQ277429, Zhang et al. (2012) | 559 |
| OSRC27 | 4 | 0.5 | JQ277433, Zhang et al. (2012) | 572–575 |
| OSRC28 | 4 | 0.5 | JQ277437, Zhang et al. (2012) | 723 |
| OSRC31 | 4 | 0.5 | JQ277441, Zhang et al. (2012) | 400 |
| OSRC32 | 4 | 0.5 | JQ277445, Zhang et al. (2012) | 614–638 |
| Microsatellites | 30 | 3.4 | JF781128, Wang et al. (2012) | 56–204 |
| Total | 874 |
| Gene . | No. INSD entries . | Percentage (%) . | Accession no., author, and year of the first entry in INSD . | Length (bp) . |
|---|---|---|---|---|
| nrDNA ITS | 397 | 45.4 | AF056582, Kang (1998) (AF122027, Chao and Li 1999)a | 261–687b |
| nrSSU | 105 | 12.0 | D86053, Ito and Hirano (1996) (AJ007566, Chen and Hseu 1997)a | 260–1,791 |
| nrLSU | 53 | 6.1 | AB067704, Kinjo (2001) | 461–3,315 |
| tub | 73 | 8.4 | HM804956, Pandey et al. (2010) | 433–1,201 |
| tef1 | 31 | 3.5 | EF468767, Sung et al. (2007) | 533–674 |
| rpb1 | 29 | 3.3 | EF468874, Sung et al. (2007) | 521–703 |
| rpb2 | 2 | 0.2 | EF468924, Sung et al. (2007) | 890–1,040 |
| MAT1-2-1 | 60 | 6.9 | FJ654150, Zhang et al. (2009) | 877–882 (7,996)c |
| DNA lyase gene | 1d | – | HM212637, Zhang et al. (2010) | 898 |
| csp1 | 1 | 0.1 | EU282382, Zhang et al. (2007) | 2,143 |
| csp2 | 1 | 0.1 | EU282383, Zhang et al. (2007) | 2,566 |
| OSRC1 | 4 | 0.5 | JQ277357, Zhang et al. (2012) | 604 |
| OSRC2 | 4 | 0.5 | JQ277361, Zhang et al. (2012) | 665 |
| OSRC3 | 4 | 0.5 | JQ277365, Zhang et al. (2012) | 570–573 |
| OSRC4 | 4 | 0.5 | JQ277369, Zhang et al. (2012) | 717 |
| OSRC7 | 4 | 0.5 | JQ277373, Zhang et al. (2012) | 461–464 |
| OSRC9 | 4 | 0.5 | JQ277377, Zhang et al. (2012) | 377 |
| OSRC11 | 4 | 0.5 | JQ277381, Zhang et al. (2012) | 471–478 |
| OSRC13 | 4 | 0.5 | JQ277385, Zhang et al. (2012) | 584–588 |
| OSRC14 | 4 | 0.5 | JQ277389, Zhang et al. (2012) | 577–590 |
| OSRC16 | 4 | 0.5 | JQ277393, Zhang et al. (2012) | 513 |
| OSRC17 | 4 | 0.5 | JQ277397, Zhang et al. (2012) | 480 |
| OSRC18 | 4 | 0.5 | JQ277401, Zhang et al. (2012) | 378 |
| OSRC19 | 4 | 0.5 | JQ277405, Zhang et al. (2012) | 525 |
| OSRC21 | 4 | 0.5 | JQ277409, Zhang et al. (2012) | 455 |
| OSRC22 | 4 | 0.5 | JQ277413, Zhang et al. (2012) | 485 |
| OSRC23 | 4 | 0.5 | JQ277417, Zhang et al. (2012) | 716–721 |
| OSRC24 | 4 | 0.5 | JQ277421, Zhang et al. (2012) | 534 |
| OSRC25 | 4 | 0.5 | JQ277425, Zhang et al. (2012) | 563–564 |
| OSRC26 | 4 | 0.5 | JQ277429, Zhang et al. (2012) | 559 |
| OSRC27 | 4 | 0.5 | JQ277433, Zhang et al. (2012) | 572–575 |
| OSRC28 | 4 | 0.5 | JQ277437, Zhang et al. (2012) | 723 |
| OSRC31 | 4 | 0.5 | JQ277441, Zhang et al. (2012) | 400 |
| OSRC32 | 4 | 0.5 | JQ277445, Zhang et al. (2012) | 614–638 |
| Microsatellites | 30 | 3.4 | JF781128, Wang et al. (2012) | 56–204 |
| Total | 874 |
aThe first records of nrDNA ITS and nrSSU in the INSD were erroneously annotated. The earliest correct ones are in parentheses
bPartial sequences of nrLSU and nrSSU were included in some nrDNA ITS entries. The actual length of the ITS region (including ITS1, 5.8S, and ITS2) was ~500 bp
cFifty-nine of MAT1-2-1 entries were 877–882 bp long except for HM212637, which was 6,996 bp long because both flanking sequences were included in this entry
dThe sequence of the DNA lyase gene was embedded in a MAT1-2-1 entry, and they share the accession no. of HM212637

Increase in INSD entries annotated as O. sinensis between 1996 and 2012. The entries for 2012 are probably incomplete because the data were collected up to October 15, 2012 and the entries submitted in 2012 may need some time to become open to public
Although O. sinensis is mainly distributed in China, scientists from more than ten countries submitted O. sinensis sequences to the INSD (Table 5), suggesting a worldwide interest in the fungus. Given the medicinal, cultural, and economic importance of Chinese cordyceps in China, it is not surprising that Chinese researchers submitted 78.8 % (689 records) of the total entries. In China, more than 24 research groups or institutes have submitted O. sinensis sequences to the INSD, and this number accounted for 60.0 % of all research groups or institutes submitting O. sinensis sequences to the INSD (Table 5). However, 62.0 % (542 records) of the overall entries were marked as unpublished even though some (ca. 71 entries as far as we know) were published.
