-
PDF
- Split View
-
Views
-
Cite
Cite
D Palmero Llamas, M de Cara Gonzalez, C Iglesias Gonzalez, G Ruíz Lopez, J C Tello Marquina, Effects of water potential on spore germination and viability of Fusarium species, Journal of Industrial Microbiology and Biotechnology, Volume 35, Issue 11, 1 November 2008, Pages 1411–1418, https://doi.org/10.1007/s10295-008-0441-7
Close - Share Icon Share
Abstract
Germination of macroconidia and/or microconidia of 24 strains of Fusarium solani, F. chlamydosporum, F. culmorum, F. equiseti, F. verticillioides, F. sambucinum, F. oxysporum and F. proliferatum isolated from fluvial channels and sea beds of the south-eastern coast of Spain, and three control strains (F. oxysporum isolated from affected cultures) was studied in distilled water in response to a range of water potentials adjusted with NaCl. (0, −13.79, −41.79, −70.37, −99.56 and −144.54 bars). The viability (UFC/ml) of suspensions was also tested in three time periods (0, 24 and 48 h). Conidia always germinated in distilled water. The pattern of conidial germination observed of F. verticilloides, F. oxysporum, F. proliferatum, F. chlamydosporum and F. culmorum was similar. A great diminution of spore germination was found in −13.79 bars solutions. Spore germination percentage for F. solani isolates was maximal at 48 h and −13.79 bars with 21.33% spore germination, 16% higher than germination in distilled water. F. equiseti shows the maximum germination percentage in −144.54 bars solution in 24 h time with 12.36% germination. This results did not agree with those obtained in the viability test were maximum germination was found in distilled water. The viability analysis showed the great capacity of F. verticilloides strains to form viable colonies, even in such extreme conditions as −144.54 bars after 24 h F. proliferatum colony formation was prevented in the range of −70.37 bars. These results show the clear affectation of water potential to conidia germination of Fusaria. The ability of certain species of Fusarium to develop a saprophytic life in the salt water of the Mediterranean Sea could be certain. Successful germination, even under high salty media conditions, suggests that Fusarium spp. could have a competitive advantage over other soil fungi in crops irrigated with saline water. In the specific case of F. solani, water potential of −13.79 bars affected germination positively. It could indicate that F. solani has an special physiological mechanism of survival in low water potential environments.
Introduction
Spore germination of Fusarium genus is a quick process that can take place in short periods of 4–7 h in the majority of cases, macroconidia show lower germination speed in axenic cultures [14].
Conidial germination is characterized by the formation of one or two germ tubes [14], some authors observe swelling or increase in size during or previous to germination [19, 30]. Different authors had indicated that Fusarium culmorum macroconida contains an outer mucilaginous sheath [30], or carbohydrates of F. solani or F. avenaceum, that could act as specific receptors for plant lectins [18].
Endogenous substrates of conidia are sufficient to support germination, Fusarium conidia are known to have a high lipid content which allows it. Energetic requirements that favour conidial germination have been broadly studied, indicating that there are absolute requirements for exogenous organic carbon substrates (sugars, alcohols, organic acids, amino acids) and nitrogen sources [3, 9, 13–15, 19]. Regarding the influence of the chemical environment on germination, provision of a balanced inorganic salt medium provides a more suitable environment for germination [14].
Ragazzy and Vecchio [21] indicated that, salinities of 10 dS/m, increase both germination and length of clamidospore germination tube in F. oxysporum f. sp. vasinfectum, with extreme decrease with salinities of 15 ds/m, authors suggested that cotton fields irrigated with saline water could increase infection in cotton.
Percentage of spore germination for six isolates of F. graminearum, F. culmorum and F. avenaceum, including conidia, clamydospores and ascospores was uniformly maximal at all water potentials between −1 and −20 bars, and prevented in the range of −60 and −80 bars. [25]. In F. moniliforme, germination percentage decreased from 80.3% germination in media with no amended NaCl until 0% germination observed in 15% NaCl medium [1].
Research dedicated to the Fusarium genus in aquatic habitats is not frequent, perhaps because it is considered a ground (soil-borne) fungus. The presence of Fusarium spp. has been reported in marshy waters. F. merismoides was mentioned by Booth [5] in dirty stagnant water and mud. Articles on fungi in fluvial water mention the presence of Fusarium as a decomposer of leaves and branches from trees fallen into the channel [2, 8, 22, 31]. F. culmorum and F. aquaeductum [23, 24] and Fusarium sp. [7] have been sporadically isolated from river channels in Southeast Spain, which were considered saprophytes.
Tello and Lacasa [26] studied the presence of Fusarium sp. in uncultivated land, finding a high proportion of F. solani and F. oxysporum. These authors questioned the relationship between the isolated species and those that produced diseases in crops surrounding the uncultivated ground sampled, especially in the case of F. oxysporum. In carnation crops in the sampled area, F. oxysporum f.sp. dianthi causes a limiting mycosis. Two important mycoses were found in tomato crops, one caused by F. oxysporum f.sp. lycopersici (Fusarium wilt) and another caused by F. oxysporum f.sp. radicis-lycopersici (Fusarium crown and root rot).
The presence of Fusarium sp. in beach sand and marine sea beds on the Mediterranean coast of Spain has been broadly studied [20, 27, 28]. The study was justified to search for possible sources of pathogen inoculate for several vascular fusariosis in the sand-covered crops of Almeria (South-eastern Spain). Sand-covered cultivation is a production technique for 20,000 ha of greenhouse crops, consisting of covering the original soil with a thin layer (2–3 cm) of fresh manure and then adding a 10–15 cm thick layer of sand. This technique has been used for more than 50 years, permitting utilization of saline water (2,000–5,000 mmhos of conductivity) in susceptible crops.
This present study shows the analysis to know the effect of water potential on spore germination of seven different Fusarium sp. isolated from these habitats.
Materials and methods
Isolated used in germination tests
A total of 21 strains of F. solani, F. chlamydosporum, F. culmorum, F. equiseti, F. verticillioides, F. oxysporum and F. proliferatum isolated from fluvial channels and sea beds of the south-eastern coast of Spain and three control strains: F. oxysporum f. sp. lycopersici race 0 F. oxysporum f. sp. radicis cucumerinum and F. oxysporum f. sp. melonis race 1 (all isolated from affected cultures and coded as “AC”) were analyzed.
