-
PDF
- Split View
-
Views
-
Cite
Cite
Virgilio Giannone, Chiara Longo, Arcangelo Damigella, Domenico Raspagliesi, Alfio Spina, Massimo Palumbo, Technological properties of bakers’ yeasts in durum wheat semolina dough, Journal of Industrial Microbiology and Biotechnology, Volume 37, Issue 4, 1 April 2010, Pages 371–379, https://doi.org/10.1007/s10295-009-0682-0
Close - Share Icon Share
Abstract
Properties of 13 Saccharomyces cerevisiae strains isolated from different sources (traditional sourdoughs, industrial baking yeasts etc.) were studied in dough produced with durum wheat (Sicilian semolina, variety Mongibello). Durum wheat semolina and durum wheat flour are products prepared from grain of durum wheat (Triticum durum Desf.) by grinding or milling processes in which the bran and germ are essentially removed and the remainder is comminuted to a suitable degree of fineness. Acidification and leavening properties of the dough were evaluated. Strains isolated from traditional sourdoughs (DSM PST18864, DSM PST18865 and DSM PST18866) showed higher leavening power, valuable after the first and second hours of fermentation, than commercial baking yeasts. In particular the strain DSM PST 18865 has also been successfully tested in bakery companies for the improvement of production processes. Baking and staling tests were carried out on five yeast strains to evaluate their fermentation ability directly and their resistance to the staling process. Amplified fragment length polymorphism (fAFLP) was used to investigate genetic variations in the yeast strains. This study showed an appreciable biodiversity in the microbial populations of both wild and commercial yeast strains.
Introduction
In Italy more than 200 types of traditional breads have been classified by the Istituto Nazionale di Sociologia Rurale [5]. Only in Sicily, more than 50 genotypes of traditional durum wheat breads have been classified in the “Atlante del pane di Sicilia” [2]. In Mediterranean areas, the use of durum wheat for bread production is ever increasing [17], and durum wheat semolina is preferred to common wheat for its sensorial characteristics [14]. Different technologies of production are used, and many types of bread are obtained, whose features vary according to local habits [9–11, 13, 16, 18, 20]. The fermentation process and the yeast strains have a great influence on bread making with durum wheat and on the characteristics of the bread [19]. Particularly, the way of rising and the yeast characteristics influence the transformation process and the final product appreciably [7]. Sourdoughs are used in more than 30% of bakery products; some of them originate from a very old tradition and differ in the type of flour, ingredients, type of sourdough technology and shelf-life [3]. Yeast flora in sourdough are more homogenous. Universal sourdough yeast appears to be Candida milleri or strains closely related to it. Saccharomyces cerevisiae is also often reported [21].
Generally, baker’s yeast refers to the strains of S. cerevisiae used in the baking industry. Baker’s yeast is required to have several characteristics, such as high leavening ability, tolerance to sugars, freeze and chemicals, melibiose utilization, good storage and non-agglomeration [25]. Some of these characteristics are not necessarily required for bread making, but a high leavening ability is the most important characteristic to have for making bread of good quality and to save time in making it.
To investigate genetic variation in baker’s yeast strains, amplified fragment length polymorphism (fAFLP) was used. This technique, based on the selective PCR amplification of restriction fragments from a total digest of DNA [24], has been used as molecular markers, mostly for plant breeding programs [23], but also for mammalian species [8]. More recently, the effectiveness of fAFLP for taxonomy and genetic diversity studies has been demonstrated in a number of biological systems. In this work, the advantages of fAFLP are to use for strain differentiation and species identification in yeasts.
The aim of this study was to investigate the differences between commercial baker’s yeast strains and wild-type yeast strains by comparing their leavening ability on a Sicilian durum semolina. Semolina is made from the endosperm (the core or heart), coarsely ground and then sifted. The coarser material becomes semolina, and the finer parts become durum flour. The durum flour has the texture of other flours, whereas the semolina is close in texture to granulated white sugar or coarse cornmeal. In south Italy, finely ground semolina is used along with white flour, in equal quantities, to make quite dense, but very flavorful bread.
Materials and methods
Strains and growth media
Thirteen strains of S. cerevisiae were obtained from either commercial yeast products or from artisan sourdough (see Table 1). The strains YS7, YS8 and YS9 were deposited at the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH on 14 December 2006, with the following deposit numbers: DSM PST 18864, DSM PST 18865 and DSM PST 18866.