INSD entries submitted by scientists from different countries
| Country . | No. INSD entries . | Percentage (%) . | No. research groups . | Percentage (%) . |
|---|---|---|---|---|
| Chinaa | 689 | 78.8 | 24 | 60.0 |
| India | 97 | 11.1 | 2 | 5.0 |
| Japan | 41 | 4.7 | 3 | 7.5 |
| USA | 8 | 0.9 | 3 | 7.5 |
| Germany | 3 | 0.3 | 1 | 2.5 |
| Switzerland | 3 | 0.3 | 1 | 2.5 |
| Korea | 2 | 0.2 | 2 | 5.0 |
| Norway | 1 | 0.1 | 1 | 2.5 |
| South Africa | 1 | 0.1 | 1 | 2.5 |
| Spain | 1 | 0.1 | 1 | 2.5 |
| Unknown | 28 | 3.2 | 1 | 2.5 |
| Country . | No. INSD entries . | Percentage (%) . | No. research groups . | Percentage (%) . |
|---|---|---|---|---|
| Chinaa | 689 | 78.8 | 24 | 60.0 |
| India | 97 | 11.1 | 2 | 5.0 |
| Japan | 41 | 4.7 | 3 | 7.5 |
| USA | 8 | 0.9 | 3 | 7.5 |
| Germany | 3 | 0.3 | 1 | 2.5 |
| Switzerland | 3 | 0.3 | 1 | 2.5 |
| Korea | 2 | 0.2 | 2 | 5.0 |
| Norway | 1 | 0.1 | 1 | 2.5 |
| South Africa | 1 | 0.1 | 1 | 2.5 |
| Spain | 1 | 0.1 | 1 | 2.5 |
| Unknown | 28 | 3.2 | 1 | 2.5 |
aThis included scientists from China mainland, Hong Kong, and Taiwan
INSD entries submitted by scientists from different countries
| Country . | No. INSD entries . | Percentage (%) . | No. research groups . | Percentage (%) . |
|---|---|---|---|---|
| Chinaa | 689 | 78.8 | 24 | 60.0 |
| India | 97 | 11.1 | 2 | 5.0 |
| Japan | 41 | 4.7 | 3 | 7.5 |
| USA | 8 | 0.9 | 3 | 7.5 |
| Germany | 3 | 0.3 | 1 | 2.5 |
| Switzerland | 3 | 0.3 | 1 | 2.5 |
| Korea | 2 | 0.2 | 2 | 5.0 |
| Norway | 1 | 0.1 | 1 | 2.5 |
| South Africa | 1 | 0.1 | 1 | 2.5 |
| Spain | 1 | 0.1 | 1 | 2.5 |
| Unknown | 28 | 3.2 | 1 | 2.5 |
| Country . | No. INSD entries . | Percentage (%) . | No. research groups . | Percentage (%) . |
|---|---|---|---|---|
| Chinaa | 689 | 78.8 | 24 | 60.0 |
| India | 97 | 11.1 | 2 | 5.0 |
| Japan | 41 | 4.7 | 3 | 7.5 |
| USA | 8 | 0.9 | 3 | 7.5 |
| Germany | 3 | 0.3 | 1 | 2.5 |
| Switzerland | 3 | 0.3 | 1 | 2.5 |
| Korea | 2 | 0.2 | 2 | 5.0 |
| Norway | 1 | 0.1 | 1 | 2.5 |
| South Africa | 1 | 0.1 | 1 | 2.5 |
| Spain | 1 | 0.1 | 1 | 2.5 |
| Unknown | 28 | 3.2 | 1 | 2.5 |
aThis included scientists from China mainland, Hong Kong, and Taiwan
Reliability of sequences deposited in the INSD
As of October 15, 2012, all sequences of MAT1-2-1, DNA lyase gene, csp1, csp2, and OSRCs represented in the INSD were deposited by our research group [58, 60, 62]. They originated from authentic O. sinensis isolates, i.e., isolate identification was based on morphological analysis and sequence analysis of the ITS region of nrDNA, and so their reliability was not suspected in this study. The recently reported microsatellite sequences were not analyzed in this study, and they represent true O. sinensis sequences judging from the qualification of the publication [46]. Because sequences of nrDNA ITS, nrLSU, nrSSU, rpb1, rpb2, tef1, and tub in INSD were submitted by various independent research groups, their reliabilities were carefully examined herein.
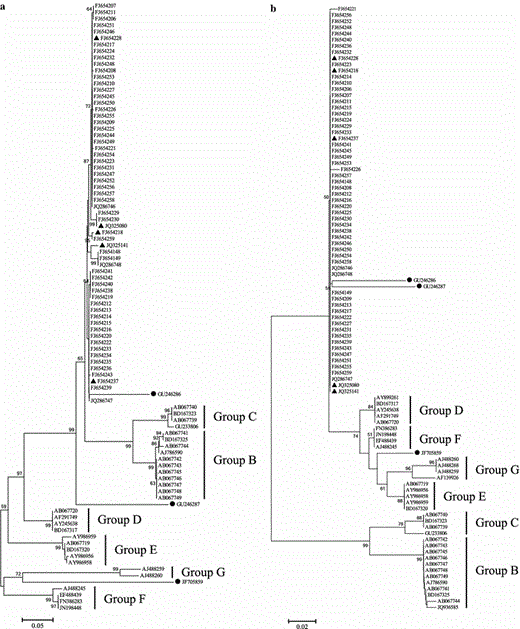
Phylogeny derived from the ITS1-5.8S-ITS2 data set (a) and the 5.8S data set (b) based on erroneously annotated sequences in the INSD and correct sequences from our research group. A total of 39 ITS entries were identified as wrongly accessioned entries, whereas only 33 or 37 were used to perform phylogenetic analyses due to incomplete sequence for some ITS entries. The three entries marked with black balls were erroneously accessioned but did not form a clade with other entries. The remaining entries were correct sequences of O. sinensis from our research group, and the five “reference sequences” used in this study were marked with black triangles
The nrSSU sequences were the second most abundant among all INSD records (Table 4). Of the 105 nrSSU sequences, 78 represented O. sinensis, and 27 were incorrectly attributed to O. sinensis (Table S1). These false entries had K2P values of 0.0117–0.0877 with reference sequences, and BLAST analyses showed that they represent species of Fusarium, Paecilomyces/Isaria, Cordyceps s.s., or others. Among all nrSSU sequences, BD167298, BD167301, BD167304, BD167307, BD167310, BD167313, BD167316, BD167319, and BD167322 were submitted as representing the ribosomal RNA gene, but they actually represent nrSSU sequences.
Of the 53 nrLSU sequences, 43 were correct O. sinensis sequences, five were incorrect, and five were indeterminate (Table S1). These indeterminate records had K2P values of 0.0044–0.0322 with reference sequences. These incorrect records had K2P values of 0.0410–0.1751 with reference sequences and represent other fungal species within the order of Hypocreales. Among all nrLSU sequences, BD167300, BD167303, BD167306, BD167309, BD167312, BD167315, BD167318, BD167321, and BD167324 were submitted as representing the ribosomal RNA gene, but they actually represented nrLSU sequences.
Of the 73 tub sequences, 59 represented O. sinensis, and 14 were indeterminate. The latter might be chimeric or represent different copies of O. sinensis tub genes (Table S1). All 31 tef1 sequences, 29 rpb1 sequences, and two rpb2 sequences in the INSD until today were correct O. sinensis sequences.