The origin and code of the isolates of Fusarium tested can be seen on Table 1. All strains used in this study are stored in the University of Almería (Plant Prod. Dept.) and in the Polytecnic University of Madrid (E.U.I.T. Agrícola) culture collections.
Origin of Fusarium isolates used in tests (D: depth)
| Analysis code . | Fusarium spp. . | Location . | ||
|---|---|---|---|---|
| Sample depth . | UTM coordinates . | |||
| FUS 2 | F. verticillioides (Sacc.) Nirenberg | Mouth of Andarax River bed D = 1.5 m | 551238 | 4074229 |
| FUS 7 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 13 | F. solani (Martius) Saccardo | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 18 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 21 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 22 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 28 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 31 | F. solani (Martius) Saccardo | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 111 | F. equiseti (Corda) Sacc. | Albuñol River Highway N342 | 551041 | 4074596 |
| FUS. 52 | F. equiseti (Corda) Sacc. | Sea bed at 6 m in depth | 550786 | 4073856 |
| FUS 60 | F. solani (Martius) Saccardo | Sea bed at 6 m in depth | 550844 | 4073833 |
| FUS 101 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 102 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 106 | F. proliferatum (Matsushima) Nirenberg | Andarax River | 545284 | 4090359 |
| FUS 115 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 545284 | 4090359 |
| FUS 118 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 119 | F. sambucinum Fuckel sensu lato | Andarax River | 549372 | 4086274 |
| FUS 121 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 130 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 132 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 133 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| Analysis code . | Fusarium spp. . | Location . | ||
|---|---|---|---|---|
| Sample depth . | UTM coordinates . | |||
| FUS 2 | F. verticillioides (Sacc.) Nirenberg | Mouth of Andarax River bed D = 1.5 m | 551238 | 4074229 |
| FUS 7 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 13 | F. solani (Martius) Saccardo | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 18 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 21 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 22 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 28 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 31 | F. solani (Martius) Saccardo | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 111 | F. equiseti (Corda) Sacc. | Albuñol River Highway N342 | 551041 | 4074596 |
| FUS. 52 | F. equiseti (Corda) Sacc. | Sea bed at 6 m in depth | 550786 | 4073856 |
| FUS 60 | F. solani (Martius) Saccardo | Sea bed at 6 m in depth | 550844 | 4073833 |
| FUS 101 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 102 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 106 | F. proliferatum (Matsushima) Nirenberg | Andarax River | 545284 | 4090359 |
| FUS 115 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 545284 | 4090359 |
| FUS 118 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 119 | F. sambucinum Fuckel sensu lato | Andarax River | 549372 | 4086274 |
| FUS 121 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 130 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 132 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 133 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
Origin of Fusarium isolates used in tests (D: depth)
| Analysis code . | Fusarium spp. . | Location . | ||
|---|---|---|---|---|
| Sample depth . | UTM coordinates . | |||
| FUS 2 | F. verticillioides (Sacc.) Nirenberg | Mouth of Andarax River bed D = 1.5 m | 551238 | 4074229 |
| FUS 7 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 13 | F. solani (Martius) Saccardo | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 18 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 21 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 22 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 28 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 31 | F. solani (Martius) Saccardo | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 111 | F. equiseti (Corda) Sacc. | Albuñol River Highway N342 | 551041 | 4074596 |
| FUS. 52 | F. equiseti (Corda) Sacc. | Sea bed at 6 m in depth | 550786 | 4073856 |
| FUS 60 | F. solani (Martius) Saccardo | Sea bed at 6 m in depth | 550844 | 4073833 |
| FUS 101 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 102 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 106 | F. proliferatum (Matsushima) Nirenberg | Andarax River | 545284 | 4090359 |
| FUS 115 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 545284 | 4090359 |
| FUS 118 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 119 | F. sambucinum Fuckel sensu lato | Andarax River | 549372 | 4086274 |
| FUS 121 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 130 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 132 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 133 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| Analysis code . | Fusarium spp. . | Location . | ||
|---|---|---|---|---|
| Sample depth . | UTM coordinates . | |||
| FUS 2 | F. verticillioides (Sacc.) Nirenberg | Mouth of Andarax River bed D = 1.5 m | 551238 | 4074229 |
| FUS 7 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 13 | F. solani (Martius) Saccardo | Mouth of Andarax River bed D = 0.1 m | 551169 | 4074231 |
| FUS 18 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 21 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 22 | F. verticillioides (Sacc.) Nirenberg | Mouth of Albuñol River bed D = 4.5 m | 485881 | 4066493 |
| FUS 28 | F. oxysporum Schlechtendahl emend. Snyder & Hansen | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 31 | F. solani (Martius) Saccardo | Mouth of Albuñol River bed D = 1.5 m | 485772 | 4066598 |
| FUS 111 | F. equiseti (Corda) Sacc. | Albuñol River Highway N342 | 551041 | 4074596 |
| FUS. 52 | F. equiseti (Corda) Sacc. | Sea bed at 6 m in depth | 550786 | 4073856 |
| FUS 60 | F. solani (Martius) Saccardo | Sea bed at 6 m in depth | 550844 | 4073833 |
| FUS 101 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 102 | F. proliferatum var. minus | Andarax River | 545284 | 4090359 |
| FUS 106 | F. proliferatum (Matsushima) Nirenberg | Andarax River | 545284 | 4090359 |
| FUS 115 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 545284 | 4090359 |
| FUS 118 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 119 | F. sambucinum Fuckel sensu lato | Andarax River | 549372 | 4086274 |
| FUS 121 | F. culmorum (W.G. Smith) Sacc. | Andarax River | 549372 | 4086274 |
| FUS 130 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 132 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
| FUS 133 | F. chlamydosporum Wollenweber & Reinking | Andarax River | 574058 | 4090020 |
Conidial germination
Germination was tested in distilled water in response to a range of osmotic potentials adjusted with NaCl (Table 2).