List of strains used in this study
| Collection number . | Reference or source . | Description . |
|---|---|---|
| YS1 | SFY1 | American baker’s yeast (San Francisco) |
| YS2 | SAY1 | South African baker’s yeast |
| YS3 | WFY1 | New Zealand baker’s yeast |
| YS4 | RFY1 | New Zealand baker’s yeast |
| YS5 | ICY1 | Italian baker’s yeast (Camaldoli) |
| YS6 | TDY1 | Australian baker’s yeast (Tasmania) |
| YS7 | DSM PST 18865 | Wild yeast from sourdough (Pachino, Sicily) |
| YS8 | DSM PST18866 | Wild yeast from sourdough (Ispica, Sicily) |
| YS9 | DSM PST 18864 | Wild yeast from sourdough (Ispica, Sicily) |
| YS10 | ZP 1 | Chinese baker’s yeast (Zhuhai district) |
| YS11 | CS 117 | Industrial baker’s yeast (Italy) |
| YS12 | Pur | Industrial baker’s yeast (Puratos, Belgium) |
| YS13 | CL1 | Industrial baker’s yeast (Le Saffre, France) |
| Collection number . | Reference or source . | Description . |
|---|---|---|
| YS1 | SFY1 | American baker’s yeast (San Francisco) |
| YS2 | SAY1 | South African baker’s yeast |
| YS3 | WFY1 | New Zealand baker’s yeast |
| YS4 | RFY1 | New Zealand baker’s yeast |
| YS5 | ICY1 | Italian baker’s yeast (Camaldoli) |
| YS6 | TDY1 | Australian baker’s yeast (Tasmania) |
| YS7 | DSM PST 18865 | Wild yeast from sourdough (Pachino, Sicily) |
| YS8 | DSM PST18866 | Wild yeast from sourdough (Ispica, Sicily) |
| YS9 | DSM PST 18864 | Wild yeast from sourdough (Ispica, Sicily) |
| YS10 | ZP 1 | Chinese baker’s yeast (Zhuhai district) |
| YS11 | CS 117 | Industrial baker’s yeast (Italy) |
| YS12 | Pur | Industrial baker’s yeast (Puratos, Belgium) |
| YS13 | CL1 | Industrial baker’s yeast (Le Saffre, France) |
List of strains used in this study
| Collection number . | Reference or source . | Description . |
|---|---|---|
| YS1 | SFY1 | American baker’s yeast (San Francisco) |
| YS2 | SAY1 | South African baker’s yeast |
| YS3 | WFY1 | New Zealand baker’s yeast |
| YS4 | RFY1 | New Zealand baker’s yeast |
| YS5 | ICY1 | Italian baker’s yeast (Camaldoli) |
| YS6 | TDY1 | Australian baker’s yeast (Tasmania) |
| YS7 | DSM PST 18865 | Wild yeast from sourdough (Pachino, Sicily) |
| YS8 | DSM PST18866 | Wild yeast from sourdough (Ispica, Sicily) |
| YS9 | DSM PST 18864 | Wild yeast from sourdough (Ispica, Sicily) |
| YS10 | ZP 1 | Chinese baker’s yeast (Zhuhai district) |
| YS11 | CS 117 | Industrial baker’s yeast (Italy) |
| YS12 | Pur | Industrial baker’s yeast (Puratos, Belgium) |
| YS13 | CL1 | Industrial baker’s yeast (Le Saffre, France) |
| Collection number . | Reference or source . | Description . |
|---|---|---|
| YS1 | SFY1 | American baker’s yeast (San Francisco) |
| YS2 | SAY1 | South African baker’s yeast |
| YS3 | WFY1 | New Zealand baker’s yeast |
| YS4 | RFY1 | New Zealand baker’s yeast |
| YS5 | ICY1 | Italian baker’s yeast (Camaldoli) |
| YS6 | TDY1 | Australian baker’s yeast (Tasmania) |
| YS7 | DSM PST 18865 | Wild yeast from sourdough (Pachino, Sicily) |
| YS8 | DSM PST18866 | Wild yeast from sourdough (Ispica, Sicily) |
| YS9 | DSM PST 18864 | Wild yeast from sourdough (Ispica, Sicily) |
| YS10 | ZP 1 | Chinese baker’s yeast (Zhuhai district) |
| YS11 | CS 117 | Industrial baker’s yeast (Italy) |
| YS12 | Pur | Industrial baker’s yeast (Puratos, Belgium) |
| YS13 | CL1 | Industrial baker’s yeast (Le Saffre, France) |
The yeasts cells were isolated to the stationary phase at 28°C for 3–5 days in YPG medium (containing, in g l−1, glucose 20, yeast extract 10 and bacteriological peptone 10, and agar 20 was added to solidify the medium). Yeast strains were maintained on YEPG agar at 4°C in sterile vials.
Dough preparation and determination of leavening ability
For the determination of leavening ability, strains were grown in an orbital shaker (200 rev min−1) at 30°C in 2,000-ml Erlenmeyer flasks containing 1,000 ml of YPG broth, pH 6.0. Cells were harvested at stationary phase by centrifugation (4,785xg, 15 min, 4°C), washed twice with ice-cold distilled water and filtered to obtained the yeast cake. Yeasts were added to the dough at 3% (2.1–2.5 × 1010 viable cells g−1). The following ingredients were mixed in a dough mixer (Bakermix 80): 250 g of flour, 7.5 ml of yeast (wet weight), 5 g of salt and 135 ml of water at 20°C. After mixing, the doughs were put in sterile cylinders and incubated at 35°C per 4 h. The leavening power was calculated at intervals of 1 h during the incubation time as the difference (volume increase) between the values immediately post inoculation (t = 0 h) and the values after the successive time points.
Dough pH and total titratable acidity
Dough pH was taken (three repetitions) by directly immersing the electrode in the doughs. Total titratable acidity (TTA) was measured with NaOH N/9 to pH 8.6, using phenolphthalein. Fifteen grams of dough (from the center of the loaf) was shaken for 30 min in 100 ml of water, and total acidity was titrated twice (three repetitions) to pH 6.6 with NaOH N/9 [22]. TTA was expressed as meq. acetic acid g−1, calculated as: vol NaOH (ml) × Normality (meq mol ml−1)/weight of sample (g).
Durum wheat semolina
Sicilian durum wheat semolina (var. Mongibello, OGM free, W = 232.99, P/L = 1.34, water adsorption = 61.31%, total proteins = 15.10%, moisture = 14.70%, yellow index of semolina = 23.77, HMW glutenins subunits at Glu-B1 = 7 + 8) was used for dough preparation [9].