Genetic diversity within O. sinensis
Nucleotide variations among correct O. sinensis sequences in the INSD
| Gene fragment . | No. entries . | Aligned length . | No. variable sites (%)a . | No. phylogenetically informative sites (%) . | No. phylogenetically uninformative sites . | No. indel sites . | No. of alleles (Frequency of the most common allele)b . | Mean K2P . | Max. K2P . | Positionc . |
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS | 304 | 502 | 137 (27.3) | 49 (9.8) | 37 | 51 | 103 (113) | 0.0107 | 0.0513 | |
| ITS1 | 318 | 162 | 50 (30.9) | 22 (12.9) | 16 | 12 | 45 (143) | 0.0098 | 0.0808 | 1–162 |
| 5.8S | 336 | 157 | 22 (14.0) | 10 (6.4) | 10 | 2 | 23 (269) | 0.0035 | 0.0395 | 163–319 |
| ITS2 | 331 | 183 | 67 (36.6) | 19 (10.4) | 15 | 33 | 55 (130) | 0.0187 | 0.0812 | 320–502 |
| nrSSU | 17 | 1,727 | 7 (0.4) | 5 (0.3) | 1 | 1 | 5 (10) | 0.0010 | 0.0029 | |
| 41 | 381 | 3 (0.8) | 0 (0.0) | 2 | 1 | 4 (38) | 0.0003 | 0.0053 | 601–981 | |
| 41 | 268 | 2 (0.7) | 1 (0.4) | 0 | 1 | 3 (19) | 0.0019 | 0.0038 | 1,110–1,376 | |
| 34 | 335 | 2 (0.6) | 1 (0.3) | 1 | 0 | 3 (31) | 0.0007 | 0.0060 | 1,393–1,727 | |
| nrLSU | 18 | 1,360 | 10 (0.7) | 9 (0.7) | 1 | 0 | 5 (6) | 0.0021 | 0.0052 | |
| 42 | 790 | 21 (2.7) | 7 (0.9) | 7 | 7 | 15 (16) | 0.0017 | 0.0077 | 10–798 | |
| tub | 33 | 1,201 | 11 (0.9) | 9 (0.7) | 2 | 0 | 6 (14) | 0.0035 | 0.0075 | |
| 59 | 424 | 12 (2.8) | 10 (2.4) | 0 | 2 | 8 (24) | 0.0039 | 0.0119 | 1–423 | |
| tef1 | 31 | 513 | 12 (2.3) | 8 (1.6) | 4 | 0 | 9 (15) | 0.0051 | 0.0158 | |
| rpb1 | 29 | 521 | 8 (1.5) | 5 (0.9) | 3 | 0 | 7 (21) | 0.0015 | 0.0077 | |
| rpb2 | 2 | 870 | 5 (0.6) | 0 (0.0) | 5 | 0 | 2 (1) | 0.0058 | 0.0058 | |
| MAT1-2-1 | 59 | 882 | 34 (3.9) | 23 (2.6) | 6 | 5 | 13 (32) | 0.0044 | 0.0244 | |
| OSRC1 | 5 | 604 | 3 (0.5) | 3 (0.5) | 0 | 0 | 2 (3) | 0.0030 | 0.0050 | |
| OSRC2 | 5 | 665 | 0 (0.0) | 0 (0.0) | 0 | 0 | 1 (5) | 0.0000 | 0.0000 | |
| OSRC3 | 5 | 573 | 6 (1.1) | 2 (0.4) | 1 | 3 | 3 (2) | 0.0028 | 0.0035 | |
| OSRC4 | 5 | 717 | 3 (0.4) | 2 (0.3) | 1 | 0 | 3 (2) | 0.0022 | 0.0042 | |
| OSRC7 | 5 | 464 | 7 (1.5) | 0 (0.0) | 4 | 3 | 3 (3) | 0.0035 | 0.0087 | |
| OSRC9 | 5 | 377 | 7 (1.9) | 3 (0.8) | 4 | 0 | 3 (2) | 0.0091 | 0.0188 | |
| OSRC11 | 5 | 478 | 8 (1.7) | 0 (0.0) | 1 | 7 | 3 (3) | 0.0008 | 0.0021 | |
| OSRC13 | 5 | 591 | 16 (2.7) | 1 (0.2) | 5 | 10 | 3 (2) | 0.0045 | 0.0104 | |
| OSRC14 | 5 | 590 | 32 (5.4) | 7 (1.2) | 12 | 13 | 3 (2) | 0.0159 | 0.0283 | |
| OSRC16 | 5 | 513 | 7 (1.4) | 4 (0.8) | 3 | 0 | 3 (2) | 0.0071 | 0.0098 | |
| OSRC17 | 5 | 481 | 11 (2.3) | 3 (0.6) | 6 | 2 | 3 (2) | 0.0088 | 0.0169 | |
| OSRC18 | 5 | 378 | 5 (1.3) | 4 (1.1) | 1 | 0 | 3 (3) | 0.0075 | 0.0134 | |
| OSRC19 | 5 | 525 | 29 (5.5) | 28 (5.3) | 1 | 0 | 3 (2) | 0.0340 | 0.0574 | |
| OSRC21 | 5 | 454 | 1 (0.2) | 1 (0.2) | 0 | 0 | 2 (3) | 0.0013 | 0.0022 | |
| OSRC22 | 5 | 485 | 10 (2.1) | 1 (0.2) | 9 | 0 | 3 (2) | 0.0088 | 0.0211 | |
| OSRC23 | 5 | 721 | 7 (0.9) | 1 (0.1) | 1 | 5 | 3 (2) | 0.0014 | 0.0028 | |
| OSRC24 | 5 | 534 | 9 (1.7) | 2 (0.4) | 7 | 0 | 4 (2) | 0.0076 | 0.0151 | |
| OSRC25 | 5 | 564 | 10 (1.8) | 1 (0.2) | 8 | 1 | 3 (2) | 0.0068 | 0.0162 | |
| OSRC26 | 5 | 559 | 5 (0.9) | 1 (0.2) | 4 | 0 | 3 (2) | 0.0040 | 0.0090 | |
| OSRC27 | 5 | 575 | 17 (2.9) | 0 (0.0) | 14 | 3 | 2 (4) | 0.0100 | 0.0250 | |
| OSRC28 | 5 | 723 | 8 (1.1) | 2 (0.3) | 6 | 0 | 4 (2) | 0.0050 | 0.0112 | |
| OSRC31 | 5 | 400 | 3 (0.8) | 1 (0.3) | 2 | 0 | 3 (2) | 0.0035 | 0.0075 | |
| OSRC32 | 5 | 638 | 88 (13.8) | 43 (6.7) | 21 | 24 | 3 (2) | 0.0592 | 0.0864 |
| Gene fragment . | No. entries . | Aligned length . | No. variable sites (%)a . | No. phylogenetically informative sites (%) . | No. phylogenetically uninformative sites . | No. indel sites . | No. of alleles (Frequency of the most common allele)b . | Mean K2P . | Max. K2P . | Positionc . |
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS | 304 | 502 | 137 (27.3) | 49 (9.8) | 37 | 51 | 103 (113) | 0.0107 | 0.0513 | |
| ITS1 | 318 | 162 | 50 (30.9) | 22 (12.9) | 16 | 12 | 45 (143) | 0.0098 | 0.0808 | 1–162 |
| 5.8S | 336 | 157 | 22 (14.0) | 10 (6.4) | 10 | 2 | 23 (269) | 0.0035 | 0.0395 | 163–319 |
| ITS2 | 331 | 183 | 67 (36.6) | 19 (10.4) | 15 | 33 | 55 (130) | 0.0187 | 0.0812 | 320–502 |
| nrSSU | 17 | 1,727 | 7 (0.4) | 5 (0.3) | 1 | 1 | 5 (10) | 0.0010 | 0.0029 | |
| 41 | 381 | 3 (0.8) | 0 (0.0) | 2 | 1 | 4 (38) | 0.0003 | 0.0053 | 601–981 | |
| 41 | 268 | 2 (0.7) | 1 (0.4) | 0 | 1 | 3 (19) | 0.0019 | 0.0038 | 1,110–1,376 | |
| 34 | 335 | 2 (0.6) | 1 (0.3) | 1 | 0 | 3 (31) | 0.0007 | 0.0060 | 1,393–1,727 | |
| nrLSU | 18 | 1,360 | 10 (0.7) | 9 (0.7) | 1 | 0 | 5 (6) | 0.0021 | 0.0052 | |
| 42 | 790 | 21 (2.7) | 7 (0.9) | 7 | 7 | 15 (16) | 0.0017 | 0.0077 | 10–798 | |
| tub | 33 | 1,201 | 11 (0.9) | 9 (0.7) | 2 | 0 | 6 (14) | 0.0035 | 0.0075 | |
| 59 | 424 | 12 (2.8) | 10 (2.4) | 0 | 2 | 8 (24) | 0.0039 | 0.0119 | 1–423 | |
| tef1 | 31 | 513 | 12 (2.3) | 8 (1.6) | 4 | 0 | 9 (15) | 0.0051 | 0.0158 | |
| rpb1 | 29 | 521 | 8 (1.5) | 5 (0.9) | 3 | 0 | 7 (21) | 0.0015 | 0.0077 | |
| rpb2 | 2 | 870 | 5 (0.6) | 0 (0.0) | 5 | 0 | 2 (1) | 0.0058 | 0.0058 | |
| MAT1-2-1 | 59 | 882 | 34 (3.9) | 23 (2.6) | 6 | 5 | 13 (32) | 0.0044 | 0.0244 | |
