-
PDF
- Split View
-
Views
-
Cite
Cite
Xin Wang, Haijie Yang, Lingwei Ruan, Xin Liu, Fang Li, Xun Xu, Cloning and characterization of a thermostable superoxide dismutase from the thermophilic bacterium Rhodothermus sp. XMH10, Journal of Industrial Microbiology and Biotechnology, Volume 35, Issue 2, 1 February 2008, Pages 133–139, https://doi.org/10.1007/s10295-007-0274-9
Close - Share Icon Share
Abstract
A superoxide dismutase (SOD) gene was cloned from the thermophilic bacterium Rhodothermus sp. XMH10 for the first time and highly expressed in Escherichia coli. The Rhodothermus sp. XMH10 SOD (RhSOD) gene encodes 209 amino acids with a putative molecular weight of 23.6 kDa and a pI value of 5.53. The recombinant RhSOD was detected to be an iron type SOD and existed as a dimer on its natural status. Experiments revealed that this RhSOD showed high activity at 50–70 °C and pH 5.0. Compared to SODs from other thermophiles, it was highly thermostable, maintaining more than 90% of its activity after incubation at 70 °C for 12 h, only totally inactivated after more than 4-h incubation at 80 °C. It also showed much higher resistance to KCN, NaN3 and H2O2 as compared to other SODs.
Xin Wang and Haijie Yang contribute to this work equally.
Introduction
Superoxide dismutases (SODs), a kind of metalloenzymes, have the capability to convert superoxide radicals to oxygen and hydrogen peroxide [3, 12]. According to the metal located at the active site of the enzyme, SODs could be divided into three types: iron-containing SOD (FeSOD), manganese-containing SOD (MnSOD), and copper zinc-containing SOD (CuZnSOD) [14, 15], each with different sensitivity to KCN, NaN3 and H2O2 [18]. There are also some novel SODs discovered in the past decades, such as cambialistic SOD and nickel SOD (NiSOD) [25], the former could function well either with iron or manganese at its active site [10, 14].
SODs exist in most aerobic bacteria in the form of dimer or tetramer [1, 20, 21] and found to be mainly responsible for the oxidative stress resistance, controlling the oxygen concentration at an appropriate level [5, 13, 27]. SODs have also been proved to be involved in acid tolerance in Staphylococcus aureus [4]. Periplasmic CuZnSODs have been found to function as important virulence factors in Neisseria meningitides [24].
During these years, some SODs from hyperthermophiles like the archaeon Sulfolobus solfataricus [23], Aquifex pyrophilus [8], and from thermophiles like Thermus aquaticus [19], Thermothrix sp. [20], have been investigated due to their potential application in industry. The thermostability is one of the most important properties that have been discussed since thermal denaturation is a common cause of enzyme inactivation in industry [7]. In our experiments, the SOD gene was cloned from a thermophilc bacterium Rhodothermus sp. XMH10, isolated from a hot spring in Jimei, Xiamen, China. The genus of Rhodothermus, existing in various environmental conditions, has been the subject of many studies in recent years. Many R. marinus enzymes were identified searching for thermostable enzymes with biotechnological potentials [2]. Compared to the SODs from other thermophiles, this recombinant superoxide dismutase cloned from Rhodothermus sp. XMH10 (RhSOD) shows unique characteristics in its thermostability and resistance to SOD inhibitors such as KCN, NaN3 and H2O2.
Materials and methods
Bacterial strains and plasmids
The thermophilic bacteria were isolated from hot spring in Jimei, Xiamen, China, and identified as Rhodothermus sp. XMH10 in our previous work (unpublished data). The pMD18-T vector was purchased from Takara. The expression vector pET-His and Escherichia coli strain BL21(DE3)plysS were purchased from Gene Power Lab Ltd. Shenzhen office.