Experimental baking test
With the aim of studying the interaction between durum wheat semolina and yeast strains, a handling bread-making test was carried out. The baking test was carried out according to the traditional process system adopted by a local breadmaker in Messina (Villafranca T., Sicily) for obtaining three loaves (replications) using the following ingredients: 300 g of semolina derived from the cultivar Mongibello, 165 ml of water, 9 ml of yeast solution at 3% and 6 g of sodium chloride.
Fermentation was carried out at 28°C in 130 min with a relative humidity of 85%. Cooking time was 23 min at 225°C.
On the loaves the following parameters were recorded: volume, height, weight, external and internal firmness, crumb color, texture and regularity of porosity, crust thickness, color and roughness. Texture was estimated by means of the Mohs scale. Crumb and crust colors were measured by means of a CR 300 Minolta colorimeter.
In order to estimate the bread staling process, internal and external firmness, weight, height and volume of loaves were measured since the day of baking and 1, 3 and 7 days after baking. Bread was stored in a paper box at room temperature. External and internal firmness was measured using a penetrometer firmness tester (diameter metal point: 0.8 cm). Analysis of variance and Duncan tests were performed to determine differences among types of yeast strains and, for each type, among storage times.
Statistics
All statistical analyses were performed using Cohort software (798 Lighthouse Ave. PMB 320, Monterey, CA) for Windows (Costat Anova version 6.311). Leavening ability, acidification properties, baking and staling process data were analyzed by variance analysis (ANOVA) using Duncan’s multiple range test. The experimental design included three or five trials for each experimental condition.
Yeast DNA isolation and fAFLP analysis
The DNA was extracted following the protocol proposed by Guthrie and Fink [4]. DNA products were examined in a 2% agarose gel stained with ethidium bromide. The DNA was quantified by spectrophotometric reading (A260 nm).
The AFLP reactions were performed as described by Vos et al. [24] with some modifications. For the results shown in this study, T4 DNA buffer ligasi 10X (Invitrogen), NaCl 0.5 M, BSA 1 mg ml−1, EcoRI adapter 5.0 μM, MseI adapter 50.0 μM, EcoRI 20.0 U μl−1, MseI 10 U μl−1 and T4 DNA ligase 6.0 U/μl (Invitrogen) were added to 0.5 pg yeast DNA for a final volume 30.0 μl. The reactions were incubated for another 180 min at 37°C. The second-step AFLP reaction was performed in a 20.0-μl reaction volume adding the following to 3.0 μl of the previous reaction PCR buffer 10× (Invitrogen): MgCl2 20 mM, dNTPs 2.5 mM, EcoRI (+1) primer (5′-GACTGCGTACCAATTC + A-3′) 10 μM, MseI (+1) primer (5′-GATGAGTCCTGAGTAA + C-3′) 10 μM and Taq recombinant polymerase 5.0 U μl−1, performed in 13.0-μl volume. Thermocycling conditions were: 72°C for 2 min, 20 cycles of 20 s at 94°C, 30 s at 56°C, 2 min at 72°C and a final extension of 2 min at 72°C and 30 min at 60°C (Eppendorf, “Mastercycler Epgradient” Thermocycler, UK). PCR products were examined in a 2% agarose gel stained with ethidium bromide. The selective PCR reaction was performed using the primers EcoRI + AGG(5′-GACTGCGTACCAATTC + AGG-3′) and MseI + CTA(5′-GATGAGTCCTGAGTAA + CTA-3′), the primer EcoRI + AGG marked with fluorescence Cy5, PCR buffer 10x (Invitrogen), MgCl2 20 mM, dNTPs 2.5 mM, EcoRI primer (+3) marked 10 μM, MseI primer (+3) marked 10 μM, Hot Start Taq polymerase 5 U μl−1 (Invitrogen) and 2 μl for dilution 1:10 obtained in the previous PCR reaction.
Thermocycling conditions were: 94°C for 2 min, 10 cycles of 20 s at 94°C, 30 s at 66°C (decreasing 1°C for cycle), 25 cycles of 30 s at 94°C, 30 s at 56°C, 3 min at 72°C and a final cycle of 30 min at 60°C.
The selective PCR reactions were examined in a 2% agarose gel stained with ethidium bromide. PCR fragments were detected using an CEQTM 8000 Genetic Analysis System (Beckman & Coulter, Inc., UK).
For AFLP analysis, 2.0 μl selective PCR reaction was mixed with 30.0 μl of Sample Loading Solution (SLS), 0.25 μl for Ceq 600 DNA Size Standard and a drop of mineral oil. The CEQ run conditions were: denaturation at 90°C for 2 min, injection at 1 KV for 40 s and separation at 55 KV for 85 min.
Separation of individual peaks was achieved at the one base-pair resolution. AFLP patterns were determined in triplicate for each individual genotype. Profiles were analyzed by an automatic scoring of the fingerprints using a bioinformatics tool such as GenomeLab™ AFLP® Dominant Scoring software (Beckman & Coulter, Inc., UK).