| OSRC1 | 5 | 604 | 3 (0.5) | 3 (0.5) | 0 | 0 | 2 (3) | 0.0030 | 0.0050 | |
| OSRC2 | 5 | 665 | 0 (0.0) | 0 (0.0) | 0 | 0 | 1 (5) | 0.0000 | 0.0000 | |
| OSRC3 | 5 | 573 | 6 (1.1) | 2 (0.4) | 1 | 3 | 3 (2) | 0.0028 | 0.0035 | |
| OSRC4 | 5 | 717 | 3 (0.4) | 2 (0.3) | 1 | 0 | 3 (2) | 0.0022 | 0.0042 | |
| OSRC7 | 5 | 464 | 7 (1.5) | 0 (0.0) | 4 | 3 | 3 (3) | 0.0035 | 0.0087 | |
| OSRC9 | 5 | 377 | 7 (1.9) | 3 (0.8) | 4 | 0 | 3 (2) | 0.0091 | 0.0188 | |
| OSRC11 | 5 | 478 | 8 (1.7) | 0 (0.0) | 1 | 7 | 3 (3) | 0.0008 | 0.0021 | |
| OSRC13 | 5 | 591 | 16 (2.7) | 1 (0.2) | 5 | 10 | 3 (2) | 0.0045 | 0.0104 | |
| OSRC14 | 5 | 590 | 32 (5.4) | 7 (1.2) | 12 | 13 | 3 (2) | 0.0159 | 0.0283 | |
| OSRC16 | 5 | 513 | 7 (1.4) | 4 (0.8) | 3 | 0 | 3 (2) | 0.0071 | 0.0098 | |
| OSRC17 | 5 | 481 | 11 (2.3) | 3 (0.6) | 6 | 2 | 3 (2) | 0.0088 | 0.0169 | |
| OSRC18 | 5 | 378 | 5 (1.3) | 4 (1.1) | 1 | 0 | 3 (3) | 0.0075 | 0.0134 | |
| OSRC19 | 5 | 525 | 29 (5.5) | 28 (5.3) | 1 | 0 | 3 (2) | 0.0340 | 0.0574 | |
| OSRC21 | 5 | 454 | 1 (0.2) | 1 (0.2) | 0 | 0 | 2 (3) | 0.0013 | 0.0022 | |
| OSRC22 | 5 | 485 | 10 (2.1) | 1 (0.2) | 9 | 0 | 3 (2) | 0.0088 | 0.0211 | |
| OSRC23 | 5 | 721 | 7 (0.9) | 1 (0.1) | 1 | 5 | 3 (2) | 0.0014 | 0.0028 | |
| OSRC24 | 5 | 534 | 9 (1.7) | 2 (0.4) | 7 | 0 | 4 (2) | 0.0076 | 0.0151 | |
| OSRC25 | 5 | 564 | 10 (1.8) | 1 (0.2) | 8 | 1 | 3 (2) | 0.0068 | 0.0162 | |
| OSRC26 | 5 | 559 | 5 (0.9) | 1 (0.2) | 4 | 0 | 3 (2) | 0.0040 | 0.0090 | |
| OSRC27 | 5 | 575 | 17 (2.9) | 0 (0.0) | 14 | 3 | 2 (4) | 0.0100 | 0.0250 | |
| OSRC28 | 5 | 723 | 8 (1.1) | 2 (0.3) | 6 | 0 | 4 (2) | 0.0050 | 0.0112 | |
| OSRC31 | 5 | 400 | 3 (0.8) | 1 (0.3) | 2 | 0 | 3 (2) | 0.0035 | 0.0075 | |
| OSRC32 | 5 | 638 | 88 (13.8) | 43 (6.7) | 21 | 24 | 3 (2) | 0.0592 | 0.0864 |
aVariable sites consist of both indel (insertion/deletion) sites and substitution sites, and the latter contain both phylogenetically informative and uninformative sites
bAn allele refers to any variant of DNA sequence observed at a given locus (gene) with indel sites considered. “Frequency of the most common allele” indicates the number of individuals represented by the dominate allele
cPositions are given for shorter fragments of a gene relative to the longest fragment
Nucleotide variations among correct O. sinensis sequences in the INSD
| Gene fragment . | No. entries . | Aligned length . | No. variable sites (%)a . | No. phylogenetically informative sites (%) . | No. phylogenetically uninformative sites . | No. indel sites . | No. of alleles (Frequency of the most common allele)b . | Mean K2P . | Max. K2P . | Positionc . |
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS | 304 | 502 | 137 (27.3) | 49 (9.8) | 37 | 51 | 103 (113) | 0.0107 | 0.0513 | |
| ITS1 | 318 | 162 | 50 (30.9) | 22 (12.9) | 16 | 12 | 45 (143) | 0.0098 | 0.0808 | 1–162 |
| 5.8S | 336 | 157 | 22 (14.0) | 10 (6.4) | 10 | 2 | 23 (269) | 0.0035 | 0.0395 | 163–319 |
| ITS2 | 331 | 183 | 67 (36.6) | 19 (10.4) | 15 | 33 | 55 (130) | 0.0187 | 0.0812 | 320–502 |
| nrSSU | 17 | 1,727 | 7 (0.4) | 5 (0.3) | 1 | 1 | 5 (10) | 0.0010 | 0.0029 | |
| 41 | 381 | 3 (0.8) | 0 (0.0) | 2 | 1 | 4 (38) | 0.0003 | 0.0053 | 601–981 | |
| 41 | 268 | 2 (0.7) | 1 (0.4) | 0 | 1 | 3 (19) | 0.0019 | 0.0038 | 1,110–1,376 | |
| 34 | 335 | 2 (0.6) | 1 (0.3) | 1 | 0 | 3 (31) | 0.0007 | 0.0060 | 1,393–1,727 | |
| nrLSU | 18 | 1,360 | 10 (0.7) | 9 (0.7) | 1 | 0 | 5 (6) | 0.0021 | 0.0052 | |
| 42 | 790 | 21 (2.7) | 7 (0.9) | 7 | 7 | 15 (16) | 0.0017 | 0.0077 | 10–798 | |
| tub | 33 | 1,201 | 11 (0.9) | 9 (0.7) | 2 | 0 | 6 (14) | 0.0035 | 0.0075 | |
| 59 | 424 | 12 (2.8) | 10 (2.4) | 0 | 2 | 8 (24) | 0.0039 | 0.0119 | 1–423 | |
| tef1 | 31 | 513 | 12 (2.3) | 8 (1.6) | 4 | 0 | 9 (15) | 0.0051 | 0.0158 | |
| rpb1 | 29 | 521 | 8 (1.5) | 5 (0.9) | 3 | 0 | 7 (21) | 0.0015 | 0.0077 | |
| rpb2 | 2 | 870 | 5 (0.6) | 0 (0.0) | 5 | 0 | 2 (1) | 0.0058 | 0.0058 | |
| MAT1-2-1 | 59 | 882 | 34 (3.9) | 23 (2.6) | 6 | 5 | 13 (32) | 0.0044 | 0.0244 | |
| OSRC1 | 5 | 604 | 3 (0.5) | 3 (0.5) | 0 | 0 | 2 (3) | 0.0030 | 0.0050 | |
| OSRC2 | 5 | 665 | 0 (0.0) | 0 (0.0) | 0 | 0 | 1 (5) | 0.0000 | 0.0000 | |
| OSRC3 | 5 | 573 | 6 (1.1) | 2 (0.4) | 1 | 3 | 3 (2) | 0.0028 | 0.0035 | |
| OSRC4 | 5 | 717 | 3 (0.4) | 2 (0.3) | 1 | 0 | 3 (2) | 0.0022 | 0.0042 | |
| OSRC7 | 5 | 464 | 7 (1.5) | 0 (0.0) | 4 | 3 | 3 (3) | 0.0035 | 0.0087 | |
| OSRC9 | 5 | 377 | 7 (1.9) | 3 (0.8) | 4 | 0 | 3 (2) | 0.0091 | 0.0188 | |
| OSRC11 | 5 | 478 | 8 (1.7) | 0 (0.0) | 1 | 7 | 3 (3) | 0.0008 | 0.0021 | |
| OSRC13 | 5 | 591 | 16 (2.7) | 1 (0.2) | 5 | 10 | 3 (2) | 0.0045 | 0.0104 | |
| OSRC14 | 5 | 590 | 32 (5.4) | 7 (1.2) | 12 | 13 | 3 (2) | 0.0159 | 0.0283 | |
| OSRC16 | 5 | 513 | 7 (1.4) | 4 (0.8) | 3 | 0 | 3 (2) | 0.0071 | 0.0098 | |
| OSRC17 | 5 | 481 | 11 (2.3) | 3 (0.6) | 6 | 2 | 3 (2) | 0.0088 | 0.0169 | |
| OSRC18 | 5 | 378 | 5 (1.3) | 4 (1.1) | 1 | 0 | 3 (3) | 0.0075 | 0.0134 | |
| OSRC19 | 5 | 525 | 29 (5.5) | 28 (5.3) | 1 | 0 | 3 (2) | 0.0340 | 0.0574 | |
| OSRC21 | 5 | 454 | 1 (0.2) | 1 (0.2) | 0 | 0 | 2 (3) | 0.0013 | 0.0022 | |
| OSRC22 | 5 | 485 | 10 (2.1) | 1 (0.2) | 9 | 0 | 3 (2) | 0.0088 | 0.0211 | |
| OSRC23 | 5 | 721 | 7 (0.9) | 1 (0.1) | 1 | 5 | 3 (2) | 0.0014 | 0.0028 | |
