-
PDF
- Split View
-
Views
-
Cite
Cite
Emilie Adelin, Noureddine Slimani, Sylvie Cortial, Isabelle Schmitz-Alfonso, Jamal Ouazzani, Platotex: an innovative and fully automated device for cell growth scale-up of agar-supported solid-state fermentation, Journal of Industrial Microbiology and Biotechnology, Volume 38, Issue 2, 1 February 2011, Pages 299–305, https://doi.org/10.1007/s10295-010-0773-y
Close - Share Icon Share
Abstract
Among various factors that influence the production of microbial secondary metabolites (MSM), the method of cultivation is an important one that has not been thoroughly investigated. In order to increase microbial throughput and simplify the extraction and workup steps, we performed a study to compare liquid-state fermentation (LSF) with agar-supported solid-state fermentation (AgSF). We found that AgSF is not only more suitable for our applications but offers, for some microbial strains, a higher yield and broader diversity of secondary metabolites. The main limitation of AgSF is the lack of a system to allow production scale-up. In order to overcome this obstacle we developed Platotex, an original fermentation unit offering 2 m2 of cultivation surface that combines automatic sterilization, cultivation, and drying steps. Platotex is also able to support both LSF and solid-state fermentation (SSF). Platotex conforms to international security and quality requirements and benefits from total remote automation through industrial communication and control standards.
Introduction
Solid-state fermentation (SSF) involves a solid organic carrier that supports microbial growth and provides nutrients [1]. The absence of a liquid phase and the heterogeneity of the substrate prevent control and compromise reproducibility. Thus, SSF has long been considered a rough, empirical process. Recent investigations have rationally implemented SSF with successful results in the field of secondary metabolite and enzyme production [2, 3]. However, there remain no clear advantages of SSF over liquid-state fermentation (LSF). LSF can be applied to microbial, plant, and mammalian cells, remains easily scalable, can be tightly controlled, and is approved for industrial use.
Agar-supported solid-state fermentation (AgSF) is the most widely used cultivation method for isolation, primary cultivation, short-term conservation, and screening of microorganisms. However, this technique has been limited to laboratory-scale experiments due to scale-up restrictions [4]. This limitation aside, AgSF represents an ideal compromise between SSF and LSF, combing advantages of each system (Table 1). For the particular application of microbial secondary metabolite (MSM) production, AgSF avoids separate treatments of the biomass and medium and facilitates one-step analysis of the compounds present in both compartments.
Advantages of agar-supported cultivation over LSF and SSF
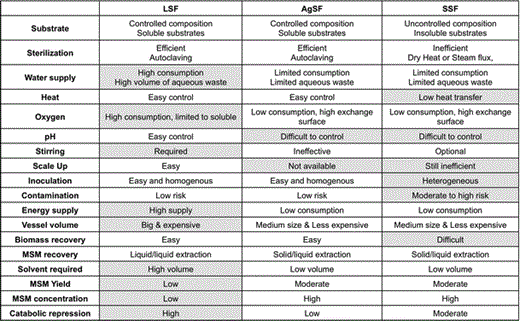
Boxes in grey show the major inconveniences of each cultivation mode
Advantages of agar-supported cultivation over LSF and SSF

Boxes in grey show the major inconveniences of each cultivation mode
We recently developed a strategy focused on the discovery of biologically active MSM. Our aims are to increase the throughput of microorganisms, decrease the evaluation time, and obtain a large quantity of the compound of interest to enable structural elucidations and preliminary biological investigations.
We have screened various cultivation methods and continue to use AgSF, as it offers, at least for the microorganisms investigated in this study, a higher yield and diversity of MSM as compared with LSF. Once convinced of the benefits of this cultivation mode, but in the absence of any dedicated scale-up system, we focused on overcoming this problem.
We report in this paper on a new cultivation device named Platotex that consists of a fermentation unit specifically developed for AgSF but that is compatible with both SSF and LSF cultivation. Platotex offers 2 m2 of agar surface and combines automatic sterilization, cultivation, and drying steps. Furthermore, Platotex supports LSF and SSF through five 7.5-L cultivation containers combined with the appropriate mixing accessories.
