-
PDF
- Split View
-
Views
-
Cite
Cite
S Badel, F Callet, C Laroche, C Gardarin, E Petit, H El Alaoui, T Bernardi, P Michaud, A new tool to detect high viscous exopolymers from microalgae, Journal of Industrial Microbiology and Biotechnology, Volume 38, Issue 2, 1 February 2011, Pages 319–326, https://doi.org/10.1007/s10295-010-0775-9
Close - Share Icon Share
Abstract
Microalgae are microorganisms often surrounded by a slime layer made of secreted polymeric substances sometimes including polysaccharides. These polysaccharides, weakly described in the literature, can constitute value-added molecules in several industrial areas. The aim of this article is to show that a new tool, the BioFilm Ring Test ®, can be used to detect viscous microalgal exopolymers. Two red microalgal strains (Rhodella violacea and Porphyridium purpureum), one cyanobacterium (Arthrospira platensis) and their excreted polymeric fractions were studied. R. violacea and P. purpureum induced a positive response with the BioFilm Ring Test ® contrary to A. platensis. Finally, the understanding of the fractions viscosity involvement in the BRT response was performed by a rheological study.
Introduction
Microalgal biofilms represent a general term to depict an assembly of species living wherever the surface gets in contact with water [28]. In these photosynthetic structures, whose average thickness is several micrometers, the microalgae are arranged into parallel layers surrounded by extracellular polymeric substances [21].
This matrix plays a part in the protection against external attacks. It also contributes to biofilm structure, keeping water and capture of nutrients or microorganisms by their adhesive properties [32]. Often described as exopolysaccharides, these exopolymeric substances are more complex and diversified as they include proteins and nucleic acids [2]. Moreover, some biofilms can be structured without secretion of exopolysaccharides. Their morphology is different but their strength is not altered [10, 33].
Only a few studies have investigated the structures of exopolymers or cell-bound polymers from microalgae, implied or not in biofilms, such as those produced by Cyanospira capsulata [17], Porphyridium sp. [19], Chlorella vulgaris [24], Arthrospira platensis [29], Dixioniella grisea [1], Botryococcus braunii [15] or Rhodella violacea [5]. In all cases, polysaccharides or proteoglycans have been identified and some of them found industrial applications as those produced by Porphyridium species [13, 34]. So, in a context where microalgae are increasingly used in industrial applications, it is important to find a new tool able to detect the ability of microalgae to excrete biopolymers such as exopolysaccharides. This new device is the BioFilm Ring Test ® whose principle is based on magnetization of magnetic beads in culture media of microorganisms. If beads are free in culture media, a spot can be observed at the center of the well after magnetization. On the other case, no spot appears because of the immobilization of beads by an extracellular matrix or an increase of viscosity. This kit is so far developed for bacterial screening only. Therefore, the aim of this study is to employ the BioFilm Ring Test ® (BRT®) to detect exopolymeric substances production by microalgae. Two red microalgae (Porphyridium purpureum, Rhodella violacea) and a cyanobacterium (Arthrospira platensis) previously described for exopolymeric substances production have been chosen as models. Our works show that the BioFilm Ring Test ® is a powerful tool to screen microalgae able to increase the viscosity of their culture media by production of high molecular weights polysaccharides.
Materials and methods
The BioFilm Ring Test ®
The principle of this kit was detailed by Chavant et al. [6]. It is performed to determine if microorganisms are able to form a biofilm. These microorganisms are cultivated in microwells of a microplate (96 wells) with magnetic particles. A microplate is divided into 12 strips, and one strip is here used for each incubation time tested. For the reading, wells are first covered with a few drops of Contrast Liquid (inert and non-toxic oil) and scanned with the Plate Reader, a dedicated scanner apparatus, to get an I0 image. Then, the strip is placed for 1 min on the Block Test and scanned again to get an I1 image. The Block Test is made of 96 magnets and designed so that each magnet is centered under the bottom of each well. After magnet contact, free beads are attracted in the center of the bottom of wells, forming a spot, while the beads blocked by the biofilm remain in place. Images of each well before (I0) and after (I1) magnetization are compared with the BioFilm Control® software, which uses an algorithm to estimate the discrepancy between the two images of the same well, giving a value named the BioFilm Indice (BFI) ranging from 0 to 30. A high BFI value corresponds to a high mobility of beads under magnet action (i.e., control wells), while a low value corresponds to a full immobilization of beads (no differences for a well between its images I0 and I1).
