-
PDF
- Split View
-
Views
-
Cite
Cite
Sasithorn Kongruang, Growth kinetics of biopigment production by Thai isolated Monascus purpureus in a stirred tank bioreactor, Journal of Industrial Microbiology and Biotechnology, Volume 38, Issue 1, 1 January 2011, Pages 93–99, https://doi.org/10.1007/s10295-010-0834-2
Close - Share Icon Share
Abstract
Monascus purpureus is a biopigment-producing fungi whose pigments can be used in many biotechnological and food industries. The growth kinetics of biopigment production were investigated in a liquid fermentation medium in a 5-l stirred tank bioreactor at 30°C, pH 7, for 8 days with 100 rpm agitation and 1.38 × 105 N/m2 aeration. Thai Monascus purpureus strains TISTR 3002, 3180, 3090 and 3385 were studied for color production, growth kinetics and productivity. Citrinin as a toxic metabolite was measured from the Monascus fermentation broth. The biopigment productions were detected from fermentation broth by scanning spectra of each strain produced. Results showed a mixture of yellow, orange and red pigments with absorption peaks of pigments occurring at different wavelengths for the four strains. It was found that for each pigment color, the color production from the strains increased in the order TISTR 3002, 3180, 3090, 3385 with 3385 production being approximately 10 times that of 3002. Similar results were found for growth kinetics and productivity. HPLC results showed that citrinin was not produced under the culture conditions of this study. The L*, a* and b* values of the CIELAB color system were also obtained for the yellow, orange and red pigments produced from the TISTR 3002, 3180, 3090 and 3385 strains. The colors of the pigments ranged from burnt umber to deep red.
This article is part of the BioMicroWorld 2009 Special Issue.
Introduction
Red pigment is one of the most important color attributes for the acceptance of many types of food. Natural colorants derived from plants and microorganisms have recently gained popularity over synthetic coloring agents, which are potential carcinogens in some cases [1]. As there is considerable concern over the consumption of foods that may pose a health hazard, natural colors are gradually gaining popularity as an alternative. The microorganisms used for fermenting red pigment are various species of filamentous fungi known as Monascus. Pigments produced by the mold Monascus purpureus offer a possible alternative to certified food dyes or other natural pigments now in use [2]. Many reports have shown that Monascus species produce at least six major related pigments, which are categorized as (1) orange pigments: rubropunctain and monascorubin; (2) yellow pigments: monascin and ankaflavin; (3) red pigments: rubropunctamine and monoscorubramine [3–5]. Compoy et al. [6] have recently shown that Monascus species can also produce two new pigments, a red pigment and a yellow pigment, that they characterized by nuclear magnetic resonance. Other functional metabolite compounds that have been proved to be produced by this fungi are monacolin K (antihypercholestemic agent), citrinin (bactericidal against gram-positive bacteria with nephrotoxic activity), г-aminobutyric acid (hypertensive agent) and dimerumic acid (antioxidant) [7]. Monascus pigments are now used in processed seafood, sausages and sauces in Asia to replace some food additives such as cochineal, potassium nitrate and nitrites [4, 8, 9]. Certain yellow pigments such as monascin have been reported as potential anti-inflammation agents [10]. Traditionally, Monascus has been cultured by solid state fermentation on rice and other cereals, such as rice bran, wheat bran, bread, oat, corn, wheat grain, jackfruit seed and dioscorea [10–16]. A carbon source that is now being successfully used in Thailand is cassava starch. It is widely used in many industries and is considered as a cheap substrate for production of, for example, pigments, monosodium glutamate, sweetener, amino acid and ethanol. Growth kinetics and production of these fungi in submerged culture have been discussed in several articles [7, 11, 17, 18]. However, there has been only limited investigation and analysis of the kinetics of growth in stirred tank bioreactors, especially for pigment production by Monascus purpureus. Thus, this study aimed to examine and compare pigment production in a stirred tank bioreactor of four Thai Monascus purpureus strains using a modified YM medium supplemented with cassava starch as the carbon source and monosodium glutamate as the nitrogen source. The level of citrinin was also evaluated.
Materials and methods
Chemicals
Citrinin was purchased from Sigma Chemical Co., (St. Louis, MO). LC grade acetonitride was purchased from Merck Co. (Darmstadt, Germany). Potato dextrose agar, yeast malt extract, yeast extract and peptone were purchased from Himedia Laboratories (PVt., Ltd., India).