Results
Leavening ability of baker’s yeast on Sicilian durum wheat semolina
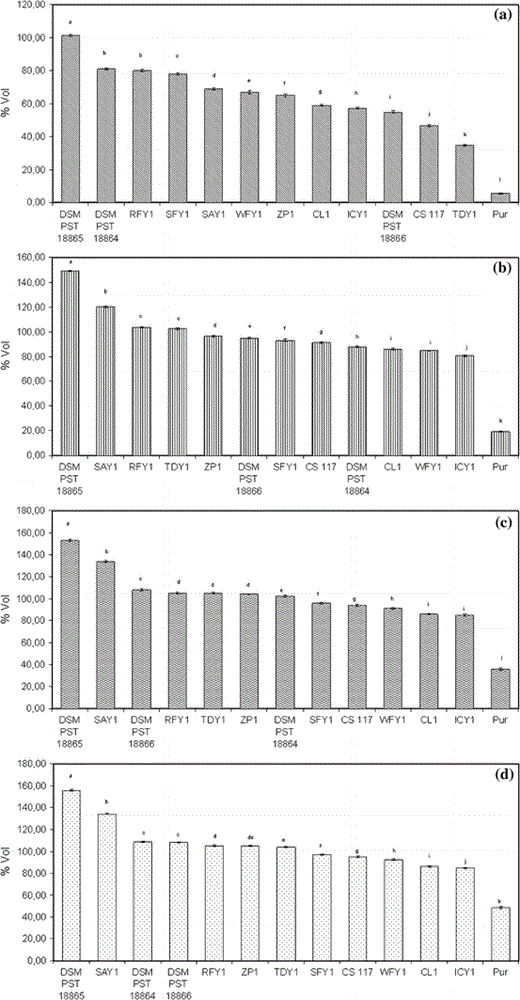
For the determination of leavening ability, strains were grown in an orbital shaker (200 rev min−1) at 30°C in 2,000-ml Erlenmeyer flasks containing 1,000 ml of YPG broth, pH 6.0. Cells were harvested at stationary phase by centrifugation at 4°C, washed twice with ice-cold distilled water and filtered to the obtained the yeast cake. Sicilian durum wheat semolina (var. Mongibello) was used for dough preparation. Yeasts were added to the dough at 3% (2.1–2.5 × 1010 viable cells g−1). The following ingredients were mixed in a dough mixer (Bakermix 80): 250 g of flour, 7.5 ml of yeast (wet weight), 5.0 g of salt and 135.0 ml of water at 20°C. After mixing, the doughs were put in sterile cylinders and incubated at 35°C per 4 h. The leavening powers were calculated at intervals of 1 h during incubation time as the difference (volume increase) between the values immediately after inoculation (t = 0 h) and the values at the successive time points. Five replications were performed for each strain of yeast. Leavening ability (volume increase ± SD) in durum wheat semolina by different yeast strains after (a) 1 h; (b) 2 h; (c) 3 h; (d) 4 h of fermentation. The results are expressed as mean values of five independent experiments, for which the SDs were below 1%, *P < 0.01. Yeast species for the different fermentations are listed in Table 1
In the 2nd h of fermentation, the dough obtained using the YS7 strain had 19.4% higher volume than strain YS2, and 30.6–31.3% higher volume than YS4 and YS6, respectively. The differences in volume increase between strain (YS7) and all other compared strains were higher than 35% (Fig. 1).
After three fermentation hours, the volume increase induced by the strain YS7 was reduced to 12.6% (always compared to strain YS2), while compared to other strains’ the volumetric increases remained high up to a maximum value of 76.4% (YS12). A similar trend was also observed at the end of the 4th fermentation h.
Dough pH and total titratable acidity
Acidification properties of the different doughs (Table 2) were defined. The control dough sample, after 2.0 h of incubation, showed an average pH of 6.36, 2.3, an average value of total titratable acidity (TTA). The lowest pH (5.66) and the highest TTA (5.0) were reached by strain YS7. Two other patented yeasts (YS8, YS9) showed pH values (5.84, 5.75) and TTA values lower than YS7 (3.8, 3.7). Yeasts selected by industrial starters generally led to a higher pH and a lower TTA values.