Microalgal strains and growth conditions
Arthrospira platensis PCC 8005 was grown in Zarrouk medium modified by Cogne et al. [8]. Rhodella violacea was isolated at the Laboratoire “Microorganismes: Génome et Environnement” (LMGE) from a natural sample. It was grown on derived Jones medium modified according to Doan et al. [11, 22]. Additional components issued from Conway medium, a modified version of Walne medium, were added [31]. They were, for 1 l of the derived Jones medium, 100 mg NaNO3; 26 mg NaH2PO4−2H2O; 100 mg Na2SiO3, 1 μl of trace metal solution. Trace metal solution was composed as follows: 0.45 g (NH4)6Mo7O24−4H2O, 1 g CuSO4−5H2O and 50 ml of distilled water.
Porphyridium purpureum CCAP 1380/1A was grown in Artificial Sea Water (ASW) composed by 3.75 ml of extra salts solution (30 g NaNO3, 1.2 g Na2HPO4 and 1 g K2HPO4 per liter); 2.65 ml of a vitamin solution (0.2 mg biotin, 20 mg calcium pantothenate, 4 mg cyanocobalamin, 0.4 mg folic acid, 1 g inositol, 20 g nicotinic acid, 0.1 g thiamine HCl and 0.6 g thymine per liter); 25 ml of soil extract; 0.5 g of tricine diluted in water qsp 1 l. All cultures are inoculated at 10% (v/v) of the total volume and incubated without shaking at 22°C under strip lighting (18 W) using a photoperiod of 16 h followed by a dark phase of 8 h.
Evaluation of microalgal biofilms with the BioFilm Ring Test ®
Tests were made with non-inoculated culture media (Zarrouk modified, ASW, Jones medium modified) in the presence or not of Tween 20, added at 0.002% (w/v) in final concentration. The toner was added in each culture media to get a final concentration of 10 μl ml−1 and 200 μl per well were loaded. Plates were incubated at 22°C. After 0, 24, 48, 72, 96, and/or 168 h, strips were analyzed with the BFC Elements ® as described previously.
Arthrospira platensis, Rhodella violacea and Porphyridium purpureum initial microalgal suspensions (IMS) were diluted until obtaining a clear spot at T0 with the BioFilm Ring Test ®. Two wells per strip composed of culture media with magnetic beads and without microorganisms were used as controls. Plates were incubated at 22°C under light as described above and strips were analyzed at different times of incubation with the BFC Elements® as explained previously.
Growth of Arthrospira platensis was followed from wells loaded without toner and incubated in the same conditions by measure of A600. This was not possible for the two red algae due to high level of large aggregates formation.
Production and extraction of exocellular fractions
Microalgae were cultivated in 500-ml flasks containing 200 ml of culture media under artificial light as described above between 6 and 10 weeks before extracellular fraction extraction. All flasks were inoculated at 10% (v/v) and incubated at 22°C without shaking.
Rhodella violacea and Porphyridium purpureum cultures were centrifuged at 10,000 × g for 20 min at room temperature to eliminate biomass (a dilution of P. purpureum culture was necessary to reduce the too high viscosity before the centrifugation), while the culture of Arthrospira platensis was filtrated through a glass filter (10–16 μm).
Supernatant or filtrates were concentrated by evaporation (Büchi RII) under 72 mbar, 60°C, and dialyzed (10,000 Da) against distilled water at 4°C for 48 h. Finally, the products were dried under vacuum and exopolymers were stored at room temperature.