Microorganism and growth medium
Monascus purpureus TISTR 3002, 3180, 3090 and 3385 were obtained from the Thailand Institute of Scientific and Technology Research, Bangkok, Thailand. Stocks of cultures in freeze-dried ampoules were activated in YM broth at 30°C for 2 days and transferred to a PDA and further incubated for 7 days. A spore suspension of 106 spores/ml of 0.1% tween 80 was initially transferred to a 30-ml modified YM broth (5% peptone, 3% yeast extract powder, 3% monosodium glutamate and 10% cassava starch in distilled water). Culture was grown on the shaking incubator at 200 rpm, 30°C, for 7 days. All cultivation broths were then transferred to 300 ml of modified YM broth and incubated again under the same conditions as above.
A 10% culture inoculum was added to 3 l fermentation broth in a 5-l stirred tank bioreactor (Bio Flow 3000, New Brunswick, NJ). Then 5 ml of antifoam was added, and the fermentation conditions were set at pH 7, 30°C, 1.38 × 105 N/m2 aeration rate and 100 rpm agitation rate for 8 days. During pigment production, the pH was maintained at 7.0 by automatic addition of 4 M NaOH/4 M H2SO4; 20-ml samples were taken every 2 days for measurement of fermentation parameters.
Measurements of fermentation parameters
Culture broths were filtrated through filter paper no. 1 and washed twice with distilled water. The filter paper containing the cells from the broth was dried for 24 h at 80°C in the hot air oven. The cell dry weight was then determined as the difference between the dry weight of the filter paper before and after the filtration. The supernatant was collected to determine the residual sugar content using the Anthrone method [19]. Biomass concentration was estimated by measuring absorbance at 600 nm. Production of yellow, orange and red pigments was estimated by using a spectrophotometer to measure absorption at 400, 460 and 500 nm. The growth kinetics were reported in terms of rate of production of yellow, orange and red pigment (rpy, rpo and rpr, UA/d), specific rate of pigment formation (Qpy, Qpo, Qpr, UA-L/d), biomass yield; Yx/s (g/g), product yield from substrate; Yp/s (UA/g), product yield from biomass, Yp/x (UA/g) and productivity, P (UA/g/d).
Citrinin determination
The fungal extracts were filtered through a 0.45-μm filter and analyzed for citrinin content by HPLC. Standard citrinin solutions were prepared by dissolving citrinin (Sigma Co. Ltd.) in 100% acetonitrile and accurately diluting to 5, 10, 20, 30, 40 and 50 μg/ml. The injection volume for HPLC was 20 μl. An HPLC system (Waters Chromatography Division, Milford, MA) was used to perform an HPLC analysis using the method previously described by Lee et al. [20]. A C18 column, 25 cm × 4.6 mm, i.d. 5 μm, was used as the analytical column. The mobile phase contained acetonitrile 55%, nano water 45% and trifluoroacetate 0.1%. The flow rate was set at 1 ml/min, and citrinin was detected using a UV detector set at 238 nm. A calibration curve was determined with the concentrations and the relevant peak area.
Color analysis
For the color analysis, the concentration of pigment solutions of the medium for M. purpureus at 500 nm was adjusted to obtain absorbance values in the range of 1–2. The values of L*, a* and b* were measured by a Hunter Lab Color Quest (Memmert, Germany) colorimeter with the CIELAB color system. These values were then used to calculate chroma (C*) and hue angle (h ab) values. L* indicates lightness from 0 (black) to 100 (white). Positives and negatives in a* represent red and green, respectively, whereas positives and negatives in b* represent yellow and blue, respectively. Chroma values denote the saturation or purity of the color. Values close to the center at the same L* value indicate dull or gray colors, whereas values near the circumference represent vivid or bright colors. Hue angle values represent 0 for redness, 90 for yellowness, 180 for greenness and 270 for blueness.
Statistical analyses
Each treatment was conducted in triplicate, and all experiments were repeated at least twice. The statistical significance of the evaluated data was analyzed by one-way analysis of variance. Differences among the mean values were tested using the least significant difference multiple range test. Values were considered significant when P < 0.05, except when otherwise indicated.
