-
PDF
- Split View
-
Views
-
Cite
Cite
M A Berbert-Molina, A M R Prata, L G Pessanha, M M Silveira, Kinetics of Bacillus thuringiensis var. israelensis growth on high glucose concentrations, Journal of Industrial Microbiology and Biotechnology, Volume 35, Issue 11, 1 November 2008, Pages 1397–1404, https://doi.org/10.1007/s10295-008-0439-1
Close - Share Icon Share
Abstract
The kinetic and general growth features of Bacillus thuringiensis var. israelensis were evaluated. Initial glucose concentration (S 0) in fermentation media varied from 10 to 152 g/l. The results afforded to characterize four morphologically and physiologically well-defined culture phases, independent of S 0 values: Phase I, vegetative growth; Phase II, transition to sporulation; Phase III, sporulation; and Phase IV, spores maturation and cell lysis. Important process parameters were also determined. The maximum specific growth rates (μ X,m) were not affected with S 0 up to 75 g/l (1.0–1.1 per hour), but higher glucose concentrations resulted in growth inhibition by substrate, revealed by a reduction in μ X,m values. These higher S 0 values led to longer Phases III and IV and delayed sporulation. Similar biomass concentrations (X m = 15.2–15.9 g/l) were achieved with S 0 over 30.8 g/l, with increasing residual substrate, suggesting a limitation in some other nutrients and the use of glucose to form other metabolites. In this case, with S 0 from 30.8 to 152 g/l, cell yield (Y X/S) decreased from 0.58 to 0.41 g/g. On the other hand, with S 0 = 10 g/l growth was limited by substrate, and Y X/S has shown its maximum value (0.83 g/g).
Introduction
Bacillus thuringiensis var. israelensis (Bti) is recognized by its high toxicity against larvae of some human disease vectors, such as Aedes aegypti, the dengue fever vector [17]. The larvicidal activity of Bti is due to a crystal body composed by different proteins, named δ-endotoxins, which are produced during the sporulation cycle and deposited in the bacterial cytoplasm [8]. The safety for non-target organisms and environment has favored the continuous increase in the use of Bti formulations for insect control programs in the last decades [14]. However, little information is available about some physiological and kinetic growth aspects of the fermentation process involving this microorganism. Moreover, the literature data suggested that the effect of operational conditions and media composition on fermentation results depends on the B. thuringiensis (Bt) variety or on the strain used [5, 7, 19, 22]. There are, for example, contradictions concerning the type and the substrate concentration for the process, variables that affect the cell, spores and toxin production by Bt. According to Lacey et al. [13], the improvement of the production processes and the medium formulation are indispensable aspects for the future development of the insecticides market with Bt.
Although other carbon sources, as sucrose and maltose, allowed the appropriate growth of some Bt varieties [10, 19], glucose is considered by some authors as the most suitable carbohydrate for Bt biolarvicide production [19, 24]. Substrate concentrations of 10 g/l have been used in most fermentation studies, although higher concentrations were tested in a few cases. For B. thuringiensis var. galleriae, it was observed that glucose concentrations of about 28 g/l led to a maximum biomass production, but the use of 35 g/l of this substrate resulted in a heterogeneous population, composed by vegetative and sporulated cells and free spores [1]. On the other hand, for B. thuringiensis var. kurstaki a maximum glucose concentration of 56 g/l can be utilized [2]. Holmberg and Sievanen [9] reported that high concentration of nutrients inhibit the growth of B. thuringiensis var. thuringiensis, harming the sporulation and toxin synthesis. According to the authors, the substrate concentration should not exceed 23 g/l.
The inhibitory effects of high glucose concentrations on Bt growth can be avoided by the use of fed-batch fermentation system. High biomass concentrations were obtained by this way, but the sporulation efficiency and toxicity depended on the feeding nutrient strategy [11]. For B. thuringiensis var. israelensis, with a linear gradient continuous fed-batch culture it was verified low yields of spores and a decline in toxicity compared to batch system [4]. For B. thuringiensis var. kurstaki fed-batch cultures with constant feeding suppressed the sporulation [11].
In view of these facts, this work aimed to study the Bti kinetic growth, in batch system, employing media with glucose concentration higher than that usually evaluated for this fermentation process. Concentrations from 10 to 150 g/l were assayed, in a semi-defined medium contained yeast extract and salts. The main morphological and physiological characteristics of Bti culture under these conditions were also assessed.
Materials and methods
Microorganism and cultivation media
B. thuringiensis var. israelensis IPS 82 was used in all assays. The strain was maintained as a sporulated culture on nutrient agar at 4 °C. LB medium (Luria Bertani Broth), with the following composition (g/l), was used for the preparation of the pre-inoculum: peptone, 10.0; yeast extract, 5.0; NaCl, 5.0. For inoculum preparation and fermentation assays, a modified glucose/yeast extract/salts (GYS) medium was used containing the following (g/l): yeast extract, 12.0; (NH4)2SO4, 3.0; CaCl2·2H2O, 0.12; MgSO4·7H2O, 1.5; MnSO4·H2O, 0.09; K2HPO4, 1.5; KH2PO4, 1.5. The initial glucose concentration (S 0) in the medium for inoculum preparation was 20 g/l, whereas for bioreactor assays it varied from 10 to 152 g/l. Concentrated glucose solutions were prepared and sterilized separately and added to the medium before inoculation.