Test of exopolymers with the BioFilm Ring Test ®
Different concentrations of exopolymeric fractions were tested with the BRT® after their solubilization in specific culture media of their microalgae producer.
Toner was added in each solution at 10 μl ml−1. Plate reading was carried out instantaneously. Controls were composed by media and toner without any exocellular fraction.
Quantitative analyses
Protein concentrations were determined by the Bradford method [3] using BioRad reagent and bovine serum albumin (BSA) as standard.
Total organic nitrogen was measured after a digestion procedure with sulfuric acid and hydrogen peroxide followed by colorimetric assay as developed by Hach [20].
Concentrations of acidic and neutral monosaccharides were determined by colorimetric assays. Uronic acids were quantified by absorbance at 520 nm (A520) after reaction with m-hydroxydiphenyl [4] and neutral sugars by A450 after addition of resorcinol in the presence of sulfuric acid [12, 23]. The results were expressed in equivalent of d-glucose for neutral sugars and d-glucuronic acid for acidic sugars.
SEC–MALLS analyses
Average molecular weights and molecular weight distributions were determined by high-pressure size exclusion chromatography (HPSEC) with on line multi-angle laser light scattering (MALLS) filled with a K5 cell (50 μl) and two detectors: a He–Ne laser (λ = 690 nm) and a differential refractive index (DRI). Columns [OHPAK SB-G guard column, OHPAK SB806, 804 and 803 HQ columns (Shodex)] were eluted with NaNO3 0.1 M at 0.7 ml.min−1. Solvent was filtered through 0.1-μm filter, degassed and filtered through a 0.45-μm filter upstream column. The sample was injected through a 100-μl full loop. The collected data were analyzed using the Astra 4.50 software package.
Spectroscopy
Dried exopolymer samples (3 mg) were dispersed in 100 mg of anhydrous KBr and pressed. The IR spectra were recorded at room temperature in the wave number range of 400–4,000 cm−1 and referenced against air with a Nicolet 380 FT-IR instrument (Thermoelectron Corporation). A total of 50 scans were averaged for each sample at 4 cm−1 resolution.
Rheological measurements
Rheological measurements were performed with double concentric cylinders geometry on a stress-controlled Rheometer AR-G2 (TA Instrument) with a Peltier temperature control system. Temperature was controlled at 25°C and the viscosity was evaluated depending on the shear rate comprised between 10−3 and 103s−1. Extracellular fractions were dissolved in pure water at concentrations of 1.5 g l−1, 0.8 g l−1 and 0.5 g l−1 by gentle stirring at 40°C. Finally, 13 ml of solution was inserted in the instrument. All data were analyzed by the Rheology Advance software.
Results and discussion
Adaptation of microalgae culture media to the BioFilm Ring Test ®
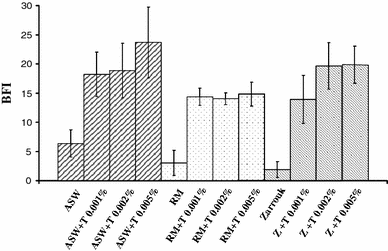
Tests of media with the BioFilm Ring Test ®. ASW artificial sea water, RM Rhodella medium, Z Zarrouk modified medium, T Tween 20. All experiments were performed in triplicate
Evaluation of exopolymer production with the BioFilm Ring Test ®
Among the three microalgae species, Rhodella sp. and Porphyridium sp. were well depicted as viscous exopolysaccharides producers [14, 18], although, there was an ambivalence concerning the abundant literature describing exopolymers from Arthrospira platensis [16, 29]. Effectively, no increase of apparent viscosity of culture media was detected after incubation of Arthrospira platensis PCC 8005 during 168 h.