Results and discussion
Effect of strain on pigment production
In this study, Thai isolated strains of Monascus purpureus were studied in a stirred tank bioreactor, which was maintained with a high oxygen-transfer capacity and with good mixing. The aim was to compare pigment production by different strains under the same conditions of agitation rate and oxygen transfer rate. We used an agitation rate of 100 rpm and aeration rate of 1.38 × 105 N/m2. Under these conditions, the fungi appeared to be evenly distributed in the tank, there was no evidence of clumping, and a high pigment yield was obtained. We found that at 100 rpm agitation, high cell activities and low viscosities could be maintained without serious cell aggregation or cell shortening. Under conditions of strong agitation, small pellets and short mycelia have been reported by previous authors [21–24]. Kim et al. [9] have studied the effects of different agitation rates. They reported that cells grown at 200 rpm consisted of highly branched and elongated mycelia. As the rotational speed increased to 500 rpm, the mycelia became shorter and less branched. When the rotation rate was further increased to 700 rpm, a large number of short, seriously damaged mycelia fragments were observed. Therefore, 100 rpm seems to be an acceptable choice for this study as the cell damage is low and adequate mixing appears to be obtained.
The M. purpureus strains TISTR 3002, 3180, 3090 and 3385 grew on the media, and each of them produced yellow, orange and red pigments (Table 1). The spectrophotometric profiles of the fungal extracts produced from all strains were qualitatively similar (spectra not shown). However, as shown in Table 1, there were appreciable quantitative differences in the kinetics of pigment productions, biomass yields, pigment yield from biomass and calculated values of pigment yield from substrate.
The growth kinetic parameters during the fermentation of Monascus purpureus strains grown in modified medium supplement at 30°C for 2 weeks
| Strains . | Pigment . | Yp/x (UA/g) . | Yp/s (UA/g) . | rpy (UA/d) . | rpo (UA/d) . | rpr (UA/d) . | Yx/s (g/g) . |
|---|---|---|---|---|---|---|---|
| TISTR 3002 | Yellow | 0.76 | 509.83 | 0.064 | 0.023 | 0.011 | 0.07 |
| Orange | 0.72 | 557.26 | |||||
| Red | 0.66 | 580.97 | |||||
| TISTR 3180 | Yellow | 2.03 | 157.97 | 1.161 | 0.632 | 0.473 | 0.08 |
| Orange | 2.00 | 155.35 | |||||
| Red | 2.05 | 159.34 | |||||
| TISTR 3090 | Yellow | 3.99 | 2.68 | 0.090 | 0.119 | 0.065 | 0.67 |
| Orange | 3.99 | 2.68 | |||||
| Red | 4.19 | 2.82 | |||||
| TISTR 3385 | Yellow | 8.62 | 4.52 | 0.073 | 0.079 | 0.064 | 0.52 |
| Orange | 10.47 | 5.49 | |||||
| Red | 10.18 | 5.34 |
| Strains . | Pigment . | Yp/x (UA/g) . | Yp/s (UA/g) . | rpy (UA/d) . | rpo (UA/d) . | rpr (UA/d) . | Yx/s (g/g) . |
|---|---|---|---|---|---|---|---|
| TISTR 3002 | Yellow | 0.76 | 509.83 | 0.064 | 0.023 | 0.011 | 0.07 |
| Orange | 0.72 | 557.26 | |||||
| Red | 0.66 | 580.97 | |||||
| TISTR 3180 | Yellow | 2.03 | 157.97 | 1.161 | 0.632 | 0.473 | 0.08 |