Relations between the osmotic potential of the medium (ψ)and the concentrations of KCl and NaCl
| ψ (bars) . | Product amount (g/l of PDA) . | |
|---|---|---|
| NaCla . | KCla . | |
| −1.50b | 0.0 | 0.0 |
| −13.79 | 17.6 | 22.2 |
| −41.79 | 52.0 | 68.8 |
| −70.37 | 84.8 | 112.0 |
| −99.56 | 115.2 | 152.8 |
| −144.54 | 156.6 | 212.5 |
| ψ (bars) . | Product amount (g/l of PDA) . | |
|---|---|---|
| NaCla . | KCla . | |
| −1.50b | 0.0 | 0.0 |
| −13.79 | 17.6 | 22.2 |
| −41.79 | 52.0 | 68.8 |
| −70.37 | 84.8 | 112.0 |
| −99.56 | 115.2 | 152.8 |
| −144.54 | 156.6 | 212.5 |
Relations between the osmotic potential of the medium (ψ)and the concentrations of KCl and NaCl
| ψ (bars) . | Product amount (g/l of PDA) . | |
|---|---|---|
| NaCla . | KCla . | |
| −1.50b | 0.0 | 0.0 |
| −13.79 | 17.6 | 22.2 |
| −41.79 | 52.0 | 68.8 |
| −70.37 | 84.8 | 112.0 |
| −99.56 | 115.2 | 152.8 |
| −144.54 | 156.6 | 212.5 |
| ψ (bars) . | Product amount (g/l of PDA) . | |
|---|---|---|
| NaCla . | KCla . | |
| −1.50b | 0.0 | 0.0 |
| −13.79 | 17.6 | 22.2 |
| −41.79 | 52.0 | 68.8 |
| −70.37 | 84.8 | 112.0 |
| −99.56 | 115.2 | 152.8 |
| −144.54 | 156.6 | 212.5 |
Each of the isolates was sub-cultured from selective medium Komada [17] to 20 ml PDA-KCl (6 g/l) medium [29]. Twelve cultures per isolate were incubated under ultra violet light (12.000 lux) for 10 days. After that, Petri dishes were washed twice with 5 ml of each salt solution in distilled water and the control without salt addition.
Germination percentages were calculated as mean germination values measured in hematocytometer (Malassez 0.00050 mm3) at 0, 24 and 48 h in 12 replications per isolate for each osmotic pressure.
Viability test
The viability of suspensions was also tested in three time periods (0, 24 and 48 h) by placking 0.25 μl washing solution (five replicates per osmotic pressure and isolate) in 15 ml PDA media. Results were calculated as mean number of colony former unit (CFU) per ml followed by standard deviation.
Statistical analysis of data
The statistical treatment of data was carried out using STATGRAPHICS Plus 5.1 statistical package software (StatPoint, Inc. 2325 Dulles Corner Boulevard, Suite 500 Herndon, Virginia 20171). Analysis of variance were carried out for the germination rates so that numbers with the same letter do not differ significantly.
Results and discussion
Germination test
Fusarium conidia showed germination in tests in aqueous medium. Results show how conidial germination, for the genus as a whole, increases with germination period up to an average of 2.81% of germination 48 h later.
The percentage of germinated spores was significantly different among incubation periods (Table 3). Conidial germination increases with time from an average rate of 0.939 ± 5.54 at 0 h incubation to 2.093 ± 7.12 and 2.815 ± 8.96 at 24 and 48 h, respectively. Significant differences between the different incubation periods studied were noted.
Variance analysis of germination depending on incubation period
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 805.902 | 2 | 402.951 | 7.47 | 0.0006 |
| Intra groups | 72675.2 | 1347 | 52.953 | ||
| Total (Corr.) | 73481.1 | 1349 |
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 805.902 | 2 | 402.951 | 7.47 | 0.0006 |
| Intra groups | 72675.2 | 1347 | 52.953 | ||
| Total (Corr.) | 73481.1 | 1349 |
FD freedom degrees
P: signification of mean difference with previous value
Variance analysis of germination depending on incubation period
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 805.902 | 2 | 402.951 | 7.47 | 0.0006 |
| Intra groups | 72675.2 | 1347 | 52.953 | ||
| Total (Corr.) | 73481.1 | 1349 |
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 805.902 | 2 | 402.951 | 7.47 | 0.0006 |
| Intra groups | 72675.2 | 1347 | 52.953 | ||
| Total (Corr.) | 73481.1 | 1349 |
FD freedom degrees
P: signification of mean difference with previous value
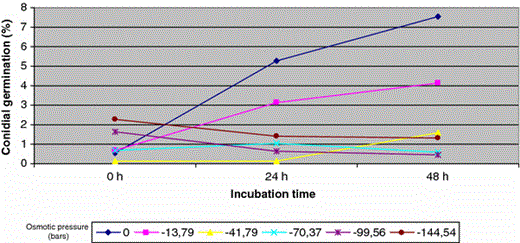
Fusarium germination (%) in response to a range of water potentials
Germination in distilled water increases with incubation period up to a maximum of 48 h (10.67%). Less conidial germination was observed in solutions with osmotic potential of −13.79 bars, although conidia kept on showing germination, which increased in parallel with incubation period up to 5.75%, 48 h later.
These results are coincident in part with those obtained by Gilbert [11] for ascospores germination of Giberella zeae (anamorph F. graminearum), germination rates were highest at 90% RH at 15 and 20 °C.
Beyer [3, 4] demonstrated that relative humidity was a key factor in germination of G. zeae ascospores. At RH over 84% and 20 °C almost 100% of the freshly discharged ascospores germinated. Successful germination, even under extreme conditions, suggests that ascospores are sufficiently robust to constitute a source of inoculum under most environmental conditions.
In the same way as in the germination of ascospores and, although the germination rates of asexual spores are not so high, germination raised over time. The percentages of conidial germination observed in this study may indicate that the asexual spores are not the main form of survival in aqueous media. Although it allows their survival in aqueous environments.
The rest of the saline solutions tested, with lower osmotic potential, did not show effects in germination, which in any case exceeded 2.4% of germination during tested incubation periods, but it is necessary to underline that they showed germination in all the osmotic pressures tested.
The germination results were analysed distinguishing the three tested incubation periods and Fusarium sp.