| OSRC24 | 5 | 534 | 9 (1.7) | 2 (0.4) | 7 | 0 | 4 (2) | 0.0076 | 0.0151 | |
| OSRC25 | 5 | 564 | 10 (1.8) | 1 (0.2) | 8 | 1 | 3 (2) | 0.0068 | 0.0162 | |
| OSRC26 | 5 | 559 | 5 (0.9) | 1 (0.2) | 4 | 0 | 3 (2) | 0.0040 | 0.0090 | |
| OSRC27 | 5 | 575 | 17 (2.9) | 0 (0.0) | 14 | 3 | 2 (4) | 0.0100 | 0.0250 | |
| OSRC28 | 5 | 723 | 8 (1.1) | 2 (0.3) | 6 | 0 | 4 (2) | 0.0050 | 0.0112 | |
| OSRC31 | 5 | 400 | 3 (0.8) | 1 (0.3) | 2 | 0 | 3 (2) | 0.0035 | 0.0075 | |
| OSRC32 | 5 | 638 | 88 (13.8) | 43 (6.7) | 21 | 24 | 3 (2) | 0.0592 | 0.0864 |
| Gene fragment . | No. entries . | Aligned length . | No. variable sites (%)a . | No. phylogenetically informative sites (%) . | No. phylogenetically uninformative sites . | No. indel sites . | No. of alleles (Frequency of the most common allele)b . | Mean K2P . | Max. K2P . | Positionc . |
|---|---|---|---|---|---|---|---|---|---|---|
| nrDNA ITS | 304 | 502 | 137 (27.3) | 49 (9.8) | 37 | 51 | 103 (113) | 0.0107 | 0.0513 | |
| ITS1 | 318 | 162 | 50 (30.9) | 22 (12.9) | 16 | 12 | 45 (143) | 0.0098 | 0.0808 | 1–162 |
| 5.8S | 336 | 157 | 22 (14.0) | 10 (6.4) | 10 | 2 | 23 (269) | 0.0035 | 0.0395 | 163–319 |
| ITS2 | 331 | 183 | 67 (36.6) | 19 (10.4) | 15 | 33 | 55 (130) | 0.0187 | 0.0812 | 320–502 |
| nrSSU | 17 | 1,727 | 7 (0.4) | 5 (0.3) | 1 | 1 | 5 (10) | 0.0010 | 0.0029 | |
| 41 | 381 | 3 (0.8) | 0 (0.0) | 2 | 1 | 4 (38) | 0.0003 | 0.0053 | 601–981 | |
| 41 | 268 | 2 (0.7) | 1 (0.4) | 0 | 1 | 3 (19) | 0.0019 | 0.0038 | 1,110–1,376 | |
| 34 | 335 | 2 (0.6) | 1 (0.3) | 1 | 0 | 3 (31) | 0.0007 | 0.0060 | 1,393–1,727 | |
| nrLSU | 18 | 1,360 | 10 (0.7) | 9 (0.7) | 1 | 0 | 5 (6) | 0.0021 | 0.0052 | |
| 42 | 790 | 21 (2.7) | 7 (0.9) | 7 | 7 | 15 (16) | 0.0017 | 0.0077 | 10–798 | |
| tub | 33 | 1,201 | 11 (0.9) | 9 (0.7) | 2 | 0 | 6 (14) | 0.0035 | 0.0075 | |
| 59 | 424 | 12 (2.8) | 10 (2.4) | 0 | 2 | 8 (24) | 0.0039 | 0.0119 | 1–423 | |
| tef1 | 31 | 513 | 12 (2.3) | 8 (1.6) | 4 | 0 | 9 (15) | 0.0051 | 0.0158 | |
| rpb1 | 29 | 521 | 8 (1.5) | 5 (0.9) | 3 | 0 | 7 (21) | 0.0015 | 0.0077 | |
| rpb2 | 2 | 870 | 5 (0.6) | 0 (0.0) | 5 | 0 | 2 (1) | 0.0058 | 0.0058 | |
| MAT1-2-1 | 59 | 882 | 34 (3.9) | 23 (2.6) | 6 | 5 | 13 (32) | 0.0044 | 0.0244 | |
| OSRC1 | 5 | 604 | 3 (0.5) | 3 (0.5) | 0 | 0 | 2 (3) | 0.0030 | 0.0050 | |
| OSRC2 | 5 | 665 | 0 (0.0) | 0 (0.0) | 0 | 0 | 1 (5) | 0.0000 | 0.0000 | |
| OSRC3 | 5 | 573 | 6 (1.1) | 2 (0.4) | 1 | 3 | 3 (2) | 0.0028 | 0.0035 | |
| OSRC4 | 5 | 717 | 3 (0.4) | 2 (0.3) | 1 | 0 | 3 (2) | 0.0022 | 0.0042 | |
| OSRC7 | 5 | 464 | 7 (1.5) | 0 (0.0) | 4 | 3 | 3 (3) | 0.0035 | 0.0087 | |
| OSRC9 | 5 | 377 | 7 (1.9) | 3 (0.8) | 4 | 0 | 3 (2) | 0.0091 | 0.0188 | |
| OSRC11 | 5 | 478 | 8 (1.7) | 0 (0.0) | 1 | 7 | 3 (3) | 0.0008 | 0.0021 | |
| OSRC13 | 5 | 591 | 16 (2.7) | 1 (0.2) | 5 | 10 | 3 (2) | 0.0045 | 0.0104 | |
| OSRC14 | 5 | 590 | 32 (5.4) | 7 (1.2) | 12 | 13 | 3 (2) | 0.0159 | 0.0283 | |
| OSRC16 | 5 | 513 | 7 (1.4) | 4 (0.8) | 3 | 0 | 3 (2) | 0.0071 | 0.0098 | |
| OSRC17 | 5 | 481 | 11 (2.3) | 3 (0.6) | 6 | 2 | 3 (2) | 0.0088 | 0.0169 | |
| OSRC18 | 5 | 378 | 5 (1.3) | 4 (1.1) | 1 | 0 | 3 (3) | 0.0075 | 0.0134 | |
| OSRC19 | 5 | 525 | 29 (5.5) | 28 (5.3) | 1 | 0 | 3 (2) | 0.0340 | 0.0574 | |
| OSRC21 | 5 | 454 | 1 (0.2) | 1 (0.2) | 0 | 0 | 2 (3) | 0.0013 | 0.0022 | |
| OSRC22 | 5 | 485 | 10 (2.1) | 1 (0.2) | 9 | 0 | 3 (2) | 0.0088 | 0.0211 | |
| OSRC23 | 5 | 721 | 7 (0.9) | 1 (0.1) | 1 | 5 | 3 (2) | 0.0014 | 0.0028 | |
| OSRC24 | 5 | 534 | 9 (1.7) | 2 (0.4) | 7 | 0 | 4 (2) | 0.0076 | 0.0151 | |
| OSRC25 | 5 | 564 | 10 (1.8) | 1 (0.2) | 8 | 1 | 3 (2) | 0.0068 | 0.0162 | |
| OSRC26 | 5 | 559 | 5 (0.9) | 1 (0.2) | 4 | 0 | 3 (2) | 0.0040 | 0.0090 | |
| OSRC27 | 5 | 575 | 17 (2.9) | 0 (0.0) | 14 | 3 | 2 (4) | 0.0100 | 0.0250 | |
| OSRC28 | 5 | 723 | 8 (1.1) | 2 (0.3) | 6 | 0 | 4 (2) | 0.0050 | 0.0112 | |
| OSRC31 | 5 | 400 | 3 (0.8) | 1 (0.3) | 2 | 0 | 3 (2) | 0.0035 | 0.0075 | |
| OSRC32 | 5 | 638 | 88 (13.8) | 43 (6.7) | 21 | 24 | 3 (2) | 0.0592 | 0.0864 |
aVariable sites consist of both indel (insertion/deletion) sites and substitution sites, and the latter contain both phylogenetically informative and uninformative sites
bAn allele refers to any variant of DNA sequence observed at a given locus (gene) with indel sites considered. “Frequency of the most common allele” indicates the number of individuals represented by the dominate allele
cPositions are given for shorter fragments of a gene relative to the longest fragment
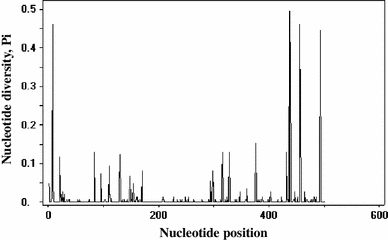
Nucleotide diversity of different nucleotide sites of nrDNA ITS from O. sinensis. This figure was plotted by DnaSP software based on 304 correct O. sinensis INSD sequences with the full-length ITS region (i.e., ITS1, 5.8S, and ITS2). The 5.8S region is located between the 164th and 319th position. Almost all sites in the 5.8S region had nucleotide diversity indices lower than 0.1, whereas several sites in the ITS1 and ITS2 regions had nucleotide diversity indices larger than 0.1
Genetic difference between O. sinensis and other related taxa