| Orange | 2.00 | 155.35 | |||||
| Red | 2.05 | 159.34 | |||||
| TISTR 3090 | Yellow | 3.99 | 2.68 | 0.090 | 0.119 | 0.065 | 0.67 |
| Orange | 3.99 | 2.68 | |||||
| Red | 4.19 | 2.82 | |||||
| TISTR 3385 | Yellow | 8.62 | 4.52 | 0.073 | 0.079 | 0.064 | 0.52 |
| Orange | 10.47 | 5.49 | |||||
| Red | 10.18 | 5.34 |
The growth kinetic parameters during the fermentation of Monascus purpureus strains grown in modified medium supplement at 30°C for 2 weeks
| Strains . | Pigment . | Yp/x (UA/g) . | Yp/s (UA/g) . | rpy (UA/d) . | rpo (UA/d) . | rpr (UA/d) . | Yx/s (g/g) . |
|---|---|---|---|---|---|---|---|
| TISTR 3002 | Yellow | 0.76 | 509.83 | 0.064 | 0.023 | 0.011 | 0.07 |
| Orange | 0.72 | 557.26 | |||||
| Red | 0.66 | 580.97 | |||||
| TISTR 3180 | Yellow | 2.03 | 157.97 | 1.161 | 0.632 | 0.473 | 0.08 |
| Orange | 2.00 | 155.35 | |||||
| Red | 2.05 | 159.34 | |||||
| TISTR 3090 | Yellow | 3.99 | 2.68 | 0.090 | 0.119 | 0.065 | 0.67 |
| Orange | 3.99 | 2.68 | |||||
| Red | 4.19 | 2.82 | |||||
| TISTR 3385 | Yellow | 8.62 | 4.52 | 0.073 | 0.079 | 0.064 | 0.52 |
| Orange | 10.47 | 5.49 | |||||
| Red | 10.18 | 5.34 |
| Strains . | Pigment . | Yp/x (UA/g) . | Yp/s (UA/g) . | rpy (UA/d) . | rpo (UA/d) . | rpr (UA/d) . | Yx/s (g/g) . |
|---|---|---|---|---|---|---|---|
| TISTR 3002 | Yellow | 0.76 | 509.83 | 0.064 | 0.023 | 0.011 | 0.07 |
| Orange | 0.72 | 557.26 | |||||
| Red | 0.66 | 580.97 | |||||
| TISTR 3180 | Yellow | 2.03 | 157.97 | 1.161 | 0.632 | 0.473 | 0.08 |
| Orange | 2.00 | 155.35 | |||||
| Red | 2.05 | 159.34 | |||||
| TISTR 3090 | Yellow | 3.99 | 2.68 | 0.090 | 0.119 | 0.065 | 0.67 |
| Orange | 3.99 | 2.68 | |||||
| Red | 4.19 | 2.82 | |||||
| TISTR 3385 | Yellow | 8.62 | 4.52 | 0.073 | 0.079 | 0.064 | 0.52 |
| Orange | 10.47 | 5.49 | |||||
| Red | 10.18 | 5.34 |
Maximum production for all three colors of pigment was found in M. purpureus TISTR 3385. The total yields of orange pigment from TISTR 3385 were 15, 5 and 2 times, respectively, and higher than from TISTR 3002, 3180 and 3090. However, the rate of orange pigment production from TISTR 3180 was higher than from 3385 and the other two strains. The measured value of Yx/s = 0.52 g/g for TISTR 3385 was higher than for the other strains, demonstrating that 3385 shows more effective growth. This results in a higher proportion of yellow, orange and red pigment associated with the mycelium cells of 3385. From the results it was evident that as the mycelium growth increased, the amount of pigment produced also increased.
Effect of fermentation time on pigment production
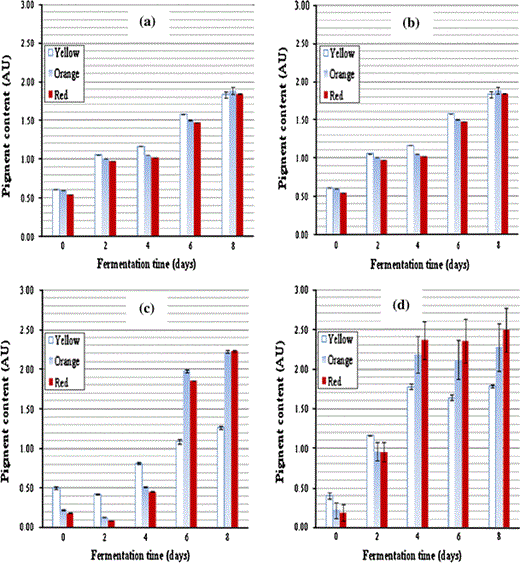
Pigment production at different fermentation time by Monascus purpureus a TISTR 3002, b TISTR 3180, c TISTR 3090 and d TISTR 3385
Pigment productivity
The calculated productivities of yellow, orange and red pigment production are shown in Table 2. The productivities show the same pattern as reported in the previous section. The productivities increased in the order: TISTR 3002, 3180, 3090 and 3385 with productivity of 3385 being approximately 10 times greater than 3002.