Acidification properties of dough leavened by different starter cultures
| Starters . | Dough . | |
|---|---|---|
| pH . | TTA (× 10−2 meq mol g−1) . | |
| DSM PST 18865 | 5.66 ± 0.040 h | 5.0 ± 0.001a |
| ICY1 | 5.68 ± 0.025 g,h | 4.4 ± 0.002b,c |
| LC1 | 5.75 ± 0.025f,g | 4.0 ± 0.003c,d |
| WFY1 | 5.77 ± 0.020e,f | 4.7 ± 0.004a,b |
| DSM PST 18864 | 5.75 ± 0.049e,f | 3.7 ± 0.001d,e |
| TDY1 | 5.79 ± 0.075d,e,f | 4.3 ± 0.004b,c |
| RFW1 | 5.80 ± 0.074d,e,f | 4.3 ± 0.005b,c |
| SFY1 | 5.83 ± 0.055d,e,f | 4.0 ± 0.004c,d |
| DSM PST 18866 | 5.84 ± 0.038d,e | 3.8 ± 0.002d,e |
| PUR | 5.87 ± 0.031d | 3.5 ± 0.003e |
| SAY1 | 5.98 ± 0.025c | 3.5 ± 0.002e |
| ZP1 | 6.05 ± 0.015b | 2.4 ± 0.000f |
| CS117 | 6.08 ± 0.035b | 2.0 ± 0.004f,g |
| Control | 6.36 ± 0.051a | 1.9 ± 0.001 g |
| Starters . | Dough . | |
|---|---|---|
| pH . | TTA (× 10−2 meq mol g−1) . | |
| DSM PST 18865 | 5.66 ± 0.040 h | 5.0 ± 0.001a |
| ICY1 | 5.68 ± 0.025 g,h | 4.4 ± 0.002b,c |
| LC1 | 5.75 ± 0.025f,g | 4.0 ± 0.003c,d |
| WFY1 | 5.77 ± 0.020e,f | 4.7 ± 0.004a,b |
| DSM PST 18864 | 5.75 ± 0.049e,f | 3.7 ± 0.001d,e |
| TDY1 | 5.79 ± 0.075d,e,f | 4.3 ± 0.004b,c |
| RFW1 | 5.80 ± 0.074d,e,f | 4.3 ± 0.005b,c |
| SFY1 | 5.83 ± 0.055d,e,f | 4.0 ± 0.004c,d |
| DSM PST 18866 | 5.84 ± 0.038d,e | 3.8 ± 0.002d,e |
| PUR | 5.87 ± 0.031d | 3.5 ± 0.003e |
| SAY1 | 5.98 ± 0.025c | 3.5 ± 0.002e |
| ZP1 | 6.05 ± 0.015b | 2.4 ± 0.000f |
| CS117 | 6.08 ± 0.035b | 2.0 ± 0.004f,g |
| Control | 6.36 ± 0.051a | 1.9 ± 0.001 g |
Yeast species for the different fermentations are listed in Table 1. Values with the same superscript letter within a row are not significantly different (P < 0.05)
Acidification properties of dough leavened by different starter cultures
| Starters . | Dough . | |
|---|---|---|
| pH . | TTA (× 10−2 meq mol g−1) . | |
| DSM PST 18865 | 5.66 ± 0.040 h | 5.0 ± 0.001a |
| ICY1 | 5.68 ± 0.025 g,h | 4.4 ± 0.002b,c |
| LC1 | 5.75 ± 0.025f,g | 4.0 ± 0.003c,d |
| WFY1 | 5.77 ± 0.020e,f | 4.7 ± 0.004a,b |
| DSM PST 18864 | 5.75 ± 0.049e,f | 3.7 ± 0.001d,e |
| TDY1 | 5.79 ± 0.075d,e,f | 4.3 ± 0.004b,c |
| RFW1 | 5.80 ± 0.074d,e,f | 4.3 ± 0.005b,c |
| SFY1 | 5.83 ± 0.055d,e,f | 4.0 ± 0.004c,d |
| DSM PST 18866 | 5.84 ± 0.038d,e | 3.8 ± 0.002d,e |
| PUR | 5.87 ± 0.031d | 3.5 ± 0.003e |
| SAY1 | 5.98 ± 0.025c | 3.5 ± 0.002e |
| ZP1 | 6.05 ± 0.015b | 2.4 ± 0.000f |
| CS117 | 6.08 ± 0.035b | 2.0 ± 0.004f,g |
| Control | 6.36 ± 0.051a | 1.9 ± 0.001 g |
| Starters . | Dough . | |
|---|---|---|
| pH . | TTA (× 10−2 meq mol g−1) . | |
| DSM PST 18865 | 5.66 ± 0.040 h | 5.0 ± 0.001a |
| ICY1 | 5.68 ± 0.025 g,h | 4.4 ± 0.002b,c |
| LC1 | 5.75 ± 0.025f,g | 4.0 ± 0.003c,d |
| WFY1 | 5.77 ± 0.020e,f | 4.7 ± 0.004a,b |
| DSM PST 18864 | 5.75 ± 0.049e,f | 3.7 ± 0.001d,e |
| TDY1 | 5.79 ± 0.075d,e,f | 4.3 ± 0.004b,c |
| RFW1 | 5.80 ± 0.074d,e,f | 4.3 ± 0.005b,c |
| SFY1 | 5.83 ± 0.055d,e,f | 4.0 ± 0.004c,d |
| DSM PST 18866 | 5.84 ± 0.038d,e | 3.8 ± 0.002d,e |
| PUR | 5.87 ± 0.031d | 3.5 ± 0.003e |
| SAY1 | 5.98 ± 0.025c | 3.5 ± 0.002e |
| ZP1 | 6.05 ± 0.015b | 2.4 ± 0.000f |
| CS117 | 6.08 ± 0.035b | 2.0 ± 0.004f,g |
| Control | 6.36 ± 0.051a | 1.9 ± 0.001 g |
Yeast species for the different fermentations are listed in Table 1. Values with the same superscript letter within a row are not significantly different (P < 0.05)
Experimental baking test
The main parameters of the bread-making test are reported in Table 3. The results showed that yeast strains influenced the bread volume significantly: the highest volumes of the loaf were obtained using the yeast strain YS2; on the contrary, the lowest loaf volume was recorded using the yeast strain YS4, which scarcely showed fermentation, probably because of the high relative humidity. Loaf weight is an important economic parameter because it indicates the bread yield, but low differences were recorded among the sample. Regarding the texture, the yeast strain YS4 showed an extremely compact crumb with no valuable porosity. On the contrary, the loaves obtained from yeast strains YS2 and YS7 showed a good level of texture (values 4 and 5) and regular porosity (value 1). Crust thickness ranged from 0.5 to 2.0 mm. As for crumb color, durum wheat bread usually has a golden crumb and a lighter color than bread made with common wheat flour. Sample with yeast strains YS2, which pointed out the highest loaf volume, showed the lowest yellow index, while for sample containing yeast strain YS4, with scarce fermentation and low bread volume, the highest b* value was recorded.
