-
PDF
- Split View
-
Views
-
Cite
Cite
M Guadalupe Sánchez-Otero, Gerardo Valerio-Alfaro, Hugo S García-Galindo, Rosa María Oliart-Ros, Immobilization in the presence of Triton X-100: modifications in activity and thermostability of Geobacillus thermoleovorans CCR11 lipase, Journal of Industrial Microbiology and Biotechnology, Volume 35, Issue 12, 1 December 2008, Pages 1687–1693, https://doi.org/10.1007/s10295-008-0433-7
Close - Share Icon Share
Abstract
A partially purified lipase produced by the thermophile Geobacillus thermoleovorans CCR11 was immobilized by adsorption on porous polypropylene (Accurel EP-100) in the presence and absence of 0.1% Triton X-100. Lipase production was induced in a 2.5% high oleic safflower oil medium and the enzyme was partially purified by diafiltration (co. 500,000 Da). Immobilization conditions were established at 25 °C, pH 6, and a protein concentration of 0.9 mg/mL in the presence and absence of 0.1% Triton X-100. Immobilization increased enzyme thermostability but there was no change in neither the optimum pH nor in pH resistance irrelevant to the presence of the detergent during immobilization. Immobilization with or without Triton X-100 allowed the reuse of the lipase preparation for 11 and 8 cycles, respectively. There was a significant difference between residual activity of immobilized and soluble enzyme after 36 days of storage at 4 °C (P < 0.05). With respect to chain length specificity, the immobilized lipase showed less activity over short chain esters than the soluble lipase. The immobilized lipase showed good resistance to desorption with phosphate buffer and NaCl; minor loses with detergents were observed (less than 50% with Triton X-100 and Tween-80), but activity was completely lost with SDS. Immobilization of G. thermoleovorans CCR11 lipase in porous polypropylene is a simple and easy method to obtain a biocatalyst with increased stability, improved performance, with the possibility for re-use, and therefore an interesting potential use in commercial conditions.
Introduction
Lipases (triacylglycerol hydrolases E.C. 3.1.1.3) are ubiquitous enzymes that catalyze in vivo the hydrolysis of triacylglycerols. These enzymes have the ability to catalyze a broad spectrum of bioconversion reactions due to their ability to synthesize soluble or insoluble carboxylic acid esters and other compounds with ester bonds analogs or amido bonds. Lipases have a tremendous biotechnological potential in areas such as food technology, biomedical sciences and fine chemical industry [1–3]. Despite the advantages of these biocatalysts, its use has been limited because of the instability of lipases under industrial conditions that require the use of solvents or moderately high temperatures to dissolve substrates; however, this inconvenience can be circumvented by the use of thermophilic lipases, whose naturally developed resistance to drastic reaction conditions have made them subjects of special interest in biotechnological research [4]. Additionally, enzyme stability can be enhanced by immobilization, a method that also has the advantage of allowing the repeated use of the biocatalyst. This can also favor continuous processing, thereby facilitating process control via manipulation of the feed flow rate. Immobilized enzymes can be easily recovered from the reaction medium, and are potentially useful to obtain a high product yield and purity, and to minimize both downstream processing costs as well as environmental impact [5]. It can also help in the establishment of catalytic sequences. Methods of immobilization can be divided into two major groups: entrapment and surface binding. Among the latter, adsorption, in which the enzyme binds to the support through ionic interactions, Van der Waals forces and hydrogen bonds, has several advantages: simple preparation, low cost, no conformational changes in the enzyme and the generation of stable derivatives [3, 6]. Lipases are commonly activated by interactions with hydrophobic interfaces, due to conformational changes in their secondary structures. Thus adsorption to hydrophobic supports such as polypropylene, have been the most widely used methods for lipase immobilization [7–13].
Recently, it has been shown that lipase activity in aqueous and anhydrous media can be improved in the presence of detergents [14–17] probably due to the breakage of lipase aggregates and/or to the shift on the closed–open equilibrium of the individual lipase molecules [16]. In immobilized lipases, the detergent Triton X-100 is commonly used to desorb the enzyme from the support, but in some instances it has been added during the immobilization procedure resulting in good immobilization yields (60–100%) [18–20].