The calculated productivities of yellow, orange and red pigment production of Monascus strains
| Strains . | Py (UA/g/d) . | P0 (UA/g/d) . | Pr (UA/g/d) . |
|---|---|---|---|
| TISTR 3002 | 6.37 | 6.97 | 7.26 |
| TISTR 3180 | 19.75 | 19.42 | 19.92 |
| TISTR 3090 | 38.64 | 38.40 | 40.26 |
| TISTR 3385 | 66.28 | 80.20 | 77.98 |
| Strains . | Py (UA/g/d) . | P0 (UA/g/d) . | Pr (UA/g/d) . |
|---|---|---|---|
| TISTR 3002 | 6.37 | 6.97 | 7.26 |
| TISTR 3180 | 19.75 | 19.42 | 19.92 |
| TISTR 3090 | 38.64 | 38.40 | 40.26 |
| TISTR 3385 | 66.28 | 80.20 | 77.98 |
The calculated productivities of yellow, orange and red pigment production of Monascus strains
| Strains . | Py (UA/g/d) . | P0 (UA/g/d) . | Pr (UA/g/d) . |
|---|---|---|---|
| TISTR 3002 | 6.37 | 6.97 | 7.26 |
| TISTR 3180 | 19.75 | 19.42 | 19.92 |
| TISTR 3090 | 38.64 | 38.40 | 40.26 |
| TISTR 3385 | 66.28 | 80.20 | 77.98 |
| Strains . | Py (UA/g/d) . | P0 (UA/g/d) . | Pr (UA/g/d) . |
|---|---|---|---|
| TISTR 3002 | 6.37 | 6.97 | 7.26 |
| TISTR 3180 | 19.75 | 19.42 | 19.92 |
| TISTR 3090 | 38.64 | 38.40 | 40.26 |
| TISTR 3385 | 66.28 | 80.20 | 77.98 |
Citrinin contamination
We examined the chromatograms for the pigments obtained from the four M. purpureus strains and could not detect this citrinin peak. Therefore, we concluded that the fungal extracts for all strains did not produce citrinin during fermentation in the bioreactor under the above conditions. Hajjaj et al. [25] found that in M. ruber ATCC 96218, pigment and citrinin use the same precursor and then move to two different pathways. They showed that citrinin could be produced from the biosynthesis of the tetraketide M. ruber as well as from pentaketides such as Penicillum and Aspergillus species [26]. Although the biosynthesis pathways of pigments and citrinin are related, our results show that among Monascus species the two pathways are independent under the conditions of these experiments. However, we have also conducted experiments (not reported in this paper) on another Monascus strain TISTR 3541 under stress conditions of severely lower pH or ultrasonic induction and found that citrinin could be produced [27]. Similar results showing increased citrinin productions under stress have been reported previously [28–30]. Genetic attributes and culture conditions have also been reported as influencing citrinin production by Monascus strains. Some commercial strains of Monascus that contain no citrinin or only a very low amount have been reported [31, 32].
Comparison analysis of colorimetric values of fungal extract
Some fungal extracts from each strain were selected for further color analysis using the CIELAB colorimetric system. Table 3 shows results of this analysis. Chroma values and hue angles were calculated from the values of L*, a* and b*. When the CIELab values for all fungal extracts were plotted on a polar scatter plot, all values were found to be scattered in the first quadrant. In the present study, the colors of fungal extract were correlated with commercially available standard reference colors. TISTR 3002 was found to be similar to a Br7 raw umber. TISTR 3180 was in the region of Br7 burnt umber, R 101 venetian red and Indian red. TISTR 3090 was similar to Rue perylene scarlet and Q48 quinacridone orange, while TISTR 3385 was the same color as Br7 mar violet (Table 3).