Cloning strategy of RhSOD
(1) SOD fragment was amplified using two degenerate primers: SODF: 5′-CSTAYGANGCNYTNSARCC-3′ and SODR: 5′-ADRTARTANGCRTGYTCCCA-3′. The primers were designed according to two amino-acid-conservative areas of SOD genes from several thermophiles (amino acid sequences: AYDALEP and WEHAYYL). The fragment was cloned following a touch-down PCR procedure with denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 56 °C for 30 s, and extension at 72 °C for 40 s. In every cycle, the annealing temperature decreased by 0.06 °C. (2) Based on the obtained SOD fragment sequence, inverse PCR [16] was carried out to clone the upstream and downstream sequences of RhSOD. Two pairs of primers were designed following the obtained sequence as follows:
5′-TGTTCTGCAGCTCAGGGTAGCCCT-3′; 5′-TCTATTCGACGCCGAACCAGGAC-3′
5′-GCAGCAGTTCTTCGATCGACTTGTT-3′; 5′-ACGAAAACGGCAAGCTCCAGGTCTA-3′
Expression and purification of recombinant RhSOD
Two PCR primers based on the obtained SOD gene sequence were synthesized as follows:
SODF 5′-GGATCCATGGCTTTCACGCTGCC-3′
SODR 5′-GAATTCTCAGGCCGCCGCGAC-3′
Crude enzyme preparation
The bacteria of Rhodothermus sp. XMH10 was grown in 200 mL of culture medium (Yeast extract 2 g/L, Tryptone 2 g/L, PIPES 6.05 g/L, pH 7.2) at 75 °C in a laboratory shaker until OD600 came to about 1.5. The cells were collected by centrifugation (8,000g, 10 min), washed once with phosphate buffer (0.01 M, pH 7.2), and centrifuged again to get the precipitate. The precipitate was then suspended in 10 ml of 0.01 M PB, pH 7.2 containing 1 mM EDTA and ruptured with the ultrasonic disintegrator. The suspension was centrifuged (12,000g, 15 min) and the resulting clear supernatant was used.
The cell-free extract was then treated with solid (NH4)2SO4 in two steps. First, solid (NH4)2SO4 was added to the supernatant to 60% saturation in an ice bath, then the mixture was stirred for 15 min, and left at 4 °C for 60 min. The precipitate was removed by centrifugation. In the second step, the supernatant was treated with solid (NH4)2SO4 to 80% saturation, stirred for 15 min, and left at 4 °C for 60 min. The precipitate with SOD activity was centrifuged at 8,000g for 30 min and then dissolved in a minimal volume of phosphate buffer saline (PBS), pH 7.2 and dialyzed at 4 °C against the same buffer.
Superoxide dismutase assay
SOD activity was assayed based on its ability to inhibit the reduction of nitroblue tetrazolium (NBT) [22]. One unit of SOD activity was defined as the amount of enzyme which causes 50% of maximum inhibition of NBT reduction [20, 22]. The SOD will show a grey band under the blue background in the nondenaturing PAGE gel after staining in the PMS-MTT solution in daylight for half an hour [9]. The protein concentration was determined using the Coomassie (Bradford) Protein Assay Kit (Pierce).
Molecular weight
The purified recombinant RhSOD was subjected to gel filtration using a Superose G-75 column (1 cm × 100 cm) by FPLC (Amersham AKTA). The elution volume of standard proteins of BSA (66 kDa), carbonic anhydrase (30 kDa) and lysozyme (14.4 kDa) (5 mg each) were first detected with the flow rate of 0.3 mL/min, followed by gel filtration of recombinant RhSOD under the same condition. The molecular weight curve of standard proteins was thus constructed based on the data above and the molecular weight of RhSOD was calculated.
Inhibition test
The crude SOD enzyme and purified recombinant RhSOD was preincubated with different concentrations of KCN, NaN3 and H2O2 at room temperature for 1 h, and electrophoresed on nondenaturing PAGE gel. After a brief wash in distilled water, the gel was stained in PMS-MTT solution to detect the SOD activity.
Atomic absorption spectrophotometry
To confirm the metal content of the RhSOD, atomic absorption spectrum was carried out. RhSOD was obtained with the LB media supplemented with and without 10 μM FeSO4 and 10 μM MnCl2 when the inducement was initiated. The purified RhSODs were then introduced to absorption spectrum to assay the Fe and Mn contents.