The purpose of this study was to investigate the effect of immobilization on porous polypropylene, in the presence and absence of Triton X-100, on enzyme activity and stability of a thermoalkaliphilic lipase produced by the thermophilic strain Geobacillus thermoleovorans CCR11.
Materials and methods
Bacterial strain
The aerobic thermophilic strain G. thermoleovorans CCR11 (EMBL # AJ536599), isolated from “El Carrizal” hot spring in Veracruz, México, was used for thermoalkaliphilic lipase production [15].
Chemicals
Microporous polypropylene powder Accurel EP-100 (particle size 200–1,000 mm) was obtained from Akzo Chemical. Chemical reagents, solvents and culture media were purchased from Sigma-Aldrich, Bioxon, J.T. Baker and Merck; all of them were of analytical grade and used without any further purification.
Lipase production and partial purification
Lipase production was induced in a liquid medium containing 200 mL of nutrient broth 0.325% (w/v), CaCl2 0.1% (w/v), high oleic safflower oil 2.5%, and arabic gum 1%, pH 6.5 [15]. Geobacillus thermoleovorans CCR11 was grown until the stationary phase was reached (38 h), and cells were collected by centrifugation (14,000×g, 15–20 min, 10 °C). The supernatant was then filtered through a 0.45 μm pore size membrane filter (Millipore) at 25 °C, and then extracted with 200 mL of hexane to remove all the lipid constituents. The crude extract was diafiltered with phosphate buffer 0.05 M, pH 6.5, and concentrated through a polyethersulfone ultrafiltration membrane, co. 500,000 Da (Millipore) in a 200 mL stirred cell (Amicon) [15].
Enzyme assay
Lipolytic activity of free and immobilized lipase preparation was determined by a spectrophotometric assay [21] with slight modifications: 5 mg of the immobilized lipase preparation or 100 mL of the diluted crude enzyme were mixed with 0.9 mL of 0.05 M phosphate buffer, pH 6.5, and 0.1 mL of 0.01 M p-nitrophenyl-laurate in ethanol. The hydrolytic reaction proceeded for 30 min at 60 °C, and was stopped by the addition of 250 mL of Na2CO3 [15]. One unit of lipase activity was defined as the amount of enzyme that released 1 mMol of p-nitrophenol (e = 4.6 mM/cm) from p-nitrophenyl-laurate in 30 min, under the assay conditions. The enzyme extract and the immobilized lipase were stored at −4 °C.
Protein assay
Protein content was evaluated by Lowry [22] using bovine serum albumin (0.1–1.0 mg/mL) as standard.
Lipase immobilization
Solutions of various lipase concentrations containing 0.16–1.6 mg protein/mL were prepared in phosphate buffer 0.05 M at pH 6.5. Polypropylene powder (Accurel EP-100, 0.025 g) was placed in glass vials and pre-wetted with 0.150 mL of 30% ethanol in water (v/v), which was evaporated for 10 min at 50 °C. Then 1.5 mL of each lipase preparation was added. Blanks of enzyme and support were included, and all the vials were incubated at temperatures 25–60 °C under orbital shaking (250 rpm). The time required to reach adsorption equilibrium for each temperature was determined in preliminary experiments. The immobilized enzyme was separated by filtration (Whatman #2), washed three times with 0.05 M phosphate buffer pH 6.5 and dried under vacuum overnight at room temperature. The amount of protein adsorbed was calculated as the difference between the initial and final protein content in the lipase solution. Langmuir adsorption parameters were estimated by nonlinear regression analysis.
Immobilization in the presence of detergent Triton X-100
Immobilization in the presence of 0.1% Triton X-100 was carried out at the optimal conditions obtained in the absence of Triton X-100.
Effect of temperature on enzyme activity and stability of free and immobilized lipase
Enzyme activity was assayed at seven temperatures (25–80 °C), as described above. The effect of temperature on enzyme stability was checked by measuring residual activity after 1 h at 4–100 °C.
Effect of pH on enzyme activity and stability of free and immobilized lipase
Lipase activity was assayed in buffers at pH 4.0–12.0. Lipase pH stability was determined by incubating the enzyme preparations in 0.05 M phosphate buffer at pH 4.0–12.0 for 12 h at room temperature. The residual activity was determined by the spectrophotometric assay at pH 6.5.