Results of the bread-making test
| Yeast strains . | Loaf volume (cm3) . | Loaf weight (g) . | Specific volume (cm3) . | Texture (1–8)(1) . | Crumb structure (1–2)(2) . | Crust thickness (mm) . | Crumb color (b*) . |
|---|---|---|---|---|---|---|---|
| DSM PST 18864 | 301.00 ± 3.6c | 139.30 ± 0.3ab | 2.16 ± 0.0c | 6.0 ± 0.0c | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.63 ± 0.1b |
| SAY 1 | 377.67 ± 2.5a | 140.00 ± 0.4a | 2.70 ± 0.0a | 4.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0c | 21.73 ± 0.2c |
| RFY | 153.33 ± 5.8e | 139.07 ± 0.8ab | 1.10 ± 0.0e | 8.0 ± 0.0e | – | 0.5 ± 0.0d | 25.07 ± 0.8a |
| DSM PST 18865 | 356.00 ± 3.6b | 139.77 ± 0.6a | 2.55 ± 0.0b | 5.0 ± 0.0b | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.43 ± 0.3b |
| DSM PST 18866 | 276.00 ± 8.5d | 138.17 ± 1.0b | 2.00 ± 0.1d | 7.0 ± 0.0d | 2.0 ± 0.0b | 2.0 ± 0.0a | 23.57 ± 0.1b |
| MEANS | 292.80 | 139.26 | 2.10 | 4.4 | 1.0 | 1.3 | 23.49 |
| Yeast strains . | Loaf volume (cm3) . | Loaf weight (g) . | Specific volume (cm3) . | Texture (1–8)(1) . | Crumb structure (1–2)(2) . | Crust thickness (mm) . | Crumb color (b*) . |
|---|---|---|---|---|---|---|---|
| DSM PST 18864 | 301.00 ± 3.6c | 139.30 ± 0.3ab | 2.16 ± 0.0c | 6.0 ± 0.0c | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.63 ± 0.1b |
| SAY 1 | 377.67 ± 2.5a | 140.00 ± 0.4a | 2.70 ± 0.0a | 4.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0c | 21.73 ± 0.2c |
| RFY | 153.33 ± 5.8e | 139.07 ± 0.8ab | 1.10 ± 0.0e | 8.0 ± 0.0e | – | 0.5 ± 0.0d | 25.07 ± 0.8a |
| DSM PST 18865 | 356.00 ± 3.6b | 139.77 ± 0.6a | 2.55 ± 0.0b | 5.0 ± 0.0b | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.43 ± 0.3b |
| DSM PST 18866 | 276.00 ± 8.5d | 138.17 ± 1.0b | 2.00 ± 0.1d | 7.0 ± 0.0d | 2.0 ± 0.0b | 2.0 ± 0.0a | 23.57 ± 0.1b |
| MEANS | 292.80 | 139.26 | 2.10 | 4.4 | 1.0 | 1.3 | 23.49 |
(1)1: most porous; 8: less porous
(2)1: regular; 2: irregular
Values with the same superscript letter within a row are not significantly different (P < 0.05)
Results of the bread-making test
| Yeast strains . | Loaf volume (cm3) . | Loaf weight (g) . | Specific volume (cm3) . | Texture (1–8)(1) . | Crumb structure (1–2)(2) . | Crust thickness (mm) . | Crumb color (b*) . |
|---|---|---|---|---|---|---|---|
| DSM PST 18864 | 301.00 ± 3.6c | 139.30 ± 0.3ab | 2.16 ± 0.0c | 6.0 ± 0.0c | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.63 ± 0.1b |
| SAY 1 | 377.67 ± 2.5a | 140.00 ± 0.4a | 2.70 ± 0.0a | 4.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0c | 21.73 ± 0.2c |
| RFY | 153.33 ± 5.8e | 139.07 ± 0.8ab | 1.10 ± 0.0e | 8.0 ± 0.0e | – | 0.5 ± 0.0d | 25.07 ± 0.8a |
| DSM PST 18865 | 356.00 ± 3.6b | 139.77 ± 0.6a | 2.55 ± 0.0b | 5.0 ± 0.0b | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.43 ± 0.3b |
| DSM PST 18866 | 276.00 ± 8.5d | 138.17 ± 1.0b | 2.00 ± 0.1d | 7.0 ± 0.0d | 2.0 ± 0.0b | 2.0 ± 0.0a | 23.57 ± 0.1b |
| MEANS | 292.80 | 139.26 | 2.10 | 4.4 | 1.0 | 1.3 | 23.49 |
| Yeast strains . | Loaf volume (cm3) . | Loaf weight (g) . | Specific volume (cm3) . | Texture (1–8)(1) . | Crumb structure (1–2)(2) . | Crust thickness (mm) . | Crumb color (b*) . |
|---|---|---|---|---|---|---|---|
| DSM PST 18864 | 301.00 ± 3.6c | 139.30 ± 0.3ab | 2.16 ± 0.0c | 6.0 ± 0.0c | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.63 ± 0.1b |
| SAY 1 | 377.67 ± 2.5a | 140.00 ± 0.4a | 2.70 ± 0.0a | 4.0 ± 0.0a | 1.0 ± 0.0a | 1.0 ± 0.0c | 21.73 ± 0.2c |
| RFY | 153.33 ± 5.8e | 139.07 ± 0.8ab | 1.10 ± 0.0e | 8.0 ± 0.0e | – | 0.5 ± 0.0d | 25.07 ± 0.8a |
| DSM PST 18865 | 356.00 ± 3.6b | 139.77 ± 0.6a | 2.55 ± 0.0b | 5.0 ± 0.0b | 1.0 ± 0.0a | 1.5 ± 0.0b | 23.43 ± 0.3b |
| DSM PST 18866 | 276.00 ± 8.5d | 138.17 ± 1.0b | 2.00 ± 0.1d | 7.0 ± 0.0d | 2.0 ± 0.0b | 2.0 ± 0.0a | 23.57 ± 0.1b |
| MEANS | 292.80 | 139.26 | 2.10 | 4.4 | 1.0 | 1.3 | 23.49 |
(1)1: most porous; 8: less porous
(2)1: regular; 2: irregular
Values with the same superscript letter within a row are not significantly different (P < 0.05)
A factorial analysis was calculated to value the significance level between the two factors and their interaction (Table 4).