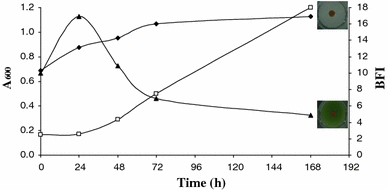
Growth (square) of Arthrospira platensis incubated in Zarrouk medium during 168 h at 22°C and biofilm formation (filled triangle), (filled diamond) Control (Zarrouk medium non-inoculated)
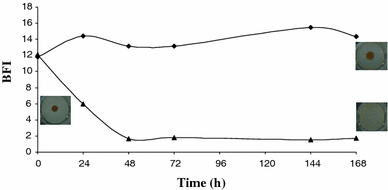
Biofilm formation by Rhodella violacea (filled triangle), (filled diamond) Control (Rhodella medium non inoculated)
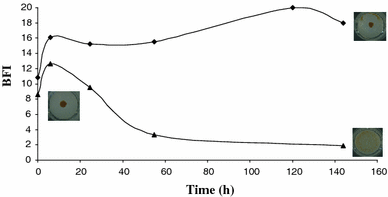
Biofilm formation by Porphyridium purpureum (filled triangle), (filled diamond) control (ASW non-inoculated)
Implication of exopolymers into BFI
To evaluate the role of putative exopolymers in BFI values, the three microalgae were grown in their specific culture media and their exocellular fractions were extracted. Yields of polymers were 0.12, 0.26, and 0.42 g l−1 for R. violacea, P. purpureum and A. platensis, respectively (Table 1). Even if yields are depending not only on culture duration but also on light intensity and light/dark phases, it was possible to correlate them with bibliographic data. Considering A. platensis, our results agree with a yield of 0.21 g l−1 after 21 days, as published by Trabelsi et al. [29]. From Porphyridium sp. cultures, yields were comprised between 0.13 and 1.3 g l−1 [25–27, 30]. Our yield of 0.26 g l−1 was also consistent. A polysaccharide production from Rhodella reticulata attaining 1.63 g l−1 when its culture medium was added by nitrates [35] was obviously higher than our yield of 0.12 g l−1. However, our experiences showed a co-precipitation of salts which may induce some overvalued yields when alcoholic precipitation was employed to extract exopolymers. That was the reason why our samples were concentrated and dialyzed but not precipitated by polar solvents such as ethanol or isopropanol.
Composition of sugars and nitrogen according to microalgal fractions
| Microorganisms . | Arthrospira platensis . | Rhodella violacea . | Porphyridium purpureum . |
|---|---|---|---|
| Yields (g l−1) | 0.42 | 0.12 | 0.26 |
| Proteins (g g−1) | 0.06 | – | 0.02 |
| Nitrogen (g g−1) | 0.04 | 0.01 | 0.01 |
| Uronic acids (g g−1) | 0.09 | 0.07 | 0.08 |
| Neutral monosaccharides (g g−1) | 0.39 | 0.41 | 0.48 |
| Microorganisms . | Arthrospira platensis . | Rhodella violacea . | Porphyridium purpureum . |
|---|---|---|---|
| Yields (g l−1) | 0.42 | 0.12 | 0.26 |
| Proteins (g g−1) | 0.06 | – | 0.02 |
| Nitrogen (g g−1) | 0.04 | 0.01 | 0.01 |
| Uronic acids (g g−1) | 0.09 | 0.07 | 0.08 |
| Neutral monosaccharides (g g−1) | 0.39 | 0.41 | 0.48 |
Composition of sugars and nitrogen according to microalgal fractions
| Microorganisms . | Arthrospira platensis . | Rhodella violacea . | Porphyridium purpureum . |
|---|---|---|---|
| Yields (g l−1) | 0.42 | 0.12 | 0.26 |
| Proteins (g g−1) | 0.06 | – | 0.02 |
| Nitrogen (g g−1) | 0.04 | 0.01 | 0.01 |
| Uronic acids (g g−1) | 0.09 | 0.07 | 0.08 |
| Neutral monosaccharides (g g−1) | 0.39 | 0.41 | 0.48 |
| Microorganisms . | Arthrospira platensis . | Rhodella violacea . | Porphyridium purpureum . |
|---|---|---|---|
| Yields (g l−1) | 0.42 | 0.12 | 0.26 |
| Proteins (g g−1) | 0.06 | – | 0.02 |
| Nitrogen (g g−1) | 0.04 | 0.01 | 0.01 |
| Uronic acids (g g−1) | 0.09 | 0.07 | 0.08 |
| Neutral monosaccharides (g g−1) | 0.39 | 0.41 | 0.48 |
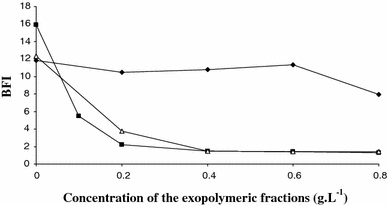
Biofilm formation by exopolymeric fractions extracted from Porphyridium purpureum (triangle), Rhodella violacea (filled square) and Arthrospira platensis (filled diamond)
Chemical and physico-chemical characterizations of exopolymeric fractions
To understand the behavior of extracted and dialyzed exocellular fractions, their nature was elucidated by colorimetric assays (Table 1).