Substrate specificity of free and immobilized lipase
Substrate specificity was analyzed by the spectrophotometric assay using 0.01 M p-nitrophenyl esters (C3, C4, C6, C8, C10, C12, C16 and C18) dissolved in ethanol as substrates, at optimal assay conditions.
Desorption of the immobilized lipase
The leaching of lipolytic activity was studied by incubating the immobilized lipase in 0.05 M phosphate buffer pH 6.5 with either 1.0 M NaCl, 1.0% Triton X-100, 1.0% SDS, 1.0% Tween 80 or 96% ethanol for 12 h at room temperature under orbital shaking (250 rpm). Residual activity was determined by the spectrophotometric assay as described above.
Reutilization of immobilized lipase
Reuse of the immobilized lipase preparation was determined by measuring activity up to 12 cycles. After each cycle, immobilized lipase was washed three times with 0.05 M phosphate buffer pH 6.5 and dried overnight under vacuum.
Storage stability
Immobilized and soluble lipases were stored at either −4 °C or room temperature and residual activity was determined by the spectrophotometric assay.
Results and discussion
Lipase immobilization
After the crude lipolytic preparation of G. thermoleovorans was diafiltered (co 500,000 Da), a partially purified lipase was obtained, as demonstrated by Castro-Ochoa and co-workers [15]. This preparation was used for the immobilization experiments with protein concentrations up to 1.5 mg/mL at 25–60 °C. Immobilization time was set at 3 h since after 120 min adsorption reached equilibrium at all temperatures tested. Lipase appeared to bind rapidly to the support; after 30 min at 25 °C as much as 63% of the initial activity disappeared from the solution, which corresponded to 39% of the total protein content (data not shown).
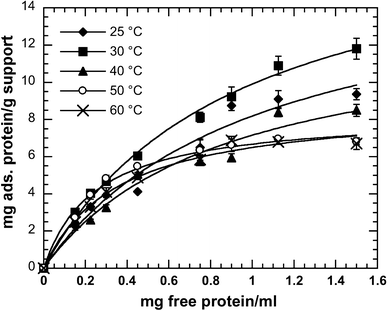
Experimental data and Langmuir isotherm fits for protein adsorption of the partially purified lipase from Geobacillus thermoleovorans CCR11 on microporous polypropylene at different temperatures: (filled diamond) 25 °C, (filled square) 30 °C, (filled triangle) 40 °C, (open square) 50 °C, (times) 60 °C
K prot and C prot ads max values calculated for the different protein adsorption isotherms
| Temperature . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K prot (mg/mL) | 1.03 | 0.92 | 0.86 | 0.25 | 0.35 |
| C prot ads max (mg/g) | 16.59 | 19.03 | 13.32 | 8.34 | 8.81 |
| Temperature . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K prot (mg/mL) | 1.03 | 0.92 | 0.86 | 0.25 | 0.35 |
| C prot ads max (mg/g) | 16.59 | 19.03 | 13.32 | 8.34 | 8.81 |
K prot and C prot ads max values calculated for the different protein adsorption isotherms
| Temperature . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K prot (mg/mL) | 1.03 | 0.92 | 0.86 | 0.25 | 0.35 |
| C prot ads max (mg/g) | 16.59 | 19.03 | 13.32 | 8.34 | 8.81 |
| Temperature . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K prot (mg/mL) | 1.03 | 0.92 | 0.86 | 0.25 | 0.35 |
| C prot ads max (mg/g) | 16.59 | 19.03 | 13.32 | 8.34 | 8.81 |
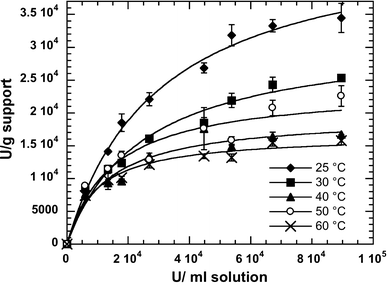
Experimental data and Langmuir isotherm fits for adsorption of lipolytic activity of the partially purified lipase from Geobacillus thermoleovorans CCR11 on microporous polypropylene at different temperatures: (filled diamond) 25 °C, (filled square) 30 °C, (filled triangle) 40 °C, (open square) 50 °C, (times) 60 °C
K act and A ads max values obtained for the different lipolytic activity adsorption isotherms
| . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K act (U/mL) | 47,313 | 31,792 | 19,585 | 23,808 | 16,866 |
| A ads max (U/g) | 30,284 | 24,992 | 13,040 | 14,970 | 9,493 |
| . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K act (U/mL) | 47,313 | 31,792 | 19,585 | 23,808 | 16,866 |