Four records annotated as O. robertsii were found in the INSD, and they included two nrDNA ITS records, one nrLSU record, and one tef1 record. The two nrDNA ITS records had high sequence dissimilarity (K2P = 0.1396). According to BLAST analyses, one (AJ309335) represents O. robertsii and the other (AJ309336) represents Trichoderma sp. Based on correct O. robertsii sequences, we found that the genetic distances between O. sinensis and O. robertsii were larger than the intraspecific variation of O. sinensis (Tables 3, 6, and 7).
Comparison of genetic distance between O. sinensis and related fungi
| Fungal taxa . | Accession no. . | Gene fragment . | Original length (bp) . | Aligned length (bp) . | Min. K2P . | Max. K2P . | Comment . |
|---|---|---|---|---|---|---|---|
| O. robertsii | AJ309335 | nrDNA ITS | 623 | 557 | 0.0567 | 0.0651 | |
| AJ309336 | nrDNA ITS | 625 | 576 | 0.1847 | 0.1964 | May represent Trichoderma sp. | |
| EF468826 | nrLSU | 894 | 863 | 0.0237 | 0.0261 | ||
| EF468766 | tef1 | 689 | 687 | 0.0298 | 0.0360 | ||
| O. crassispora | AB067714 | nrDNA ITS | 647 | 544 | 0.2013 | 0.2098 | May represent Neonectria sp. |
| FJ025150 | nrDNA ITS | 634 | 537 | 0.2043 | 0.2098 | May represent Neonectria sp. | |
| AB067697 | nrSSU | 1,728 | 1,667 | 0.0226 | 0.0251 | May represent Myrothecium sp. | |
| AB067706 | nrLSU | 1,413 | 1,387 | 0.0659 | 0.0683 | May represent Hypocreales sp. | |
| O. gansuensis | AF056583 | nrDNA ITS | 301 | 315 | 0.4743 | 0.5021 | May represent Penicillium sp. |
| AF139929 | nrDNA ITS | 260 | 286 | 0.2116 | 0.2277 | May represent Penicillium sp. | |
| O. nepalensis | AJ309358 | nrDNA ITS | 601 | 538 | 0.0038 | 0.0209 | |
| O. multiaxialis | AJ309359 | nrDNA ITS | 602 | 538 | 0.0000 | 0.0229 | |
| Group B | AB067741 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | |
| AB067742 | nrDNA ITS | 604 | 507 | 0.1181 | 0.1403 | ||
| AB067743 | nrDNA ITS | 604 | 507 | 0.1206 | 0.1429 | ||
| AB067744 | nrDNA ITS | 604 | 507 | 0.1308 | 0.1535 | ||
| AB067745 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067746 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067747 | nrDNA ITS | 561 | 507 | 0.1209 | 0.1432 | ||
| AB067748 | nrDNA ITS | 685 | 507 | 0.1228 | 0.1452 | ||
| AB067749 | nrDNA ITS | 687 | 507 | 0.1206 | 0.1429 | ||
| AB067750 | nrDNA ITS | 505 | 507 | 0.1231 | 0.1455 | ||
| BD167325 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | ||
| JQ936585 | nrDNA ITS | 359 | 360 | 0.1301 | 0.1561 | ||
| Group C | AB067739 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | |
| AB067740 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| BD167323 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| GU233806 | nrDNA ITS | 604 | 537 | 0.1495 | 0.1697 |
| Fungal taxa . | Accession no. . | Gene fragment . | Original length (bp) . | Aligned length (bp) . | Min. K2P . | Max. K2P . | Comment . |
|---|---|---|---|---|---|---|---|
| O. robertsii | AJ309335 | nrDNA ITS | 623 | 557 | 0.0567 | 0.0651 | |
| AJ309336 | nrDNA ITS | 625 | 576 | 0.1847 | 0.1964 | May represent Trichoderma sp. | |
| EF468826 | nrLSU | 894 | 863 | 0.0237 | 0.0261 | ||
| EF468766 | tef1 | 689 | 687 | 0.0298 | 0.0360 | ||
| O. crassispora | AB067714 | nrDNA ITS | 647 | 544 | 0.2013 | 0.2098 | May represent Neonectria sp. |
| FJ025150 | nrDNA ITS | 634 | 537 | 0.2043 | 0.2098 | May represent Neonectria sp. | |
| AB067697 | nrSSU | 1,728 | 1,667 | 0.0226 | 0.0251 | May represent Myrothecium sp. | |
| AB067706 | nrLSU | 1,413 | 1,387 | 0.0659 | 0.0683 | May represent Hypocreales sp. | |
| O. gansuensis | AF056583 | nrDNA ITS | 301 | 315 | 0.4743 | 0.5021 | May represent Penicillium sp. |
| AF139929 | nrDNA ITS | 260 | 286 | 0.2116 | 0.2277 | May represent Penicillium sp. | |
| O. nepalensis | AJ309358 | nrDNA ITS | 601 | 538 | 0.0038 | 0.0209 | |
| O. multiaxialis | AJ309359 | nrDNA ITS | 602 | 538 | 0.0000 | 0.0229 | |
| Group B | AB067741 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | |
| AB067742 | nrDNA ITS | 604 | 507 | 0.1181 | 0.1403 | ||
| AB067743 | nrDNA ITS | 604 | 507 | 0.1206 | 0.1429 | ||
| AB067744 | nrDNA ITS | 604 | 507 | 0.1308 | 0.1535 | ||
| AB067745 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067746 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067747 | nrDNA ITS | 561 | 507 | 0.1209 | 0.1432 | ||
| AB067748 | nrDNA ITS | 685 | 507 | 0.1228 | 0.1452 | ||
| AB067749 | nrDNA ITS | 687 | 507 | 0.1206 | 0.1429 | ||
| AB067750 | nrDNA ITS | 505 | 507 | 0.1231 | 0.1455 | ||
| BD167325 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | ||
| JQ936585 | nrDNA ITS | 359 | 360 | 0.1301 | 0.1561 | ||
| Group C | AB067739 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | |
| AB067740 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| BD167323 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| GU233806 | nrDNA ITS | 604 | 537 | 0.1495 | 0.1697 |
Comparison of genetic distance between O. sinensis and related fungi
| Fungal taxa . | Accession no. . | Gene fragment . | Original length (bp) . | Aligned length (bp) . | Min. K2P . | Max. K2P . | Comment . |
|---|---|---|---|---|---|---|---|
| O. robertsii | AJ309335 | nrDNA ITS | 623 | 557 | 0.0567 | 0.0651 | |
| AJ309336 | nrDNA ITS | 625 | 576 | 0.1847 | 0.1964 | May represent Trichoderma sp. | |
| EF468826 | nrLSU | 894 | 863 | 0.0237 | 0.0261 | ||