Effect of pH on RhSOD activity
The enzyme was diluted in the relevant universal buffer with the ratio of 10:1 between the buffer and the enzyme. Effect of pH on RhSOD activity was then measured after incubating the enzyme in the universal buffer [17] with pH ranging from 2 to 12 at 50 °C for 30 min. The remaining activity was detected using the NBT test under standard conditions and calculated as the percentage of the maximum SOD activity.
Effect of temperature on RhSOD activity
The enzyme was diluted in the universal buffer of pH 5 with the ratio of 10:1 between the buffer and the enzyme. Effect of temperature on RhSOD activity was detected after incubating the enzyme at different temperatures (40, 50, 60, 70, 80 and 90 °C) for 30 min. The remaining activity was tested under standard conditions and calculated as the percentage of maximum SOD activity.
Thermal stability
The enzyme was diluted in the universal buffer of pH 5 with the ratio of 10:1 between the buffer and the enzyme. The enzyme was incubated at 50, 60, 70, 80, and 90 °C, separately, for up to 12 h. Samples were removed at certain time intervals and the remaining activity was assayed under standard conditions.
The nucleotide sequence reported in this paper has been deposited in the GenBank database under the accession number DQ286579.
Results
Cloning and identification of RhSOD and sequence analysis
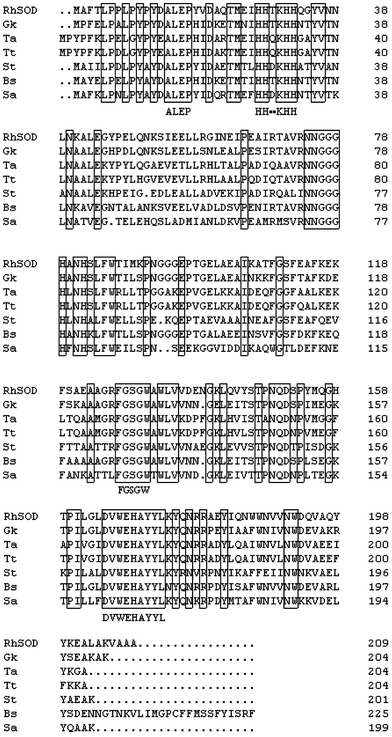
Multiple alignment of SOD amino acid sequences from Rhodothermus sp. XMH10 and other mesophiles and thermophiles. SOD amino acid sequences from RhSOD (Rhodothermus sp. XMH10), Gk (Geobacillus kaustophilus), Ta (Thermus aquaticus), Tt (Thermus thermophilus), St (Streptococcus thermophilus), Bs (Bacillus subtilis) and Sa (Staphylococcus aureus subsp.) are aligned. The solid boxes indicate conserved domains
Expression and purification of recombinant RhSOD
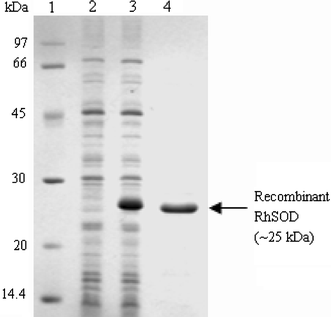
SDS-PAGE gel analysis of the recombinant RhSOD expressed in E. coli BL21(DE3)plysS. Lane 1: molecular weight marker, lane 2: total cell protein extracts of E. coli BL21(DE3)plysS containing pET-His expression vector induced by IPTG for 4 h, lane 3: total cell protein extracts of E. coli BL21(DE3)plysS containing recombinant pET-His expression vector induced by IPTG for 4 h, lane 4: approximate 4 μg of the purified recombinant RhSOD
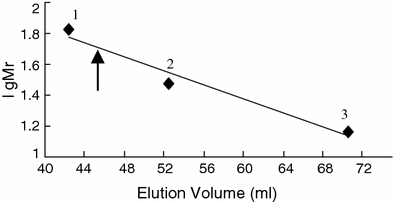
Determination of the molecular weight of RhSOD. Gel filtration was performed on a column (1 cm × 100 cm) of Sepharose G-75. Standard proteins: (1) BSA (66 kDa), (2) carbonic anhydrase (30 kDa), (3) lysozyme (14.4 kDa). The arrow indicates the elution volume of Rhodothermus sp. SOD
Inhibition test
SODs are generally divided into three types by their metal content at the active site and each type of SOD is sensitive to different inhibitors [18]. Generally, 1 mM KCN can inhibit the activity of CuZnSOD but not the other two, while 5 mM H2O2 can inhibit the activity of FeSOD and CuZnSOD. Addition of 10 mM NaN3, which can inhibit all three SODs activity, is used to detect the type of MnSOD when the SOD is inhibited neither by KCN nor by H2O2 [11].