Analysis at 0 h with every isolate
Conidia did not show practically germination in distilled water, with an average germination of 0.939521 ± 5.54, mostly because of F. equiseti, as it will be shown distinguishing results for each of the tested species.
F. equiseti was the only species that was able to germinate at 0 h time (Table 4). These results could indicate both higher germination rate of conidia of this species in question, opposite with the rest of the studied species, and lower requirement of exogenous sources of energy to unleash germination. However, it is surprising that 0 h of incubation produced germination. A speculation could be the fact that those conidia were germinated in Petri dish of origin and they were swept out during the washing.
Conidial germination percentages in each tested species at 0 h
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. culmorum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.129 ± 0.38a |
| F. equiseti | 4.629 ± 9.41b | 6.075 ± 9.44b | 1.234 ± 3.70b | 6.076 ± 9.32b | 14.629 ± 22.10b | 13.308 ± 21.43b |
| F. oxysporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.01 ± 0.04a |
| m | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. solani | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. verticillioides | 0.155 ± 0.12a | 0.187 ± 0.13a | 0.112 ± 0.01a | 0.092 ± 0.07a | 0.106 ± 0.08a | 0.108 ± 0.09a |
| AC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.101 ± 0.30a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. culmorum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.129 ± 0.38a |
| F. equiseti | 4.629 ± 9.41b | 6.075 ± 9.44b | 1.234 ± 3.70b | 6.076 ± 9.32b | 14.629 ± 22.10b | 13.308 ± 21.43b |
| F. oxysporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.01 ± 0.04a |
| m | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. solani | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. verticillioides | 0.155 ± 0.12a | 0.187 ± 0.13a | 0.112 ± 0.01a | 0.092 ± 0.07a | 0.106 ± 0.08a | 0.108 ± 0.09a |
| AC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.101 ± 0.30a | 0.0 ± 0.0a | 0.0 ± 0.0a |
Conidial germination percentages in each tested species at 0 h
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. culmorum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.129 ± 0.38a |
| F. equiseti | 4.629 ± 9.41b | 6.075 ± 9.44b | 1.234 ± 3.70b | 6.076 ± 9.32b | 14.629 ± 22.10b | 13.308 ± 21.43b |
| F. oxysporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.01 ± 0.04a |
| m | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. solani | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. verticillioides | 0.155 ± 0.12a | 0.187 ± 0.13a | 0.112 ± 0.01a | 0.092 ± 0.07a | 0.106 ± 0.08a | 0.108 ± 0.09a |
| AC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.101 ± 0.30a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. culmorum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.129 ± 0.38a |
| F. equiseti | 4.629 ± 9.41b | 6.075 ± 9.44b | 1.234 ± 3.70b | 6.076 ± 9.32b | 14.629 ± 22.10b | 13.308 ± 21.43b |
| F. oxysporum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.01 ± 0.04a |
| m | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. solani | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. verticillioides | 0.155 ± 0.12a | 0.187 ± 0.13a | 0.112 ± 0.01a | 0.092 ± 0.07a | 0.106 ± 0.08a | 0.108 ± 0.09a |
| AC | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.101 ± 0.30a | 0.0 ± 0.0a | 0.0 ± 0.0a |
Analysis at 24 h with every isolate
Results verify that there are significant differences between averages of germination (Table 5). There are differences between treatment 0 and treatment 1 as well as between both and the others (Table 6). Germination rates for each species at 24 h incubation are shown in Table 7.
Descriptives of germination of Fusarium depending on osmotic pressure
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 1626.9 | 5 | 325.379 | 6.82 | 0.0 |
| Intra groups | 21182.5 | 444 | 47.708 | ||
| Total (Corr.) | 22809.4 | 449 |
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 1626.9 | 5 | 325.379 | 6.82 | 0.0 |
| Intra groups | 21182.5 | 444 | 47.708 | ||
| Total (Corr.) | 22809.4 | 449 |
FD freedom degrees
P: signification of mean difference with previous value
Descriptives of germination of Fusarium depending on osmotic pressure
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 1626.9 | 5 | 325.379 | 6.82 | 0.0 |
| Intra groups | 21182.5 | 444 | 47.708 | ||
| Total (Corr.) | 22809.4 | 449 |
| Variable . | Sums of squares . | DF . | Mean squares . | F . | P value . |
|---|---|---|---|---|---|
| Among groups | 1626.9 | 5 | 325.379 | 6.82 | 0.0 |
| Intra groups | 21182.5 | 444 | 47.708 | ||
| Total (Corr.) | 22809.4 | 449 |
FD freedom degrees
P: signification of mean difference with previous value
Variance analysis of Fusarium conidial germination depending on osmotic pressure at 24 h incubation
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 5.694 ± 10.65a |
| 1 | 75 | 3.392 ± 8.69b |
| 2 | 75 | 0.148 ± 0.32c |
| 3 | 75 | 1.151 ± 5.43c |
| 4 | 75 | 0.673 ± 2.60c |
| 5 | 75 | 1.401 ± 7.78c |
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 5.694 ± 10.65a |
| 1 | 75 | 3.392 ± 8.69b |
| 2 | 75 | 0.148 ± 0.32c |
| 3 | 75 | 1.151 ± 5.43c |
| 4 | 75 | 0.673 ± 2.60c |
| 5 | 75 | 1.401 ± 7.78c |