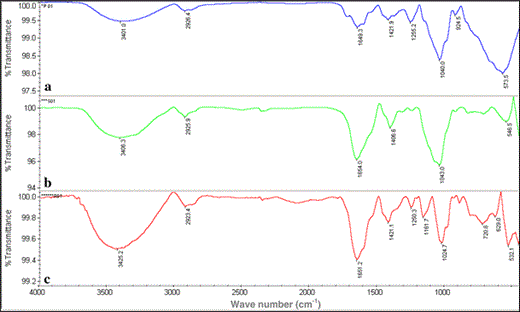
FTIR analyses of exopolymers extracted from P. purpureum (a), A. platensis (b) and R. violacea (c)
The relatively close chemical composition of the three exopolymers associated with their polysaccharidic nature macromolecules was not sufficient to explain their different behaviors with magnetic particles of the BioFilm Ring Test ®. Therefore, to validate their polymeric structures, SEC–MALLS analysis was done to appreciate their molecular weights and their polydispersities.
The concentration of polysaccharide macromolecules in the samples was 42% for R. violacea, 19% for P. purpureum, and 32% for A. platensis. This low carbohydrate content led to expect a non-negligible part of salts associated with exopolymers. The relative percentage of each macromolecular family was expressed on behalf of total macromolecules identified for each microalgae (Table 2). Curiously, three families of high-molecular-weight polymers were significantly detected in the extract from A. platensis and one of them had the highest molecular weight of all polymers detected in the three microalgal extracts. The two red algae extracts contained polysaccharides with molecular weights of 2.3 × 106 g mol−1 for R. violacea and between 4.07 and 2.14 × 106 g mol−1 for P. purpureum. Note the heterogeneity of P. purpureum and A. platensis extracts which contained, respectively, four and three exopolymers families by comparison to R. violacea producing only one exopolymer. In order to progress in the understanding of interactions between exopolymers and magnetic particles of the BioFilm Ring Test ®, rheological investigations were conducted. The aim of this study was to prove that microviscosities created in the well by exopolymers were at the origin of BFI decreases.