Colorimetric values of fermentation extracts depending on Monascus strains
| Strains . | L* . | a* . | b* . | C . | hab . |
|---|---|---|---|---|---|
| TISTR 3002 | 64.90 | 3.08 | 25.74 | 25.92 | 83.18 |
| TISTR 3180 | 47.30 | 15.78 | 29.42 | 33.39 | 61.80 |
| TISTR 3090 | 32.47 | 38.84 | 3.26 | 51.14 | 40.57 |
| TISTR 3385 | 14.08 | 20.74 | 1.80 | 20.82 | 4.96 |
| Strains . | L* . | a* . | b* . | C . | hab . |
|---|---|---|---|---|---|
| TISTR 3002 | 64.90 | 3.08 | 25.74 | 25.92 | 83.18 |
| TISTR 3180 | 47.30 | 15.78 | 29.42 | 33.39 | 61.80 |
| TISTR 3090 | 32.47 | 38.84 | 3.26 | 51.14 | 40.57 |
| TISTR 3385 | 14.08 | 20.74 | 1.80 | 20.82 | 4.96 |
Colorimetric values of fermentation extracts depending on Monascus strains
| Strains . | L* . | a* . | b* . | C . | hab . |
|---|---|---|---|---|---|
| TISTR 3002 | 64.90 | 3.08 | 25.74 | 25.92 | 83.18 |
| TISTR 3180 | 47.30 | 15.78 | 29.42 | 33.39 | 61.80 |
| TISTR 3090 | 32.47 | 38.84 | 3.26 | 51.14 | 40.57 |
| TISTR 3385 | 14.08 | 20.74 | 1.80 | 20.82 | 4.96 |
| Strains . | L* . | a* . | b* . | C . | hab . |
|---|---|---|---|---|---|
| TISTR 3002 | 64.90 | 3.08 | 25.74 | 25.92 | 83.18 |
| TISTR 3180 | 47.30 | 15.78 | 29.42 | 33.39 | 61.80 |
| TISTR 3090 | 32.47 | 38.84 | 3.26 | 51.14 | 40.57 |
| TISTR 3385 | 14.08 | 20.74 | 1.80 | 20.82 | 4.96 |
Changes in color during fermentation time
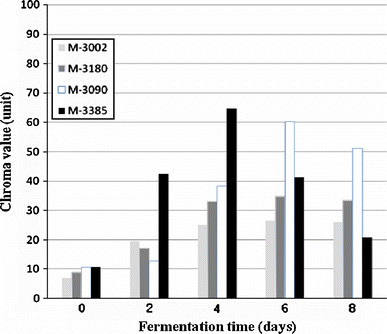
Comparison of time course of chroma values from fermentation broths in each Monascus strain
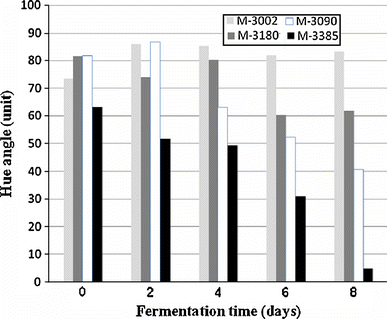
Comparison of time course of hue values from fermentation broths in each Monascus strain
Cassava starch is a relatively cheap source of raw material containing a high concentration of starch (dry-matter basis) that can equal or surpass the properties offered by other starches (maize, wheat, sweet potato and rice). In this study, results showed that cassava starch is a good carbon source as the alternative substrate while using monosodium glutamate as the nitrogen source for pigment production. However, the substrate preference depends on the Monascus strain. As far as the substrate is concerned, tropical agro-industrial residues such as rice bran, wheat bran, coconut oil cake, sesame oil cake, palm kernel cake, groundnut oil cake, cassava powder, spent brewing grain, jackfruit seed powder and tamarind seed powder have proven that all can be used as the substrate for pigment production [33]. The utilization of cheaply available substrates in liquid fermentation could be a good strategy for achieving significant pigment production. Using a stirred tank bioreactor is also one of the options to choose from for pigment production other than air-lit and roller bottle bioreactors. It can enhance the productivity under the consideration of minimizing the mycelial damage.
Acknowledgments
The author is grateful to Dr. Elvin J. Moore for helpful suggestions and a critical reading of this manuscript. This study was partly financed by the Thailand Reserach Fund through a research grant.
References
Rashbaum SA, Barrington EMY (1983) Natural red coloring prepared from an oat substrate. US Patent 4418081
Suktham K, Suwansungsa O, Wonganu B, Kongruang S, Roytrakul S (2010) Identification of citrinin biosynthesis gene under the ultrasonic induction of Monascus purpureus. International Conference on Chemistry and Chemical Engineering. pp 35–39