Variance analysis of Fusarium conidial germination depending on osmotic pressure at 24 h incubation
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 5.694 ± 10.65a |
| 1 | 75 | 3.392 ± 8.69b |
| 2 | 75 | 0.148 ± 0.32c |
| 3 | 75 | 1.151 ± 5.43c |
| 4 | 75 | 0.673 ± 2.60c |
| 5 | 75 | 1.401 ± 7.78c |
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 5.694 ± 10.65a |
| 1 | 75 | 3.392 ± 8.69b |
| 2 | 75 | 0.148 ± 0.32c |
| 3 | 75 | 1.151 ± 5.43c |
| 4 | 75 | 0.673 ± 2.60c |
| 5 | 75 | 1.401 ± 7.78c |
Results of variance analysis of conidial germination for each studied species at 24 h of incubation
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 7.246 ± 10.83ab | 2.871 ± 4.36ab | 0.055 ± 0.09a | 0.129 ± 0.38a | 1.084 ± 1.72a | 2.2E-16 ± 0.0a |
| F. culmorum | 11.985 ± 9.12bc | 0.338 ± 0.61a | 0.0 ± 0.0a | 0.175 ± 0.27a | 2.2E-16 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 2.681 ± 3.88a | 8.562 ± 9.78bc | 0.460 ± 0.66b | 9.046 ± 0.06b | 4.408 ± 6.39b | 12.369 ± 20.25b |
| F. oxysporum | 0.831 ± 1.19a | 0.101 ± 0.09a | 0.081 ± 0.07a | 0.051 ± 0.0a | 0.0 ± 0.0a | 0.029 ± 0.08a |
| F. proliferatum | 4.335 ± 4.75ab | 0.140 ± 0.21a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39ab | 8.4E-15 ± 0.0ab | −2.2E-16 ± 0.0a | −1.5E-15 ± 0.0a | −6.6E-16 ± 0.0a | −4.4E-16 ± 0.0a |
| F. solani | 1.875 ± 3.70a | 14.555 ± 18.88c | 0.465 ± 0.40b | 0.110 ± 0.14a | −1.1E-16 ± 0.0a | −2.2E-16 ± 0.0a |
| F. verticillioides | 1.631 ± 2.33a | 0.335 ± 0.33a | 0.074 ± 0.02a | 0.075 ± 0.04a | 0.107 ± 0.13a | 0.105 ± 0.14a |
| AC | 16.791 ± 22.81c | 1.361 ± 1.71a | 0.098 ± 0.15a | 0.005 ± 0.01a | 0.011 ± 0.02a | 0.009 ± 0.02a |
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 7.246 ± 10.83ab | 2.871 ± 4.36ab | 0.055 ± 0.09a | 0.129 ± 0.38a | 1.084 ± 1.72a | 2.2E-16 ± 0.0a |
| F. culmorum | 11.985 ± 9.12bc | 0.338 ± 0.61a | 0.0 ± 0.0a | 0.175 ± 0.27a | 2.2E-16 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 2.681 ± 3.88a | 8.562 ± 9.78bc | 0.460 ± 0.66b | 9.046 ± 0.06b | 4.408 ± 6.39b | 12.369 ± 20.25b |
| F. oxysporum | 0.831 ± 1.19a | 0.101 ± 0.09a | 0.081 ± 0.07a | 0.051 ± 0.0a | 0.0 ± 0.0a | 0.029 ± 0.08a |
| F. proliferatum | 4.335 ± 4.75ab | 0.140 ± 0.21a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39ab | 8.4E-15 ± 0.0ab | −2.2E-16 ± 0.0a | −1.5E-15 ± 0.0a | −6.6E-16 ± 0.0a | −4.4E-16 ± 0.0a |
| F. solani | 1.875 ± 3.70a | 14.555 ± 18.88c | 0.465 ± 0.40b | 0.110 ± 0.14a | −1.1E-16 ± 0.0a | −2.2E-16 ± 0.0a |
| F. verticillioides | 1.631 ± 2.33a | 0.335 ± 0.33a | 0.074 ± 0.02a | 0.075 ± 0.04a | 0.107 ± 0.13a | 0.105 ± 0.14a |
| AC | 16.791 ± 22.81c | 1.361 ± 1.71a | 0.098 ± 0.15a | 0.005 ± 0.01a | 0.011 ± 0.02a | 0.009 ± 0.02a |
Results of variance analysis of conidial germination for each studied species at 24 h of incubation
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 7.246 ± 10.83ab | 2.871 ± 4.36ab | 0.055 ± 0.09a | 0.129 ± 0.38a | 1.084 ± 1.72a | 2.2E-16 ± 0.0a |
| F. culmorum | 11.985 ± 9.12bc | 0.338 ± 0.61a | 0.0 ± 0.0a | 0.175 ± 0.27a | 2.2E-16 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 2.681 ± 3.88a | 8.562 ± 9.78bc | 0.460 ± 0.66b | 9.046 ± 0.06b | 4.408 ± 6.39b | 12.369 ± 20.25b |
| F. oxysporum | 0.831 ± 1.19a | 0.101 ± 0.09a | 0.081 ± 0.07a | 0.051 ± 0.0a | 0.0 ± 0.0a | 0.029 ± 0.08a |
| F. proliferatum | 4.335 ± 4.75ab | 0.140 ± 0.21a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39ab | 8.4E-15 ± 0.0ab | −2.2E-16 ± 0.0a | −1.5E-15 ± 0.0a | −6.6E-16 ± 0.0a | −4.4E-16 ± 0.0a |
| F. solani | 1.875 ± 3.70a | 14.555 ± 18.88c | 0.465 ± 0.40b | 0.110 ± 0.14a | −1.1E-16 ± 0.0a | −2.2E-16 ± 0.0a |
| F. verticillioides | 1.631 ± 2.33a | 0.335 ± 0.33a | 0.074 ± 0.02a | 0.075 ± 0.04a | 0.107 ± 0.13a | 0.105 ± 0.14a |
| AC | 16.791 ± 22.81c | 1.361 ± 1.71a | 0.098 ± 0.15a | 0.005 ± 0.01a | 0.011 ± 0.02a | 0.009 ± 0.02a |
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 7.246 ± 10.83ab | 2.871 ± 4.36ab | 0.055 ± 0.09a | 0.129 ± 0.38a | 1.084 ± 1.72a | 2.2E-16 ± 0.0a |
| F. culmorum | 11.985 ± 9.12bc | 0.338 ± 0.61a | 0.0 ± 0.0a | 0.175 ± 0.27a | 2.2E-16 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 2.681 ± 3.88a | 8.562 ± 9.78bc | 0.460 ± 0.66b | 9.046 ± 0.06b | 4.408 ± 6.39b | 12.369 ± 20.25b |
| F. oxysporum | 0.831 ± 1.19a | 0.101 ± 0.09a | 0.081 ± 0.07a | 0.051 ± 0.0a | 0.0 ± 0.0a | 0.029 ± 0.08a |
| F. proliferatum | 4.335 ± 4.75ab | 0.140 ± 0.21a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39ab | 8.4E-15 ± 0.0ab | −2.2E-16 ± 0.0a | −1.5E-15 ± 0.0a | −6.6E-16 ± 0.0a | −4.4E-16 ± 0.0a |
| F. solani | 1.875 ± 3.70a | 14.555 ± 18.88c | 0.465 ± 0.40b | 0.110 ± 0.14a | −1.1E-16 ± 0.0a | −2.2E-16 ± 0.0a |
| F. verticillioides | 1.631 ± 2.33a | 0.335 ± 0.33a | 0.074 ± 0.02a | 0.075 ± 0.04a | 0.107 ± 0.13a | 0.105 ± 0.14a |
| AC | 16.791 ± 22.81c | 1.361 ± 1.71a | 0.098 ± 0.15a | 0.005 ± 0.01a | 0.011 ± 0.02a | 0.009 ± 0.02a |
The pattern of conidial germination observed in F. culmorum, F. oxysporum, F. proliferatum, F. sambucinum and F. verticilloides was similar (Table 7). Germination was uniformly maximal in distilled water, drastically lower in aqueous solutions with osmotic potentials between −13.79 and −41.79 bars and prevented in the range of −70.37 and −144.54 bars. The three of the isolates used as control samples behave as an isolate of F. oxysporum isolated from aquatic habitat.