Relative distribution of molecular weights in microalgae fractions
| . | . | Peak 1 . | Peak 2 . | Peak 3 . | Peak 4 . |
|---|---|---|---|---|---|
| R. violacea | Mw (g mol−1) | 2.63 × 106 | – | – | – |
| Relative % | 100 | – | – | – | |
| Ip | 1.005 | – | – | – | |
| P. purpureum | Mw (g mol−1) | 4.07 × 106 | 3.78 × 106 | 3.13 × 106 | 2.14 × 106 |
| Relative % | 26 | 26 | 32 | 16 | |
| Ip | 1.002 | 1.001 | 1.007 | 1.029 | |
| A. platensis | Mw (g mol−1) | 1.08 × 107 | 7.42 × 105 | 1.17 × 106 | – |
| Relative % | 34 | 50 | 16 | – | |
| Ip | 1.48 | 1.24 | 1.06 | – |
| . | . | Peak 1 . | Peak 2 . | Peak 3 . | Peak 4 . |
|---|---|---|---|---|---|
| R. violacea | Mw (g mol−1) | 2.63 × 106 | – | – | – |
| Relative % | 100 | – | – | – | |
| Ip | 1.005 | – | – | – | |
| P. purpureum | Mw (g mol−1) | 4.07 × 106 | 3.78 × 106 | 3.13 × 106 | 2.14 × 106 |
| Relative % | 26 | 26 | 32 | 16 | |
| Ip | 1.002 | 1.001 | 1.007 | 1.029 | |
| A. platensis | Mw (g mol−1) | 1.08 × 107 | 7.42 × 105 | 1.17 × 106 | – |
| Relative % | 34 | 50 | 16 | – | |
| Ip | 1.48 | 1.24 | 1.06 | – |
Ip index of polydispersity
Relative distribution of molecular weights in microalgae fractions
| . | . | Peak 1 . | Peak 2 . | Peak 3 . | Peak 4 . |
|---|---|---|---|---|---|
| R. violacea | Mw (g mol−1) | 2.63 × 106 | – | – | – |
| Relative % | 100 | – | – | – | |
| Ip | 1.005 | – | – | – | |
| P. purpureum | Mw (g mol−1) | 4.07 × 106 | 3.78 × 106 | 3.13 × 106 | 2.14 × 106 |
| Relative % | 26 | 26 | 32 | 16 | |
| Ip | 1.002 | 1.001 | 1.007 | 1.029 | |
| A. platensis | Mw (g mol−1) | 1.08 × 107 | 7.42 × 105 | 1.17 × 106 | – |
| Relative % | 34 | 50 | 16 | – | |
| Ip | 1.48 | 1.24 | 1.06 | – |
| . | . | Peak 1 . | Peak 2 . | Peak 3 . | Peak 4 . |
|---|---|---|---|---|---|
| R. violacea | Mw (g mol−1) | 2.63 × 106 | – | – | – |
| Relative % | 100 | – | – | – | |
| Ip | 1.005 | – | – | – | |
| P. purpureum | Mw (g mol−1) | 4.07 × 106 | 3.78 × 106 | 3.13 × 106 | 2.14 × 106 |
| Relative % | 26 | 26 | 32 | 16 | |
| Ip | 1.002 | 1.001 | 1.007 | 1.029 | |
| A. platensis | Mw (g mol−1) | 1.08 × 107 | 7.42 × 105 | 1.17 × 106 | – |
| Relative % | 34 | 50 | 16 | – | |
| Ip | 1.48 | 1.24 | 1.06 | – |
Ip index of polydispersity
Cross model parameters
| Concentrations . | Microalgae . | K (s) . | η0 (Pa.s) . | m . |
|---|---|---|---|---|
| 1.5 g l−1 | A. platensis | 603.3 | 0.84 | 1.14 |
| R. violacea | 52.75 | 15.10 | 0.93 | |
| P. purpureum | 126.1 | 18.27 | 0.83 | |
| 0.8 g l−1 | A. platensis | 48.46 | 0.25 | 1.08 |
| R. violacea | 34.39 | 2.39 | 0.85 | |
| P. purpureum | 129.5 | 7.64 | 0.85 | |
| 0.5 g l−1 | A. platensis | 37.52 | 0.07 | 1.01 |
| R. violacea | 14.71 | 0.48 | 0.80 | |
| P. purpureum | 178.4 | 3.81 | 0.80 |
| Concentrations . | Microalgae . | K (s) . | η0 (Pa.s) . | m . |
|---|---|---|---|---|
| 1.5 g l−1 | A. platensis | 603.3 | 0.84 | 1.14 |
| R. violacea | 52.75 | 15.10 | 0.93 | |
| P. purpureum | 126.1 | 18.27 | 0.83 | |
| 0.8 g l−1 | A. platensis | 48.46 | 0.25 | 1.08 |