Variance and significance (P < 0.01) for different parameters of staling process
| . | Loaf volume . | Loaf weight . | External firmness . | Internal firmness . |
|---|---|---|---|---|
| Day (A) | 7763.9*** | 2459.0*** | 363.4*** | 554.7*** |
| Yeast strains (B) | 65483.1*** | 81.8*** | 236.3*** | 81.1*** |
| A × B | 681.7*** | 31.3*** | 4.2*** | 3.1*** |
| Error | 48.5*** | 2.5*** | 0.8*** | 0.3*** |
| . | Loaf volume . | Loaf weight . | External firmness . | Internal firmness . |
|---|---|---|---|---|
| Day (A) | 7763.9*** | 2459.0*** | 363.4*** | 554.7*** |
| Yeast strains (B) | 65483.1*** | 81.8*** | 236.3*** | 81.1*** |
| A × B | 681.7*** | 31.3*** | 4.2*** | 3.1*** |
| Error | 48.5*** | 2.5*** | 0.8*** | 0.3*** |
Variance and significance (P < 0.01) for different parameters of staling process
| . | Loaf volume . | Loaf weight . | External firmness . | Internal firmness . |
|---|---|---|---|---|
| Day (A) | 7763.9*** | 2459.0*** | 363.4*** | 554.7*** |
| Yeast strains (B) | 65483.1*** | 81.8*** | 236.3*** | 81.1*** |
| A × B | 681.7*** | 31.3*** | 4.2*** | 3.1*** |
| Error | 48.5*** | 2.5*** | 0.8*** | 0.3*** |
| . | Loaf volume . | Loaf weight . | External firmness . | Internal firmness . |
|---|---|---|---|---|
| Day (A) | 7763.9*** | 2459.0*** | 363.4*** | 554.7*** |
| Yeast strains (B) | 65483.1*** | 81.8*** | 236.3*** | 81.1*** |
| A × B | 681.7*** | 31.3*** | 4.2*** | 3.1*** |
| Error | 48.5*** | 2.5*** | 0.8*** | 0.3*** |
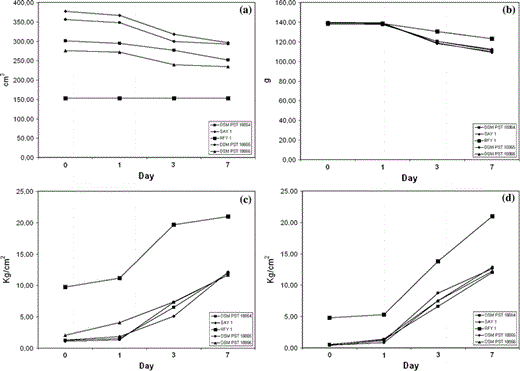
Bread staling process: trend of loaf volume, weight, bread external and internal firmness
As for bread volume, Fig. 2a shows that for sample YS4, the initial very low volume doesn’t change. The yeast strains YS2 and YS7 drastically decreased between the 1st and the 3rd day after baking, while the values of loaf volume of sample YS9 and YS8 did so gradually.
Regarding the loaf weight, using the yeast strains YS7, YS8, YS9 and YS2, Fig. 2b shows a similar decrement, while the sample YS4 decreased slowly, because its effect is anomalous.
Concerning external and internal firmness (Fig. 2c, d), the crust and crumb resistance increased in a noticeble way 1 day after baking; particularly for bread obtained using the yeast strain YS4, the firmness increased in a noticeble way, and 7 days after baking reached the maximum value measurable with the penetrometer firmness tester (21 kg/cm2).
Yeast strain differentiation
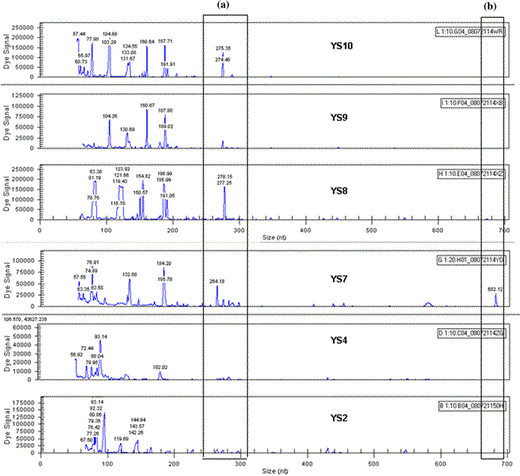
Polymorphism between strains of yeast Saccharomyces cerevisiae by primer combination E-AGG/M-CTA labeled with the Cy5 dye; (a) zoom of polymorphism among four strains at 270 bp; (b) zoom of polymorphism in a strain at 680 bp. Yeast species for the different polymorphisms are listed in Table 1
With the fAFLP technique, primer combinations EcoRI + AGG/MseI + CTA producing polymorphic peaks with distributions in the region of 264–690 bp were selected. The amplified fragments were detected by capillary gel electrophoresis in the automated DNA sequencer Beckman & Coulter CEQ 8000, enabling the “analysis fragment” option. Selective primers generated a total of 15 AFLP fragments, 3 (80%) of which were polymorphous.