Materials and methods
Chemicals, microbial strain, cultivation media, and conditions
All culture medium reagents were purchased from Difco. The strain Streptomyces sp. lma-S835 was isolated from a soil sample and identified by analyzing the 16S rRNA. Microorganisms were grown on LMA10 that corresponded to modified ISP-2. The LMA10 contained in 1 L distilled water the following components: yeast extract, 4 g; malt extract, 10 g; glucose, 4 g; tryptophan, 0.2 g; and tyrosine, 0.2 g. The agar version of the medium contained the same ingredients in addition to 20 g bacto-agar.
Platotex
The device concept, shape, and design were previously developed in our laboratory [5]. The Platotex tank was built by Pierre Guerin Technologies (Mauze, France). The fluidic circuits (steam, air, and water), the touchscreen remote panel, and the Platotex manager software (PMS) were developed in our laboratory. Communication is implemented using Omron electronic components. Three-dimensional (3D) digitization and fluidic simulations were performed by Matra-Pininfarina-Maroc (Casablanca, Marroco). Platotex was 3D-digitized using CATIA-V5 software from Dassault-System (Velizy-Villacoublay, France). Air simulations in the Platotex were performed using Fluent software (Fluent, Montigny Le Bretonneux, France). A Proline-RP845C cryothermostat (Lauda, Neuilly sur Seine-France) was used to ensure regulation of the external temperature.
Several other companies contributed components for this project as follows:
External stainless-steel accessories: ERMI (Cergy-pontoise, France)
Fluidic components: Asco/Joumatic (Rueil Malmaison, France), Swagelok (Villebon-sur-Yvette, France), Radiospares (Beauvais, France), and Vega (Erstein, France)
Automation and communication: Omron (Rosny-sous-Bois, France)
Software development: Sysmatec (Eyholz, Switzerland)
The pictures presented in this paper were taken by Sébastien Godefroy in partnership with Adele Vanot from Photothèque-CNRS.
Antibiotic screening
The inhibition zone technique was used for screening antibiotic activity. The test strains used were Escherichia coli ATCC 25922, Bacillus subtilis ATCC 6633, and Micrococcus luteus ATCC 10240. Inhibition zones were measured for 100 μg extract or pure compound adsorbed on the appropriate 6-mm paper discs and compared with antibiotic control discs containing 10 μg gentamicin for B. subtilis and 30 μg chloramphenicol for E. coli and M. luteus.
Laboratory-scale cultivation and extraction
Streptomyces sp. lma-S835 was grown in 3 L liquid LMA10 medium and five 25 × 25 cm Petri plates containing 300 mL agar complemented by LMA10. Both the liquid and agar medium were inoculated with 3-day-old liquid preculture. After 9 days of growth, the liquid medium was extracted using ethyl acetate (3 × 1 L), and the agar/biomass mixture was lyophilized and extracted with 500 mL ethyl acetate using a ASE-300 Dionex system (Voisins le Bretonneux, France). Extracts were then dried under vacuum and subjected to biological screening and analysis.
Cultivation on Platotex and the associated extraction procedure
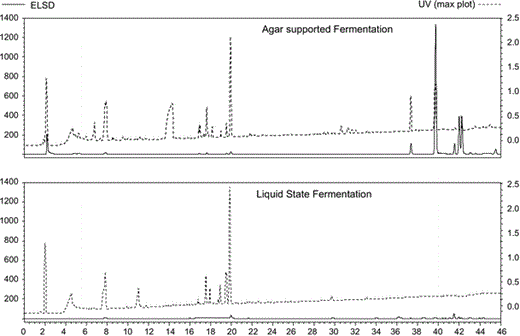
Comparison of MSM production between AgSF and LSF. Growth conditions, extraction procedure, and analytical techniques are reported in the experimental section. The compound proportions were verified by evaporative light scattering detection (ELSD) (continuous lines), while diversity was verified by both ELSD and ultraviolet (UV) detection (dashed lines)
High-performance liquid chromatography (HPLC)
HPLC was performed using an Alliance 2695 module, a 996-photodiode array detector, and an evaporative light scattering detector 2420 (ELSD), both controlled by Millennium software (Waters corporation). The column used was a C18-Sunfire (100 mm × 3.5 mm i.d.; Waters) eluted at 0.7 mL/min with a linear gradient from water (0.1% formic acid) to acetonitrile (0.1% formic acid) in 50 min.