| A ads max (U/g) | 30,284 | 24,992 | 13,040 | 14,970 | 9,493 |
K act and A ads max values obtained for the different lipolytic activity adsorption isotherms
| . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K act (U/mL) | 47,313 | 31,792 | 19,585 | 23,808 | 16,866 |
| A ads max (U/g) | 30,284 | 24,992 | 13,040 | 14,970 | 9,493 |
| . | 25 °C . | 30 °C . | 40 °C . | 50 °C . | 60 °C . |
|---|---|---|---|---|---|
| K act (U/mL) | 47,313 | 31,792 | 19,585 | 23,808 | 16,866 |
| A ads max (U/g) | 30,284 | 24,992 | 13,040 | 14,970 | 9,493 |
Finally, the maximum activity per gram of support was found at 25 °C with 0.925 mg of protein/mL. The amount of protein adsorbed was ca. 8.7 mg protein/g support in accordance to what other authors have reported for lipases on this support [3]. Under these experimental conditions we found the optimum immobilization pH at 6.0, close to the pH reported for the immobilization of lipases produced by Pseudomonas cepacia, Rhizomucor miehei, Humicola sp., Rhizopus niveus and Candida antarctica onto Accurel EP-100 (pH 7.0) [10, 23]. Consequently all the immobilization procedures were undertaken at 25 °C, pH 6.0, 250 rpm, and at a concentration of 0.925 mg of protein/mL. Under these conditions, we found up to 40% increase in lipase specific activity after immobilization. The increase in specific activity of lipases immobilized on hydrophobic supports has been reported [8, 9, 24]; the authors propose that this activity increase is caused by a strong interaction of hydrophobic areas of lipases near/on the lid with the support surface, which leads to conformational changes that result in a highly active open form.
Immobilization in the presence of Triton X-100
As previously reported [15], we observed a 5-fold increase on the specific activity of the partially purified soluble lipase in presence of 0.1% Triton X-100, probably due to the disruption of protein aggregates and/or to the shift on the closed/open equilibrium towards the open form of the lipase [16].
When 0.1% Triton X-100 was added to immobilization medium there was no difference in the immobilization yield (52.6 ± 5.3 vs. 58.8 ± 3.1) although a 4–5-fold increase in lipolytic activity per gram of support was observed with respect to the lipolytic activity of immobilized lipase without the detergent.
Effect of temperature on enzyme activity and stability of free and immobilized lipase
When the effect of temperature on enzyme activity was analyzed, a 10 °C increase in the optimum temperature of activity was observed after immobilization on porous polypropylene only when Triton X-100 was not added, from 60 °C (soluble) to 70 °C (immobilized). A similar trend has been reported for other immobilized lipases that showed increases from 1 to 11 °C in their optimal temperatures, depending on the source of the lipase and the immobilization method [8, 13, 25] suggesting that this effect may be attributed to an increase in enzyme thermal resistance due to mobility restrictions.
A significant increase in lipase thermostability was observed when the enzyme was immobilized with or without the detergent (Table 3). Immobilized lipases showed residual activity even after 1 h at 100 °C, when soluble enzyme completely lost activity after 1 h at 80 °C. This result agrees with other reports in which microbial lipases immobilized on hydrophobic supports [19, 25] have shown greater thermal stability than their free counterparts. They suggest that the restriction on protein conformational motility due to immobilization probably provokes a more rigid lipase structure that impedes the unfolding and the loss of the catalytic properties of the enzyme.