Germination pattern in F. chlamydosporum was similar except for the observed drop in germination percentages at −13.79 and −41.79 bars that was progressive. It was not observed a drastic decrease of the germination percentage in saline aqueous media.
On the other side, conidial germination of F. solani and F. equiseti was positively affected due to the osmotic pressure of aqueous medium. Conidial germination percentages of F. solani were the highest in solutions with osmotic pressures of −13.79 bars with 14.55% of germinated sample, 12% higher than germination observed in distilled water. F. equiseti shows the highest germination percentage (12.36%) in solutions with osmotic potentials of −144.54 bars.
Analysis at 48 h time with every isolate
There are differences between averages of germination at 48 h of incubation (Table 8).
Descriptives of germination of Fusarium depending on osmotic pressure
| Variable . | Sums of squares . | DF . | Mean squares . | F . | Pvalue . |
|---|---|---|---|---|---|
| Among groups | 3319.28 | 5 | 663.857 | 9.00 | 0.0 |
| Intra groups | 32734.7 | 444 | 73.7267 | ||
| Total (Corr.) | 36054.0 | 449 |
| Variable . | Sums of squares . | DF . | Mean squares . | F . | Pvalue . |
|---|---|---|---|---|---|
| Among groups | 3319.28 | 5 | 663.857 | 9.00 | 0.0 |
| Intra groups | 32734.7 | 444 | 73.7267 | ||
| Total (Corr.) | 36054.0 | 449 |
FD freedom degrees
P: signification of mean difference with previous value
Descriptives of germination of Fusarium depending on osmotic pressure
| Variable . | Sums of squares . | DF . | Mean squares . | F . | Pvalue . |
|---|---|---|---|---|---|
| Among groups | 3319.28 | 5 | 663.857 | 9.00 | 0.0 |
| Intra groups | 32734.7 | 444 | 73.7267 | ||
| Total (Corr.) | 36054.0 | 449 |
| Variable . | Sums of squares . | DF . | Mean squares . | F . | Pvalue . |
|---|---|---|---|---|---|
| Among groups | 3319.28 | 5 | 663.857 | 9.00 | 0.0 |
| Intra groups | 32734.7 | 444 | 73.7267 | ||
| Total (Corr.) | 36054.0 | 449 |
FD freedom degrees
P: signification of mean difference with previous value
Table 9 shows that there are differences between treatment 0 and treatment 1 as well as between both and the others.
Variance analysis of Fusarium conidial germination depending on osmotic pressure at 48 h incubation
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 8.128 ± 15.96a |
| 1 | 75 | 4.486 ± 9.38b |
| 2 | 75 | 1.721 ± 5.13c |
| 3 | 75 | 0.644 ± 2.65c |
| 4 | 75 | 0.503 ± 2.38c |
| 5 | 75 | 1.407 ± 7.75c |
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 8.128 ± 15.96a |
| 1 | 75 | 4.486 ± 9.38b |
| 2 | 75 | 1.721 ± 5.13c |
| 3 | 75 | 0.644 ± 2.65c |
| 4 | 75 | 0.503 ± 2.38c |
| 5 | 75 | 1.407 ± 7.75c |
Variance analysis of Fusarium conidial germination depending on osmotic pressure at 48 h incubation
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 8.128 ± 15.96a |
| 1 | 75 | 4.486 ± 9.38b |
| 2 | 75 | 1.721 ± 5.13c |
| 3 | 75 | 0.644 ± 2.65c |
| 4 | 75 | 0.503 ± 2.38c |
| 5 | 75 | 1.407 ± 7.75c |
| Treatment . | Frequency . | Average ± standard deviation . |
|---|---|---|
| 0 | 75 | 8.128 ± 15.96a |
| 1 | 75 | 4.486 ± 9.38b |
| 2 | 75 | 1.721 ± 5.13c |
| 3 | 75 | 0.644 ± 2.65c |
| 4 | 75 | 0.503 ± 2.38c |
| 5 | 75 | 1.407 ± 7.75c |
The pattern of conidial germination observed at 24 h in F. culmorum, F. oxysporum, F. proliferatum, F. sambucinum and F. verticilloides remains at 48 h (Table 10).