Reversed-phase liquid chromatography and tandem mass–mass analysis (LC-MS/MS)
LC–MS/MS analyses were undertaken on an UltiMate 3000 chromatographic system (Dionex) coupled to a hybrid LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific). A 30 μg sample was injected into a 100 mm × 3.5 mm C18-Sunfire column (3.5 micron). The sample was eluted using a gradient from solvent A (0.1% formic acid in water) to 63% solvent B (0.1% formic acid in acetonitrile) in 17 min at 150 μL/min. The sample was also detected using UV light at 310 nm with diode array detection (DAD) scanning.
Electrospray ionization was used for mass detection in both positive and negative mode. A spray was obtained while working at capillary temperature of 275°C and adjusting the voltage applied (−3.1 kV for negative mode, +4.5 kV for positive mode).
One series of MS analyses consisted of acquiring cycles composed of one MS scan in an Orbitrap analyzer (profile mode, Rs = 30,000, m/z range 295–2,000) followed by MS/MS scans triggered on the most intense species detected in the preceding MS scan.
Results and discussion
Strain Streptomyces sp. lma-S835 was cultivated using both AgSF and LSF. The media composition and cultivation time were similar. The extraction procedures were adapted to each support: solid/liquid for lyophilized agar layer and liquid/liquid for the liquid media. As shown in Table 2, the extracted quantities were similar for both methods, although AgSF required less media for cultivation and less solvent for extraction. The whole extract from AgSF exhibited a larger spectrum and antibiotic activity as compared with LSF. Comparison of the HPLC profiles showed more diversity and higher quantity of compounds for AgSF than for LSF. The latter is revealed by the ELSD detection, which is proportional to compound abundance in the extract (Fig. 1). In particular, a peak at retention time of 39.5 min is present in the AgSF extract and absent in the LSF counterpart. A portion of this peak (0.3 mg) isolated by HPLC was responsible for the antibiotic activity of the AgSF whole extract. The purification yield was too low to allow further structural or biological analysis.
Comparison between LSF and AgSF cultivation of Streptomyces sp. lma-S835
| . | Antibiotic activity (% of control) . | |||||
|---|---|---|---|---|---|---|
| Volume of medium (L) . | Volume of solvent (L) . | Whole extract (mg) . | E. coli . | B. subtlis . | M. luteus . | |
| LSF | 3 | 3 | 53 | 0 | 0 | 30 |
| AgSF | 1.5 | 0.5 | 75 | 47 | 100 | 55 |
| . | Antibiotic activity (% of control) . | |||||
|---|---|---|---|---|---|---|
| Volume of medium (L) . | Volume of solvent (L) . | Whole extract (mg) . | E. coli . | B. subtlis . | M. luteus . | |
| LSF | 3 | 3 | 53 | 0 | 0 | 30 |
| AgSF | 1.5 | 0.5 | 75 | 47 | 100 | 55 |
The inhibition zone technique was applied to screen for antibiotic activity. Inhibition diameter obtained for 100 μg extract was compared using paper discs and antibiotic control discs (10 μg gentamicin for B. subtilis, 30 μg chloramphenicol disc for E. coli and M. luteus)
Comparison between LSF and AgSF cultivation of Streptomyces sp. lma-S835
| . | Antibiotic activity (% of control) . | |||||
|---|---|---|---|---|---|---|
| Volume of medium (L) . | Volume of solvent (L) . | Whole extract (mg) . | E. coli . | B. subtlis . | M. luteus . | |
| LSF | 3 | 3 | 53 | 0 | 0 | 30 |
| AgSF | 1.5 | 0.5 | 75 | 47 | 100 | 55 |
| . | Antibiotic activity (% of control) . | |||||
|---|---|---|---|---|---|---|
| Volume of medium (L) . | Volume of solvent (L) . | Whole extract (mg) . | E. coli . | B. subtlis . | M. luteus . | |
| LSF | 3 | 3 | 53 | 0 | 0 | 30 |
| AgSF | 1.5 | 0.5 | 75 | 47 | 100 | 55 |
The inhibition zone technique was applied to screen for antibiotic activity. Inhibition diameter obtained for 100 μg extract was compared using paper discs and antibiotic control discs (10 μg gentamicin for B. subtilis, 30 μg chloramphenicol disc for E. coli and M. luteus)
At this point in the study, we faced a lack of any commercially available device to scale up AgSF, which drove us to design Platotex. During the feasibility study, we focused on how to maintain characteristics similar to a classical fermentation apparatus while keeping the system open for further improvements and applications. Our aim was not to build a pilot unit for our own purposes, but to develop an open-access AgSF scale-up facility for others. For this reason, the device was required to adhere to international security and quality requirements while enabling integrated automation and software-based remote control.