Effect of temperature on immobilized lipase activity after 1 h of incubation at temperatures above 50 °C
| Temperature (°C) . | Residual activity ± SD (%) Lipase/wo Triton X-100 . | Residual activity ± SD (%) Lipase/0.1% Triton X-100 . | ||
|---|---|---|---|---|
| Soluble . | Immobilized . | Soluble . | Immobilized . | |
| 50 | 100 ± 3.8a | 100 ± 7.2a | 100 ± 2.0a | 100 ± 2.5a |
| 60 | 91.5 ± 4.7b | 100 ± 8.1a | 89.6 ± 2.2a | 100 ± 0.4a |
| 70 | 48.3 ± 5.9c | 93.2 ± 5.7b | 34.5 ± 3.8d | 100 ± 4.6a |
| 80 | 13.0 ± 4.4e | 72.1 ± 7.6f | 8.8 ± 6.3e | 54.2 ± 6.6c |
| 90 | 0 | 56.5 ± 5.3c | 0 | 15.4 ± 11.7e |
| 100 | 0 | 15.2 ± 5.3e | 0 | 4.2 ± 2.6e |
| Temperature (°C) . | Residual activity ± SD (%) Lipase/wo Triton X-100 . | Residual activity ± SD (%) Lipase/0.1% Triton X-100 . | ||
|---|---|---|---|---|
| Soluble . | Immobilized . | Soluble . | Immobilized . | |
| 50 | 100 ± 3.8a | 100 ± 7.2a | 100 ± 2.0a | 100 ± 2.5a |
| 60 | 91.5 ± 4.7b | 100 ± 8.1a | 89.6 ± 2.2a | 100 ± 0.4a |
| 70 | 48.3 ± 5.9c | 93.2 ± 5.7b | 34.5 ± 3.8d | 100 ± 4.6a |
| 80 | 13.0 ± 4.4e | 72.1 ± 7.6f | 8.8 ± 6.3e | 54.2 ± 6.6c |
| 90 | 0 | 56.5 ± 5.3c | 0 | 15.4 ± 11.7e |
| 100 | 0 | 15.2 ± 5.3e | 0 | 4.2 ± 2.6e |
Different superscript letters indicate statistical differences between groups
P < 0.05
Effect of temperature on immobilized lipase activity after 1 h of incubation at temperatures above 50 °C
| Temperature (°C) . | Residual activity ± SD (%) Lipase/wo Triton X-100 . | Residual activity ± SD (%) Lipase/0.1% Triton X-100 . | ||
|---|---|---|---|---|
| Soluble . | Immobilized . | Soluble . | Immobilized . | |
| 50 | 100 ± 3.8a | 100 ± 7.2a | 100 ± 2.0a | 100 ± 2.5a |
| 60 | 91.5 ± 4.7b | 100 ± 8.1a | 89.6 ± 2.2a | 100 ± 0.4a |
| 70 | 48.3 ± 5.9c | 93.2 ± 5.7b | 34.5 ± 3.8d | 100 ± 4.6a |
| 80 | 13.0 ± 4.4e | 72.1 ± 7.6f | 8.8 ± 6.3e | 54.2 ± 6.6c |
| 90 | 0 | 56.5 ± 5.3c | 0 | 15.4 ± 11.7e |
| 100 | 0 | 15.2 ± 5.3e | 0 | 4.2 ± 2.6e |
| Temperature (°C) . | Residual activity ± SD (%) Lipase/wo Triton X-100 . | Residual activity ± SD (%) Lipase/0.1% Triton X-100 . | ||
|---|---|---|---|---|
| Soluble . | Immobilized . | Soluble . | Immobilized . | |
| 50 | 100 ± 3.8a | 100 ± 7.2a | 100 ± 2.0a | 100 ± 2.5a |
| 60 | 91.5 ± 4.7b | 100 ± 8.1a | 89.6 ± 2.2a | 100 ± 0.4a |
| 70 | 48.3 ± 5.9c | 93.2 ± 5.7b | 34.5 ± 3.8d | 100 ± 4.6a |
| 80 | 13.0 ± 4.4e | 72.1 ± 7.6f | 8.8 ± 6.3e | 54.2 ± 6.6c |
| 90 | 0 | 56.5 ± 5.3c | 0 | 15.4 ± 11.7e |
| 100 | 0 | 15.2 ± 5.3e | 0 | 4.2 ± 2.6e |
Different superscript letters indicate statistical differences between groups
P < 0.05
The effect of immobilization on thermal stability was affected by the presence of Triton X-100, since the lost of activity with the increase in temperature was higher in the lipase immobilized with the detergent (Table 3). This is in accordance to [19] who reported that the lipase from Thermomyces lanuginosa retained 80% of its initial activity after 30 h at 50 °C, compared to 40%, when it was immobilized in the presence of 0.6% Triton X-100. Probably the presence of oligomers and other lipase aggregates makes the enzyme immobilized without Triton X-100 more resistant to thermal deactivation.