Results of variance analysis of conidial germination for each studied species at 48 h of incubation
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 2.939 ± 4.64a | 2.663 ± 4.03a | 2.315 ± 3.02a | 0.002 ± 0.01a | 0.5 ± 0.99a | 0.925 ± 2.77a |
| F. culmorum | 17.961 ± 18.81b | 0.830 ± 1.16a | 0.118 ± 0.23a | 0.223 ± 0.35a | 5.5E-17 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 8.292 ± 7.25a | 10.042 ± 11.71b | 3.392 ± 6.29a | 4.486 ± 6.76b | 3.289 ± 6.41b | 10.761 ± 20.87b |
| F. oxysporum | 1.214 ± 1.77a | 0.163 ± 0.19a | 0.138 ± 0.15a | 0.247 ± 0.36a | 0.011 ± 0.01a | 0.0 ± 0.0a |
| F. proliferatum | 5.024 ± 7.00a | 0.171 ± 0.27a | 0.037 ± 0.05a | 0.001 ± 0.0a | 1.1E-16 ± 0.0a | 2.2E-16 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39a | −1.1E-14 ± 0.0a | −2.8E-15 ± 0.0a | −1.2E-15 ± 0.0a | −1.1E-15 ± 0.0a | −2.4E-15 ± 0.0a |
| F. solani | 4.832 ± 4.16a | 21.333 ± 14.26c | 8.026 ± 11.43b | 0.192 ± 0.29a | 0.005 ± 0.01a | −2.2E-16 ± 0.0a |
| F. verticillioides | 4.413 ± 5.78a | 0.388 ± 0.49a | 0.282 ± 0.35a | 0.214 ± 0.26a | 0.393 ± 0.56a | 0.043 ± 0.04a |
| AC | 22.985 ± 36.39b | 1.796 ± 2.72a | 0.037 ± 0.05a | 0.0009 ± 0.0a | −6.3E-16 ± 0.0a | −1.3E-15 ± 0.0a |
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 2.939 ± 4.64a | 2.663 ± 4.03a | 2.315 ± 3.02a | 0.002 ± 0.01a | 0.5 ± 0.99a | 0.925 ± 2.77a |
| F. culmorum | 17.961 ± 18.81b | 0.830 ± 1.16a | 0.118 ± 0.23a | 0.223 ± 0.35a | 5.5E-17 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 8.292 ± 7.25a | 10.042 ± 11.71b | 3.392 ± 6.29a | 4.486 ± 6.76b | 3.289 ± 6.41b | 10.761 ± 20.87b |
| F. oxysporum | 1.214 ± 1.77a | 0.163 ± 0.19a | 0.138 ± 0.15a | 0.247 ± 0.36a | 0.011 ± 0.01a | 0.0 ± 0.0a |
| F. proliferatum | 5.024 ± 7.00a | 0.171 ± 0.27a | 0.037 ± 0.05a | 0.001 ± 0.0a | 1.1E-16 ± 0.0a | 2.2E-16 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39a | −1.1E-14 ± 0.0a | −2.8E-15 ± 0.0a | −1.2E-15 ± 0.0a | −1.1E-15 ± 0.0a | −2.4E-15 ± 0.0a |
| F. solani | 4.832 ± 4.16a | 21.333 ± 14.26c | 8.026 ± 11.43b | 0.192 ± 0.29a | 0.005 ± 0.01a | −2.2E-16 ± 0.0a |
| F. verticillioides | 4.413 ± 5.78a | 0.388 ± 0.49a | 0.282 ± 0.35a | 0.214 ± 0.26a | 0.393 ± 0.56a | 0.043 ± 0.04a |
| AC | 22.985 ± 36.39b | 1.796 ± 2.72a | 0.037 ± 0.05a | 0.0009 ± 0.0a | −6.3E-16 ± 0.0a | −1.3E-15 ± 0.0a |
Results of variance analysis of conidial germination for each studied species at 48 h of incubation
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 2.939 ± 4.64a | 2.663 ± 4.03a | 2.315 ± 3.02a | 0.002 ± 0.01a | 0.5 ± 0.99a | 0.925 ± 2.77a |
| F. culmorum | 17.961 ± 18.81b | 0.830 ± 1.16a | 0.118 ± 0.23a | 0.223 ± 0.35a | 5.5E-17 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 8.292 ± 7.25a | 10.042 ± 11.71b | 3.392 ± 6.29a | 4.486 ± 6.76b | 3.289 ± 6.41b | 10.761 ± 20.87b |
| F. oxysporum | 1.214 ± 1.77a | 0.163 ± 0.19a | 0.138 ± 0.15a | 0.247 ± 0.36a | 0.011 ± 0.01a | 0.0 ± 0.0a |
| F. proliferatum | 5.024 ± 7.00a | 0.171 ± 0.27a | 0.037 ± 0.05a | 0.001 ± 0.0a | 1.1E-16 ± 0.0a | 2.2E-16 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39a | −1.1E-14 ± 0.0a | −2.8E-15 ± 0.0a | −1.2E-15 ± 0.0a | −1.1E-15 ± 0.0a | −2.4E-15 ± 0.0a |
| F. solani | 4.832 ± 4.16a | 21.333 ± 14.26c | 8.026 ± 11.43b | 0.192 ± 0.29a | 0.005 ± 0.01a | −2.2E-16 ± 0.0a |
| F. verticillioides | 4.413 ± 5.78a | 0.388 ± 0.49a | 0.282 ± 0.35a | 0.214 ± 0.26a | 0.393 ± 0.56a | 0.043 ± 0.04a |
| AC | 22.985 ± 36.39b | 1.796 ± 2.72a | 0.037 ± 0.05a | 0.0009 ± 0.0a | −6.3E-16 ± 0.0a | −1.3E-15 ± 0.0a |
| . | Treatment 0 . | Treatment 1 . | Treatment 2 . | Treatment 3 . | Treatment 4 . | Treatment 5 . |
|---|---|---|---|---|---|---|
| F. chlamydosporum | 2.939 ± 4.64a | 2.663 ± 4.03a | 2.315 ± 3.02a | 0.002 ± 0.01a | 0.5 ± 0.99a | 0.925 ± 2.77a |
| F. culmorum | 17.961 ± 18.81b | 0.830 ± 1.16a | 0.118 ± 0.23a | 0.223 ± 0.35a | 5.5E-17 ± 0.0a | 4.4E-16 ± 0.0a |
| F. equiseti | 8.292 ± 7.25a | 10.042 ± 11.71b | 3.392 ± 6.29a | 4.486 ± 6.76b | 3.289 ± 6.41b | 10.761 ± 20.87b |
| F. oxysporum | 1.214 ± 1.77a | 0.163 ± 0.19a | 0.138 ± 0.15a | 0.247 ± 0.36a | 0.011 ± 0.01a | 0.0 ± 0.0a |
| F. proliferatum | 5.024 ± 7.00a | 0.171 ± 0.27a | 0.037 ± 0.05a | 0.001 ± 0.0a | 1.1E-16 ± 0.0a | 2.2E-16 ± 0.0a |
| F. sambucinum | 0.229 ± 0.39a | −1.1E-14 ± 0.0a | −2.8E-15 ± 0.0a | −1.2E-15 ± 0.0a | −1.1E-15 ± 0.0a | −2.4E-15 ± 0.0a |
| F. solani | 4.832 ± 4.16a | 21.333 ± 14.26c | 8.026 ± 11.43b | 0.192 ± 0.29a | 0.005 ± 0.01a | −2.2E-16 ± 0.0a |
| F. verticillioides | 4.413 ± 5.78a | 0.388 ± 0.49a | 0.282 ± 0.35a | 0.214 ± 0.26a | 0.393 ± 0.56a | 0.043 ± 0.04a |
| AC | 22.985 ± 36.39b | 1.796 ± 2.72a | 0.037 ± 0.05a | 0.0009 ± 0.0a | −6.3E-16 ± 0.0a | −1.3E-15 ± 0.0a |
Glenn [12] studied the variation in spore germination of F. verticillioides, the author state two different phenotypes. In general, germination tubes immediately penetrated into agar, but other isolates formed germ tubes that grew along the surface of agar. The invasive germination was the predominant phenotype and was more virulent than other strains tested.