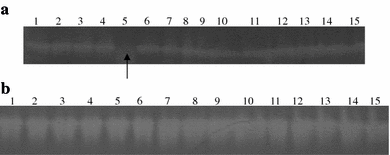
Detection of RhSOD (approximate 4 μg in each lane) and crude SOD enzyme (approximate 5 μg in each lane) activity by nondenaturing PAGE gel. a Lanes 1–5: RhSOD preincubated in water, 5 mM, 10 mM, 100 mM and 0.25 M H2O2, respectively. The arrow showed the inhibited RhSOD by 0.25 M H2O2; lanes 6–10: RhSOD preincubated in water, 10 mM, 100 mM, 0.5 M and 1 M NaN3, respectively; lanes 11–15: RhSOD preincubated in water, 1 mM, 10 mM, 100 mM, and 0.25 M KCN, respectively. b Lanes 1–5: crude SOD enzyme preincubated in water, 5 mM, 10 mM, 100 mM and 0.25 M H2O2, respectively; lanes 6–10: crude SOD enzyme preincubated in water, 1 mM, 10 mM, 100 mM, and 0.25 M KCN, respectively; lanes 11–15: crude SOD enzyme preincubated in water, 10 mM, 100 mM, 0.5 M and 1 M NaN3, respectively
Atomic absorption spectrophotometry
Since the method with low concentration of inhibitors could not suggest the metal content of RhSOD, we confirmed it through the method of atomic absorption spectrum. When supplemented with and without 10 μM FeSO4 and 10 μM MnCl2 in the LB media, similar metal quantities of the purified RhSOD were detected. In both conditions, every subunit of RhSOD was calculated to contain approximate 0.37 iron atom and 0.03 manganese atom (Table 1). Considering that 10 μM FeSO4 and 10 μM MnCl2 were enough for iron and manganese need of RhSOD, this data shown that RhSOD was an iron type SOD and one RhSOD dimer contained one iron atom (2 × 0.37).
Atomic absorption spectrophotometry for metal content
| . | Metal free . | Metal supplemented . | ||
|---|---|---|---|---|
| Fe2+ . | Mn2+ . | Fe2+ . | Mn2+ . | |
| Metal concentration (μg/L) | 338 | 23.2 | 61.5 | <5 |
| Metal quantity (/SOD) | 0.37 | 0.03 | 0.37 | 0.03 |
| SOD concentration (μg/mL) | 410 | 75 | ||
| . | Metal free . | Metal supplemented . | ||
|---|---|---|---|---|
| Fe2+ . | Mn2+ . | Fe2+ . | Mn2+ . | |
| Metal concentration (μg/L) | 338 | 23.2 | 61.5 | <5 |
| Metal quantity (/SOD) | 0.37 | 0.03 | 0.37 | 0.03 |
| SOD concentration (μg/mL) | 410 | 75 | ||
Atomic absorption spectrophotometry for metal content
| . | Metal free . | Metal supplemented . | ||
|---|---|---|---|---|
| Fe2+ . | Mn2+ . | Fe2+ . | Mn2+ . | |
| Metal concentration (μg/L) | 338 | 23.2 | 61.5 | <5 |
| Metal quantity (/SOD) | 0.37 | 0.03 | 0.37 | 0.03 |
| SOD concentration (μg/mL) | 410 | 75 | ||
| . | Metal free . | Metal supplemented . | ||
|---|---|---|---|---|
| Fe2+ . | Mn2+ . | Fe2+ . | Mn2+ . | |
| Metal concentration (μg/L) | 338 | 23.2 | 61.5 | <5 |
| Metal quantity (/SOD) | 0.37 | 0.03 | 0.37 | 0.03 |
| SOD concentration (μg/mL) | 410 | 75 | ||
Effect of pH on RhSOD activity
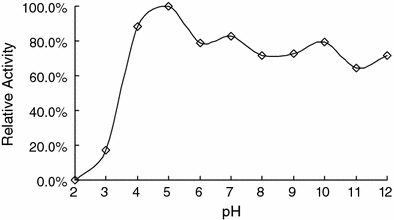
Effect of pH on SOD activity. The recombinant SOD was incubated in the universal buffer with pH ranging from 2 to 12 at 50 °C for 30 min. The remaining activity was detected under standard conditions and calculated as the percentage of the maximum SOD activity
Effect of temperatures on RhSOD activity
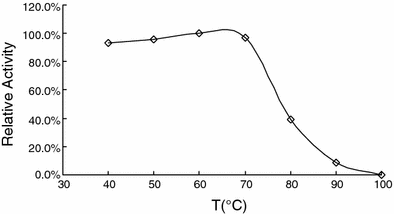
Effect of temperature on SOD activity. The recombinant SOD was incubated at different temperatures (40–90 °C) for half an hour. The remaining activity was tested under standard conditions and calculated as the percentage of maximum SOD activity
Thermal stability