Effect of pH on enzyme activity and stability of free and immobilized lipase
There was no significant change in the optimum pH of activity after immobilization (pH 9), irrelevant to the presence of Triton X-100, as has been reported for other microbial lipases immobilized on hydrophobic supports [8, 25]. Soluble and immobilized lipases showed good pH stability over a wide range of pHs (5–12) (data not shown).
Substrate specificity of free and immobilized lipase
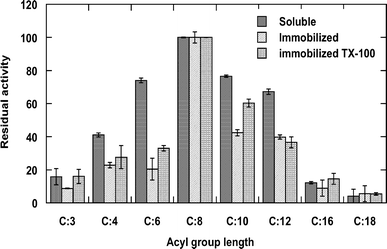
Effect of immobilization on chain length preference of Geobacillus thermoleovorans CCR11 lipase
Desorption of the immobilized lipase
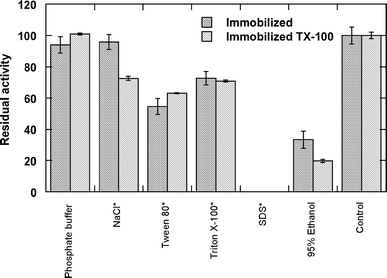
Effect of detergents and other substances on immobilized lipase activity
The detergents SDS, Tween and Triton X-100 are commonly used to disaggregate lipases, to decrease the unspecific protein adsorption during immobilization procedures, to desorb lipases after hydrophobic chromatography, and for the recovery of expensive supports [11, 18–20]. In this work, washing the immobilized lipases in the presence of 1% SDS resulted in a total lost of lipolytic activity probably due to lipase denaturalization, as has been reported by Castro-Ochoa et al. [15]. A different behavior was observed with the other detergents used; there was a 28–45% decrease in lipolytic activity in the presence of Tween 80 and Triton X-100 in both immobilized lipases, contrasting with the results of Pencreac’h et al. [11] who found a total lost of the activity of Pseudomonas cepacia lipase immobilized on Accurel EP 100 after 10 min in 0.4% Triton X-100. This suggests that the hydrophobic interaction between the lipase of G. thermoleovorans and the polypropylene support is very strong.
The immobilized lipases with and without Triton X-100 washed in 95% ethanol maintained more than 60% of its original activity. Castro-Ochoa et al. [15] reported that the same lipase lost ca. 70% of its activity when exposed to 70% ethanol for 2 h. Consequently the loss of activity observed in our work is most probably due to protein denaturalization than to the wash out from the support.
Reutilization of the immobilized lipase

Residual activity of the lipase from Geobacillus thermoleovorans CCR11 immobilized on polypropylene in continuous cycles of hydrolysis of p-nitrophenol laureate. Filled circle Immobilized (filled triangle) immobilized in presence of 0.1% Triton X-100
Storage stability
Storage stability is an important attribute of immobilized enzyme preparations [3]. In our work, immobilized lipases, with or without Triton X-100, showed high storage stability even after 36 days at 4 °C, as has been previously reported for other lipases [8].
In conclusion, a lipase from G. thermoleovorans CCR11 was immobilized on porous polypropylene from a partially purified extract. The presence of 0.1% Triton X-100 in immobilization medium did not interfere with lipase adsorption to the support, although lipase immobilized in the presence of the detergent neither show as much thermostability nor reuse ability as the one immobilized without it. However, the presence of Triton X-100 resulted in an immobilized biocatalyst with a high activity per gram of support, with a potential use in dairy fat modification.
Acknowledgments
M. Sc Guadalupe Sánchez Otero acknowledges her scholarship from the Mexican National Council for Science and Technology (Conacyt). This work was supported by grant UR-612-04 from the General Direction of Superior Technological Studies (DGEST-SEP).
References
Hill CG Jr, Otero C, García HS (2006) Immobilized Enzyme Technology. In: Encyclopedia of Chemical Processing. Tyler and Francis, pp 1367–1380
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1955) Protein measurement with the Folin phenol reagent. Department of Pharmacology, Washington University. School of Medicine, St. Louis, Missouri, pp 265–275