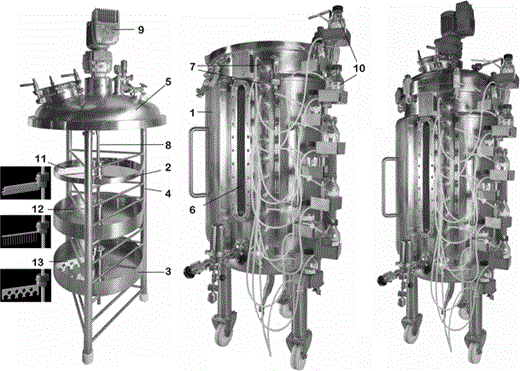
Platotex consists of three principal components: a cylindrical 500-L tank 1 that accepts a frame 4 supporting the cultivation plates and mounted with a cover 5 that fits over the tank opening to ensure tight closure
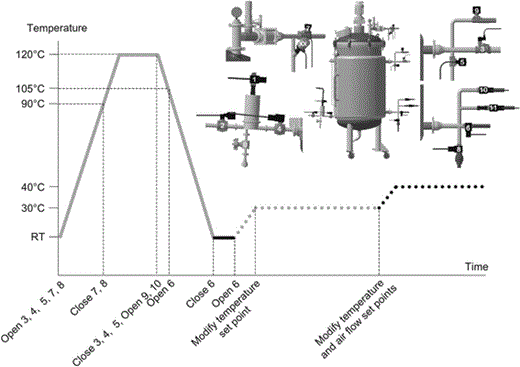
Electromagnetic valve management during Platotex operations: sterilization (grey continuous line), inoculation (black continuous line), cultivation (grey dotted line), and drying (black dotted line). All steps are automatically managed through the Platotex manager software (PMS). RT room temperature
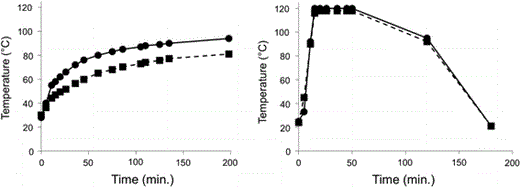
Time course of temperature during the sterilization process by heating the double envelope (left) or coupling with steam injection into the tank (right). Dark circles correspond to tank temperatures and dark squares to agar temperatures in the plates
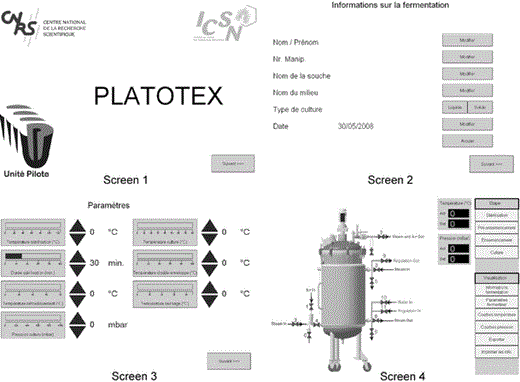
Platotex manager software (PMS) is an intuitive interface consisting of four screens dedicated to the storage of operator and culture references and monitoring of pressure and temperature during the process. At any time the operator can visualize and modify the fermentation parameters
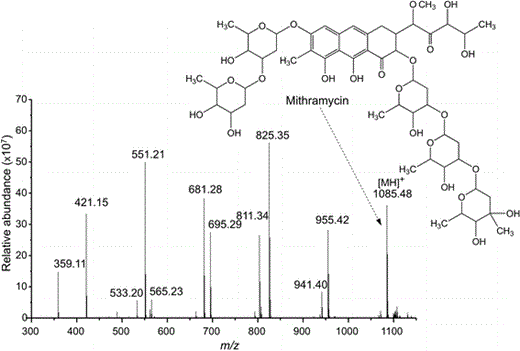
LTQ-Orbitrap MS–MS spectrum for mithramycin. Fragments correspond to the loss of glucoside units

Platotex plate after the growth of Streptomyces sp. lma-S835 in AgSF mode. The strain secretes a dark pigment that colors the agar black. The mirror-quality polish of the stainless steel avoids sticking and facilitates recovery of the agar/biomass layer (right)
In a typical experiment, agar-selected medium was introduced into the plates. Sterilization was performed as previously described. The agar was allowed to solidify by cooling to room temperature. Previously prepared liquid preculture was divided among ten inoculation bottles (7 in Fig. 2). Each bottle contained 200 mL preculture and served to inoculate one plate. Bottles were equipped with inoculation needles that crossed through the seal of each screw hole. The screw holes were positioned so as to drive the needles 15 cm inside each plate. A rotating Teflon blade fixed to a shaft (11 in Fig. 2) that rides through the frame shaft ensures spreading of the preculture on the agar surface. The shaft is connected to a motor (9 in Fig. 2) fixed