| R. violacea | 34.39 | 2.39 | 0.85 | |
| P. purpureum | 129.5 | 7.64 | 0.85 | |
| 0.5 g l−1 | A. platensis | 37.52 | 0.07 | 1.01 |
| R. violacea | 14.71 | 0.48 | 0.80 | |
| P. purpureum | 178.4 | 3.81 | 0.80 |
K consistency, η0 viscosity measured at the lowest shear rate, m rate index
Cross model parameters
| Concentrations . | Microalgae . | K (s) . | η0 (Pa.s) . | m . |
|---|---|---|---|---|
| 1.5 g l−1 | A. platensis | 603.3 | 0.84 | 1.14 |
| R. violacea | 52.75 | 15.10 | 0.93 | |
| P. purpureum | 126.1 | 18.27 | 0.83 | |
| 0.8 g l−1 | A. platensis | 48.46 | 0.25 | 1.08 |
| R. violacea | 34.39 | 2.39 | 0.85 | |
| P. purpureum | 129.5 | 7.64 | 0.85 | |
| 0.5 g l−1 | A. platensis | 37.52 | 0.07 | 1.01 |
| R. violacea | 14.71 | 0.48 | 0.80 | |
| P. purpureum | 178.4 | 3.81 | 0.80 |
| Concentrations . | Microalgae . | K (s) . | η0 (Pa.s) . | m . |
|---|---|---|---|---|
| 1.5 g l−1 | A. platensis | 603.3 | 0.84 | 1.14 |
| R. violacea | 52.75 | 15.10 | 0.93 | |
| P. purpureum | 126.1 | 18.27 | 0.83 | |
| 0.8 g l−1 | A. platensis | 48.46 | 0.25 | 1.08 |
| R. violacea | 34.39 | 2.39 | 0.85 | |
| P. purpureum | 129.5 | 7.64 | 0.85 | |
| 0.5 g l−1 | A. platensis | 37.52 | 0.07 | 1.01 |
| R. violacea | 14.71 | 0.48 | 0.80 | |
| P. purpureum | 178.4 | 3.81 | 0.80 |
K consistency, η0 viscosity measured at the lowest shear rate, m rate index
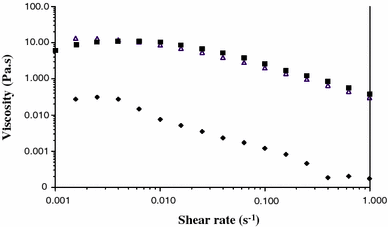
Viscosity of exopolymeric fractions (1.5 g l−1), (triangle) Porphyridium purpureum; (filled diamond) Arthrospira platensis; (filled square) Rhodella violacea
Conclusions
In conclusion, the use the BioFilm Ring Test ® with microalgae and cyanobacteria was validated after the addition of 0.002% Tween 20 in their culture media. Two red microalgae (R. violacea and P. purpureum) were able to immobilize particles of toner, inducing a positive response, while the third photosynthetic microorganism was diagnosed as negative. Production of exopolymers has been detected in culture media of the three microalgae. Two of them exhibited significant levels of viscous exopolymers. These red microalgal polymers have been identified as the agents able to immobilize magnetic particles of the BRT at concentrations equivalent to those expressed by microorganisms. This allows concluding that the BRT can be used as a new tool to detect high viscous exopolymers from microalgal cultures. The role of these macromolecules in biofilm formation will be investigated in the future after screening of enzymes efficient for their degradations. At a time when the number of publications relative to the culture of microalgae for biofuel production has increased significantly, the BRT® could be an interesting tool for screening photosynthetic microorganisms able to generate innovative high viscous exopolymers for industrial applications.
References
Walne PR (1966) Experiments in the large-scale culture of the larvae of Ostrea edulis. In: HMSO. London, 53 pp