SOD thermostability. The recombinant SOD was incubated at 50, 60, 70, 80, and 90 °C for up to 12 h. The enzyme was removed at certain time intervals and the remaining activity was assayed under standard conditions and calculated as percentage of maximum SOD activity
Discussion
RhSOD was characterized to be an iron type SOD in our experiments. Its activity was not affected by H2O2 with concentration from 10 to 100 mM, and could only be inhibited as the concentration of H2O2 went up to 250 mM. Also, its activity could not be inhibited by 250 mM KCN and even by 1 M NaN3. These results suggested that this enzyme had high resistance to all the three inhibitors of KCN, H2O2 and NaN3. In most conditions, SODs could be inhibited by low concentration of relevant inhibitors [7, 8, 11], which was why reduction of SOD activity at relatively low levels of all three inhibitors was frequently used as an indicative marker for metal content at the active site. Inhibition of SOD activity at high concentrations of inhibitors and its mechanism were seldom discovered in the past decades. Glutamines (Q69) of FeSODs were found to be involved in the inhibition of FeSODs to their inhibitors [6]. The inhibition experiments were conducted at lower concentrations of hydrogen peroxide and sodium azide, which differed from ours but shown in a certain level the responsibility of the enzyme structure for the high resistance to inhibitors.
The bacterium Rhodothermus sp. XMH10 was isolated from hot spring with the environmental pH of 4.0–5.0. The optimal pH value for the RhSOD activity was 4.0–5.0, which was in correspondence with the environmental pH condition of the bacterium habitat. Unexpectedly, this recombinant RhSOD was found to be stable in alkaline environment, retaining about 70% of its maximum activity after incubation at pH 12 for 30 min. It is a specific property of the bacterium Rhodothermus sp. XMH10 ever discovered in other bacteria before.
The recombinant RhSOD was highly thermostable as compared to SODs from other bacteria. It retained more than 90% of its activity after 12-h incubation at 70 °C. The CpSOD, a highly thermostable SOD from Chlamydia pneumoniae, could hold more than 90% activity only after 1-h incubation at 70 °C [26]. When the temperature went up to 80 °C, the RhSOD could retain its activity for more than 4 h. However, the SODs from Thermothrix sp. [20], and Thermomyces lanuginosus [7], were totally inactivated only after 1-h incubation at 80 °C. Although the mechanism responsible for protein thermostability is not clear yet, the increased number of charged residues and hydrophobic residues, increased number of ion-pairs, and the increased buried surface area are definitely involved [8, 26]. The number of charged residues of A. pyrophilus SOD and thermophilic SODs from Methanobacterium thermoautotrophicum and T. aquaticus were found much more than E. coli SOD [8]. The RhSOD was found to be enriched with some charged residues. The number of charged residues—lysine, arginine and glutamic acid (total 35)—of RhSOD was much higher than that of E. coli SOD (total 24), which explains in one way its high thermostability.
Acknowledgments
This investigation was financially supported by China Ocean Mineral Resources R&D Association (DY105-02-04-05) and Hi-Tech Research and Development Program of China (863 Program of China) (2004AA621010).