on top of the tank cover. Once inoculation was achieved, the temperature was maintained at the desired value by circulating heated water in the double envelope. After sterilization, air pressure in the tank was decreased from 1.6 to 0.2 bar to prevent contamination and avoid premature drying of the agar layers. Dial observations were used to monitor progress of the culture. Once the growth period was over, partial or total drying was accelerated by increasing the temperature and air flow. Finally, the cover and frame assembly were taken off using a hoist. Agar layers were removed gently from the plates and pooled in an appropriate vessel. After lyophilization, the dry agar and biomass mixture was ready for solvent extraction.
In order to increase the potential of Platotex, we developed particular accessories to enable LSF and SSF. This helped to rapidly compare the three cultivation modes within one culture cycle and within the same device. SSF and LSF took place in 50-cm-diameter 8-cm-deep plates (3 in Fig. 2). Special stainless-steel blades were designed to perform efficient mixing and to supply air without spattering (12–13 in Fig. 2). Perforated blades 13 were built for LSF and brush blades 12 for SSF. The engine ensured continuous stirring at 10 rpm.
Omron automation and communication components were connected to the electromagnetic valves; interfacing was managed by Cx-One and Cx-Supervisor software. A graphical user interface was implemented using a touch-sensitive screen to allow total remote control of the process through homemade software (PMS) (Fig. 5).
Cultivation of Streptomyces sp. lma-S835 on Platotex helped to easily isolate the compound responsible for biological activity. This was analyzed using reversed-phase LC–MS/MS analysis on an LTQ-Orbitrap instrument. Spectra were matched with MSM databases (AntiBase, Wiley). The compound was identified as mithramycin, an antibiotic and cytotoxic compound previously reported [7].
References
Ouazzani J, Cortial S, Sergent D, Lopes P (2007) Platotex, device for the cultivation of microorganisms and cells. (WO/2007/135098), PCT/EP2007/054834
Ouazzani J, Cortial S, Sergent D, Lopes P (2008) Method for solid/liquid extraction of substances from a mass of dry material and device for carrying out said method. (WO 2008/141943 A1), PCT/EP2008/055659, see also www.zippertex.com