The observed differences in the germination percentages of different species could be explained by the genetic variability within the strains. Further analysis should determine the possible correlation between the conidial germination and virulence of the isolates.
Germination was uniformly the maximum in distilled water. In case of F. chlamydosporum, the observed decrease in germination percentages at −13.79 and −41.79 bars is still progressive.
The two species that were positively affected by osmotic pressure of aqueous medium, keep on showing that influence. Just like at 24 h, conidial germination percentages of F. solani were the highest at 48 h in treatment 1 (−13.79 bars of osmotic pressure) with 21.33% if germinated conidia, 16% higher than observed germination in distilled water. Percentage observed in treatment 2 (−41.79 bars) is lower than treatment 1, but 3.6% higher than the one in distilled water.
F. equiseti keeps on showing the highest germination percentage (10.76%) in solutions with osmotic potentials of −144.54.
Viability test
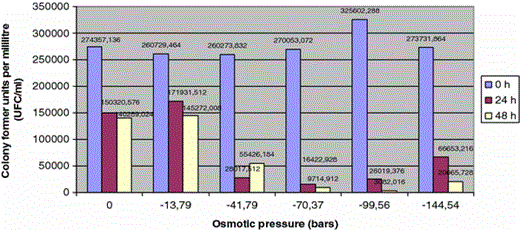
Viability of Fusarium from river and sea beds, responding to the osmotic potential, given as colony formation units per millilitre (CFU ml−1)
Following incubation periods with different saline concentration, it could be observed how viability remains undermined by the aqueous medium, with a marked reduction of CFU. The effect of the first saline concentration tested (−13.79 bars) is not different to the control treatment in distilled water and it is lower than the rest of osmotic pressures tested.
The harmful effect observed in conidial germination with osmotic pressures below −41.79 bars confirms the results of the viability studies, which showed a drastic drop in the number of UFC from the first saline concentration tested (Fig. 2).
The favorable effect of salinity observed in the germination of F. solani is not so clear in relation to viability while there are no significant differences, after 48 h incubation, among the treatment with distilled water (84486.6 UFC) and osmotic pressure of −13.79 (82806 UFC) and −41.79 bars(71560 UFC).. The adverse effect of salt is visible from −70.37 bars pressure and keeps on increasing with incubation time and lower osmotic potentials.
In the case of F. equiseti, the results show that the aquatic environment clearly favors the viability, from 1,09,000 CFU/ml at 0 h to 10,58,000 CFU/ml at 48 h.
The first saline concentration tested (ce = 24.8 mS/ml) tolerates the viability of F. equiseti, which reaches 1,32,000 CFU/ml at 48 h of incubation. Higher salinities seriously affect Fusarium and prevent the viability of the isolated studied.
We must underline the differential behaviour showed by T2 isolate against the rest of F. oxysporum studied. Just like it happened with F. solani, this isolate shows more viability at 48 h at −13.79 bars (818485.8 CFU) than in distilled water (560955.93 CFU). Finally, in the particular case of F. sambucinum viability is completely null at 24 and 48 h, then it could be said that it does not present any survival capacity in aqueous media and it could explain, partly, the low presence of the specie in the original sampling, in which is based this study.
Electric conductivity of Sea water at the mouth of the River Andarax ranges between 50 and 54.40 dS / m. Treatment 1 and 2 represent respectively approximately electric conductivities of 24.8 and 60.5 dS / m, therefore, the experimental results indicate that the conditions present at seabed are more favorable to germination of these fungi than distilled water, with no salt added to the medium. The ability of certain species of Fusarium to develop a saprophytic life in the salt water of the Mediterranean Sea could be certain.
Results show how conidial germination in aqueous medium increased with germination period. Some of the Fusarium sp. studied are potential mycotoxin producers. Mycotoxins as deoxinivalenol or zearalenone were detected in Swiss rivers [6], these and other mycotoxins exibit high solubility. The ecotoxicological effects of the presence of mycotoxins in surface waters remain to be elucidated.
Successful germination, even under high salty media conditions, suggests that Fusarium sp. could have a possible competitive advantage over other soil fungi in crops irrigated with saline water. Practical implications as the possible proliferation of Fusaria in farmland or glasshouse crops irrigated with saline water should also be attempted.
References
Tello JC, Lacasa A, Rodriguez MC (1990) Presence of some Fusarium species on Spanish beaches. Proceedings of the 8th congress of the Mediterranean phytopathological union. Agadir, Morocco, pp 137–138
Tello JC, Vares F, Lacasa A (1991) Análisis de muestras. In: Manual de laboratorio: diagnostico de hongos, bacterias y nematodos fitopatógenos. M.A.P.A. Madrid, pp 39–48