| EF468766 | tef1 | 689 | 687 | 0.0298 | 0.0360 | ||
| O. crassispora | AB067714 | nrDNA ITS | 647 | 544 | 0.2013 | 0.2098 | May represent Neonectria sp. |
| FJ025150 | nrDNA ITS | 634 | 537 | 0.2043 | 0.2098 | May represent Neonectria sp. | |
| AB067697 | nrSSU | 1,728 | 1,667 | 0.0226 | 0.0251 | May represent Myrothecium sp. | |
| AB067706 | nrLSU | 1,413 | 1,387 | 0.0659 | 0.0683 | May represent Hypocreales sp. | |
| O. gansuensis | AF056583 | nrDNA ITS | 301 | 315 | 0.4743 | 0.5021 | May represent Penicillium sp. |
| AF139929 | nrDNA ITS | 260 | 286 | 0.2116 | 0.2277 | May represent Penicillium sp. | |
| O. nepalensis | AJ309358 | nrDNA ITS | 601 | 538 | 0.0038 | 0.0209 | |
| O. multiaxialis | AJ309359 | nrDNA ITS | 602 | 538 | 0.0000 | 0.0229 | |
| Group B | AB067741 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | |
| AB067742 | nrDNA ITS | 604 | 507 | 0.1181 | 0.1403 | ||
| AB067743 | nrDNA ITS | 604 | 507 | 0.1206 | 0.1429 | ||
| AB067744 | nrDNA ITS | 604 | 507 | 0.1308 | 0.1535 | ||
| AB067745 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067746 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067747 | nrDNA ITS | 561 | 507 | 0.1209 | 0.1432 | ||
| AB067748 | nrDNA ITS | 685 | 507 | 0.1228 | 0.1452 | ||
| AB067749 | nrDNA ITS | 687 | 507 | 0.1206 | 0.1429 | ||
| AB067750 | nrDNA ITS | 505 | 507 | 0.1231 | 0.1455 | ||
| BD167325 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | ||
| JQ936585 | nrDNA ITS | 359 | 360 | 0.1301 | 0.1561 | ||
| Group C | AB067739 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | |
| AB067740 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| BD167323 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| GU233806 | nrDNA ITS | 604 | 537 | 0.1495 | 0.1697 |
| Fungal taxa . | Accession no. . | Gene fragment . | Original length (bp) . | Aligned length (bp) . | Min. K2P . | Max. K2P . | Comment . |
|---|---|---|---|---|---|---|---|
| O. robertsii | AJ309335 | nrDNA ITS | 623 | 557 | 0.0567 | 0.0651 | |
| AJ309336 | nrDNA ITS | 625 | 576 | 0.1847 | 0.1964 | May represent Trichoderma sp. | |
| EF468826 | nrLSU | 894 | 863 | 0.0237 | 0.0261 | ||
| EF468766 | tef1 | 689 | 687 | 0.0298 | 0.0360 | ||
| O. crassispora | AB067714 | nrDNA ITS | 647 | 544 | 0.2013 | 0.2098 | May represent Neonectria sp. |
| FJ025150 | nrDNA ITS | 634 | 537 | 0.2043 | 0.2098 | May represent Neonectria sp. | |
| AB067697 | nrSSU | 1,728 | 1,667 | 0.0226 | 0.0251 | May represent Myrothecium sp. | |
| AB067706 | nrLSU | 1,413 | 1,387 | 0.0659 | 0.0683 | May represent Hypocreales sp. | |
| O. gansuensis | AF056583 | nrDNA ITS | 301 | 315 | 0.4743 | 0.5021 | May represent Penicillium sp. |
| AF139929 | nrDNA ITS | 260 | 286 | 0.2116 | 0.2277 | May represent Penicillium sp. | |
| O. nepalensis | AJ309358 | nrDNA ITS | 601 | 538 | 0.0038 | 0.0209 | |
| O. multiaxialis | AJ309359 | nrDNA ITS | 602 | 538 | 0.0000 | 0.0229 | |
| Group B | AB067741 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | |
| AB067742 | nrDNA ITS | 604 | 507 | 0.1181 | 0.1403 | ||
| AB067743 | nrDNA ITS | 604 | 507 | 0.1206 | 0.1429 | ||
| AB067744 | nrDNA ITS | 604 | 507 | 0.1308 | 0.1535 | ||
| AB067745 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067746 | nrDNA ITS | 562 | 507 | 0.1206 | 0.1429 | ||
| AB067747 | nrDNA ITS | 561 | 507 | 0.1209 | 0.1432 | ||
| AB067748 | nrDNA ITS | 685 | 507 | 0.1228 | 0.1452 | ||
| AB067749 | nrDNA ITS | 687 | 507 | 0.1206 | 0.1429 | ||
| AB067750 | nrDNA ITS | 505 | 507 | 0.1231 | 0.1455 | ||
| BD167325 | nrDNA ITS | 685 | 507 | 0.1308 | 0.1535 | ||
| JQ936585 | nrDNA ITS | 359 | 360 | 0.1301 | 0.1561 | ||
| Group C | AB067739 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | |
| AB067740 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| BD167323 | nrDNA ITS | 686 | 537 | 0.1495 | 0.1697 | ||
| GU233806 | nrDNA ITS | 604 | 537 | 0.1495 | 0.1697 |
Based on morphological characters, six species similar to O. sinensis have previously been reported on the Tibetan Plateau [9, 54, 56, 57]. No sequences of O. kangdingensis and O. laojunshanensis were accessible in the INSD, and the other four species (O. crassispora, O. gansuensis, O. nepalensis, and O. multiaxialis) had one to four entries in the INSD. The K2P values between O. sinensis and sequences annotated as O. nepalensis or O. multiaxialis were smaller than the maximal K2P values among O. sinensis sequences (Tables 3, 6, and 7). This is consistent with previous conclusions that O. nepalensis and O. multiaxialis are synonyms of O. sinensis [27, 40]. The K2P values between O. sinensis and sequences annotated as O. crassispora or O. gansuensis, however, were much larger than the maximal K2P values among O. sinensis sequences (Tables 3, 6, and 7). BLAST analyses showed that the two records annotated as O. gansuensis represent Penicillium sp. and that the four entries annotated as O. crassispora represent other Hypocreales species (Table 7). In addition, INSD sequences of O. nepalensis and O. multiaxialis but not of O. crassispora or O. gansuensis originated from type specimens. Therefore, determining whether O. crassispora and O. gansuensis are synonyms of O. sinensis will require more evaluation.