When applied to strain YS7, this primer combination produced one polymorphous fragment in the region of 682.12 bp, which distinguished it from all other strains analyzed.
Two other polymorphous fragments in the region of 264–278 bp distinguished strains YS7, YS8, YS9 and YS10 from the other strains analyzed.
Discussion
In a number of regions in southern Italy, bread is mainly made from durum wheat. Traditional Sicilian breads made with durum wheat flour are considered as ready to be baked when the volume reaches 2.0, 2.5 times the initial volume. Some varieties can be recommended to the processing industry to help meet the increasing demand for durum wheat with its suitability for bread making [1, 9, 11].
Therefore, it is apparent that there are new yeast strains available for the preparation of bakery products that reduce leavening time, thus increasing the hourly production rate compared to commercially available yeasts. Results showed that yeasts selected from several bakery starters can promote the leavening processes, producing doughs with different acidification properties. The leavening time can be of the utmost importance during bread making at both the industrial and artisan levels and can allow a shorter production time; therefore, we isolated strains that reduced fermentation duration by 19–22% in comparison with industrial baker’s yeasts. The acidification properties of the different doughs also varied according to the starter used.
Moreover, the significant differences between doughs with respect to acidimetric characteristics and leavening ability could allow the selection of appropriate starter cultures for specific use in the bakery industry. Starters producing doughs with a strong acidification and a higher amount of acetic acid could be of interest for extending the shelf life of traditional breads, because they appear to possess the greatest ability to prevent the growth of molds and rope bacteria [6, 12].
In this work, a general correlation between the fAFLP strain profile and isolation origin of strains from the leavening process can be recognized. The present results confirm the powerful discriminator of this method, which distinguished among strains of S. cerevisiae YS 10, YS 9, YS 8 and YS 7, which show polymorphic peaks compared to other strains of the same yeast. This result probably correlates with different electrophoresis profiles and bread-making properties. The fAFLP analysis was shown to be highly reproducible, as reported elsewhere [15, 26]. In addition, it was shown to be a powerful tool for demonstrating the relationship among molecular profiles, strain origin and leavening ability. Even though in the future an extensive characterization must be performed with other strains, these preliminary results show the importance of using molecular techniques for the characterization of yeast strains used in bakery companies.
On the basis of its technological and molecular characteristics (high leavening power, high acidification effects), the strain named DSM PST 18865 could be used as a new microbial starter that can be readily transferred to bakery companies for the improvement of production processes. The preliminary baking and staling tests, carried out with five strains, confirmed the importance of yeast type in determining bread characteristics and property keeping.
Acknowledgments
The authors thank Mr. Tommaso Cannata (Forno Cannata SNC, Villafranca T., Sicily) for technical assistance and being available to test selected yeast strains in experimental baking.
References
Consorzio “Gian Pietro Ballatore” per la ricerca su specifici settori della filiera cerealicola (2001) Atlante del pane di Sicilia. 1a edizione. Agrigento, Italy
Palumbo M, Sciacca F, Virzì N, Boggini G (2003) Breadmaking quality of Sicilian durum wheat landraces. Proceeding of the 2nd International workshop: “Durum wheat and pasta quality: recent achievements and new trends”. Roma, pp 77–81, 19–20 novembre 2002
Palumbo M, Spina A, Cambrea M, Licciardello S, Virzì N (2005) Aspetti qualitativi di cultivar di frumento duro in funzione del processo di panificazione. Atti del XXXVI Convegno della Società Italiana di Agronomia “Ricerca ed innovazione per le produzioni vegetali e la gestione delle risorse agroambientali”. Foggia, pp 340–341, 20–22 settembre 2005
Romano AD, Gullo M, Caggia C (2001) Le tecnologie del pane in Sicilia. Tecnica agricola, anno LII, 1–2: 111–123
Romano AD, Caggia C, Spina A, Palumbo M (2002) Characterization of different Saccharomyces strains and their influence on dough fermentation in breadmaking of durum wheat. XLVI SIGA Annual congress. Giardini Naxos (ME), 18–21 settembre 2002
Spina A, Palumbo M, Boggini G (2003a) Bread-making quality of new durum wheat cultivars. Proceedings of the “Tenth International Wheat Genetics Symposium”, Paestum, pp 1394–1396, 1–6 Settembre 2003
Spina A, Virzì N, Palumbo M, Romano AD, Caggia C, Giudici P (2003b) Genotypes-yeast strains interaction in breadmaking of durum wheat. Proceedings of the 2nd International Workshop: “Durum wheat and pasta quality: recent achievements and new trends”: Roma, pp 271–274, 19–20 novembre 2002
Walker GM (1998) Baker’s yeast. In: Yeast Physiology and Biotechnology. Wiley and Sons, Chichester, pp 300–303