Groups B and C in the ITS phylogenetic trees (Fig. 2) were controversial. They were regarded as either cryptic species of O. sinensis [41] or different species [49] by different researchers. These sequences showed strong BLAST hits with correct O. sinensis although their similarity to O. sinensis was lower than 90 %. Further analysis indicated that even the minimum K2P values (0.1181–0.1495) between O. sinensis and groups B and C were much larger than the maximum K2P values between O. sinensis sequences (Tables 3, 6, and 7). Therefore, groups B and C are unlikely to be cryptic species of O. sinensis.
Discussion
Nucleic acid sequences deposited in public databases are widely used by systematic and evolutionary biologists to construct systematic frameworks and to model evolutionary pathways. According to the evaluation of fungal nrDNA sequences, as many as 20 % of the sequences in public databases may be unreliable [5, 33]. Because certain sequences in the INSD have been annotated erroneously [5, 33, 41] and because intraspecific genetic diversity is substantial in many organisms [32, 38, 63], the reliability of sequences annotated as a given species in INSD should be examined by those with substantial experience with the organism in question. The current study examined the reliability of all INSD sequences annotated as O. sinensis. We used a method that should be useful for evaluating the sequences of other organisms. In this method, genetic distances between INSD sequences and sequences from authenticated materials were carefully compared with maximal genetic distances among different authentic sequences as the criteria. In addition, BLAST searches were performed as a supplement.
As a valuable medicinal fungus, O. sinensis has become a focus of scientific research and commercialization in recent years [61]. The fungus is difficult to study, however, because it evidently grows only in harsh alpine environments on the Tibetan Plateau. O. sinensis is also difficult to study because in nature it is only found as a parasite of insects, and the parasitized insect (Chinese cordyceps) supports a complex microbial community [65]. These difficulties have generated confusion in the identification of O. sinensis. Some of this confusion has been reduced by recent research. For example, a recent study reported that of the 91 insect species associated with O. sinensis in the literature, 57 are potential hosts, eight are indeterminate hosts, and 26 are non-hosts [47]. Of the 203 distribution sites for O. sinensis recorded in publications, 106 are considered as confirmed distribution sites, 65 as possible distribution sites, 29 as “non-distribution” sites, and three as “suspicious” distribution sites [22]. Analyses of fungal materials used in 152 papers on O. sinensis from PubMed since 1998 showed that at least 116 papers (over 75 %) used unreliable, uncertain, or unspecified materials [13]. Errors in INSD sequences have been reported [41], and the reliability of all INSD sequences deposited as from O. sinensis therefore deserved careful evaluation.
Of the 874 nucleotide sequences that were in the INSD as of October 15, 2012 and that were evaluated in this study, 774 are considered as O. sinensis sequences, 71 as sequences of other fungi, seven as chimeras, and 22 as indeterminate sequences. Results of this study have provided essential reference for INSD staff and for scientists studying O. sinensis and related species. Because nrDNA ITS sequences were the most abundant sequences associated with O. sinensis in the INSD and also had the most annotation errors, we strongly recommend that, if they intend to report O. sinensis from new environments or to submit an ITS sequence under the name O. sinensis, researchers compare their sequences with our reference sequences and adhere to the criteria we have established for ITS sequences in this study (Table 3). We also strongly recommend that the quality of a sequence be carefully checked (i.e., the chromatograms should be carefully examined) before it is submitted to INSD.
Annotation error on newly generated sequences can be avoided if enough attention is paid, but how to rectify pre-existing erroneously accessioned entries in the INSD? One simple solution to this problem is that such correction can be done by submitters of those sequences. The reality, however, is that these submitters often have moved on to other projects and never get around to making the changes [35]. Some researchers have submitted a sequence of O. sinensis to INSD, but they may not want to perform an in depth research on this fungus. Another solution is that researches who have discovered inaccuracies should append corrections [4]. However, currently, third-party annotation tools are poorly developed in the INSD [35]. Anyway, appropriate measures need to be worked out as early as possible in order to prevent error propagation, which would degrade the quality of the INSD. It is obvious that these discussions have been beyond the scope of this study, and herein, we want to alert subsequent INSD users to the presence of such defective data of O. sinensis.
Why have so many sequences been erroneously accessioned in INSD? One possible reason is that researchers have incorrectly assumed that sequences obtained from Chinese cordyceps represent O. sinensis. This is a poor assumption because a natural Chinese cordyceps specimen harbors a complex fungal community in which O. sinensis is the dominant but not the only member [65]. Another possible reason is that the original material was misidentified. Too often materials have been identified based only on BLAST results of nrDNA sequences without a careful check of their morphological characteristics. Under these conditions, if a sequence had strong BLAST hits with an INSD entry erroneously annotated as O. sinensis, that sequence would have been incorrectly submitted to the INSD as O. sinensis. Considering these problems, it is understandable why many publications have reported O. sinensis from unexpected substrates and locations such as from plants and from soil outside the Tibetan Plateau.
Although O. sinensis is genetically different from other taxa, the sequence variation for many of its genes is high. Given their high sequence variation, genes like ITS, MAT1-2-1, OSRC14, OSRC17, OSRC19, OSRC22, OSRC27, and OSRC32 have the potential to serve as markers for the analysis of genetic diversity in O. sinensis. Genes like the partial nrSSU fragment, rpb1, OSRC2, and OSRC11, in contrast, are relatively conserved, and therefore have the potential to serve as markers for identification and DNA barcoding of O. sinensis.
Stensrud et al. [41] analyzed the nucleotide variation of 71 ITS sequences annotated as O. sinensis in the INSD and placed these sequences in five groups (A–E). Based on the additional ITS sequences submitted to the INSD in the following years, additional groups (F and G) were identified in the current study (Fig. 2). Group A represents true O. sinensis sequences. Groups D–G and JF705859 represent other fungi according to BLAST results. Groups B and C, GU246286, and GU246287 had high scoring hits with correct O. sinensis sequences when BLAST was performed, but their identities with correct O. sinensis sequences were less than 90 %, and they were thus treated as incorrect sequences in this study. Sequences in groups B and C, which were originally submitted by Kinjo, have been reported by different researchers, and have been detected by our laboratory during an analysis of an ITS clone library of Chinese cordyceps (unpublished data). Unfortunately, sequences of groups B and C were all reported from natural Chinese cordyceps samples rather than from isolated fungal cultures. Determining whether they are cryptic species of O. sinensis [41] or different species [49] will require the isolation of fungi with those sequences. Solving this problem seems to be urgent, considering that several papers have been published by a research group who regarded groups B and C as different mutant genotypes during maturation of O. sinensis in nature [15, 16, 52, 67, 69].
Acknowledgments
This study was supported by grants from the National Plans of Sciences and Technology (2007BAI32B04), the cooperative project between Guangdong Province and Chinese Academy of Sciences (2009B091300015), the National Science Foundation of China (81102759; 31140013), and the Specialized Research Fund for the Doctoral Program of Higher Education (20101401120007). We also thank Prof. Bruce Jaffee (University of California at Davis) for serving as a pre-submission technical editor.
References
- INSD
International Nucleotide Sequence Databases
- nrDNA
Nuclear ribosomal DNA
- ITS
Internal transcribed spacer
- nrLSU
Nuclear ribosomal large subunit DNA
- nrSSU
Nuclear ribosomal small subunit DNA
- K2P
Kimura 2-parameter
- rpb1
The largest subunit of RNA polymerase II
- rpb2
The second largest subunit of RNA polymerase II
- tef1
Elongation factor-1α gene
- tub
β-tubulin gene
- OSRC
O. sinensis random clone