Culture conditions
For pre-inoculum preparation, 125-ml Erlenmeyer flasks containing 25 ml LB medium were inoculated with a loopful of sporulated culture and statically incubated for 15 h at 30 °C. For inoculum preparation, 500-ml Erlenmeyer flasks, containing 100 ml GYS medium, were inoculated with 5% (v/v) of the pre-inoculum. The flasks were incubated in a reciprocal shaker (New Brunswick incubator shaker model 25D, USA), at 30 °C and 110 per minute, for 5–6 h, until the culture reached an absorbance of approximately 10 measured at 610 nm. The volume of inoculum corresponded to 5% of the initial volume of fermentation medium.
The assays were performed in a 5-l Biostat MD bioreactor (B. Braun Biotech, Germany) with an initial volume of 3.6 l. The temperature was automatically controlled at 30 °C. The pH was adjusted to 7.0 before inoculation, allowed to drop spontaneously to a minimum value of 5.6, and then controlled with automatic addition of 5 M KOH to prevent lower values until the moment it began to rise again naturally. The agitation speed and the aeration rate were settled at 550 per minute and 0.57 vvm, respectively, until the concentration of dissolved oxygen (DO) reached 35% of the saturation. After that, they were shifted automatically to maintain DO at a minimum of 35% of saturation. Six assays were accomplished, using S 0 values of 10.0, 30.8, 57.7, 75.0, 124 and 152 g/l.
Analytical methods
During the first 5 h, defined as vegetative growth phase in this work (see details in Sect. “Results and discussion”), cell concentration was estimated by reading absorbance (spectrophotometer Shimadzu UV-160A) of diluted suspensions of culture at 610 nm and converting it into concentration (g/l) using an equation from a calibration curve. For all subsequent samples, biomass dry weight concentration was measured gravimetrically, since the flocculation of culture that characterizes this fermentation period hinders the biomass quantification by turbidimetry. For this, a predefined volume of fermentation broth was centrifuged, washed three times and the pellet dried at 85 °C for 24 h. The general characteristics of cell morphology, the formation of inclusion bodies (crystals of δ-endotoxins and PHB granules) and sporulation development were monitored by analyzing fresh fermentation broth preparations in a phase contrast microscopy (Leitz, Laborluz S). The presence of PHB (polyhydroxybutyrate) granules in the cell was examined by microscopy according to Ostle and Holt [16]. For this, heat-fixed smears of bacterial cells were stained with the Nile blue solution (1% w/v) at 55 °C for 10 min, washed with tap water and with 8% (v/v) aqueous acetic acid for 1 min. The stained smear was washed with tap water, air-dried, remoistened with tap water and covered with a glass cover slip. The preparations were covered with immersion oil and examined in a microscope (Zeiss, Jenalumar) with an episcopic fluorescence attachment. PHB granules exhibited a strong orange fluorescence when stained with Nile blue. A sporulation rate of 90%, which determined the end of sporulation phase (see Sect. “Results and discussion”), was empirically defined as the ratio of the total sporulated cells (lysed and unlysed cells) over the total cell population. The glucose concentration was determined according to a glucose oxidase method (Celm kit, Brazil). Dissolved oxygen was continuously measured using a polarographic electrode (Ingold Co., Switzerland) connected to the digital control unit, that was calibrated in fermentation medium at 30 °C and initial settled aeration/agitation conditions just before the inoculation. The values were converted from percentage air saturation to concentrations (mmol/l) taking into account the saturation concentration of GYS medium calculated as stated by Schumpe and Quicker [23]. The molar fractions of oxygen and carbon dioxide in the bioreactor exhaust gas were measured with a Maihak (Germany) gas analyzer. By using these data and the gas flow rate, oxygen uptake rate (OUR) was calculated as described by Wang [25].
Estimation of biomass yield, cell productivity and rate coefficients
The biomass yield based on glucose (Y X/S, g/g) was calculated as the ratio of the biomass produced (g/l) to the glucose consumed (g/l) until t X,m (h), which is the time to achieve the maximum cell concentration (X m). Cell productivity (p x) was calculated as the ratio of the biomass produced to elapsed time until t X,m. The maximum specific growth rates were calculated from linear regressions of natural logarithm of biomass concentrations versus time. The angular coefficient of the linear trend corresponded to the μ X,m value.
Results and discussion
In this work, experimental data of seven Bti batch fermentations, employing initial glucose concentrations (S 0) from 10 to 152 g/l, were analyzed to investigate the kinetics of the bacterial growth and some process parameters that are important to optimize the large scale production of Bt insecticides, as biomass yield, cell productivity, growth rates and patterns of substrate consumption. The morphological and physiological changes that characterize Bt cultivations were also examined and correlated with the overall metabolic behavior observed under the different S 0 conditions.
Morphological and physiological characteristics of the culture
Main morphological and physiological characteristics of Bacillus thuringiensis var. israelensis cultures carried out under different initial glucose concentrations (S 0)
| Phase of cellular growth . | Main characteristics . |
|---|---|
| Vegetative growth (Phase I) | Fast decrease of pH values; occurrence of exponential growth phase, with higher μ X,m, followed by a period of non-exponential growth; the cell growth curve presents a linear profile; long and uniform rods; isolated, double rods and chains; high motility; high oxygen uptake rates |
| Transition to sporulation (Phase II) | Rise of pH; shorter and isolated cells; no motility; beginning of the flocculation; reduction on growth rates; drastic reduction on oxygen uptake rates |
| Sporulation (Phase III) | Spore formation; intense flocculation; decreasing growth curve; increasing dissolved oxygen concentrations; decreasing oxygen uptake rates |
| Cell lysis (Phase IV) | Complete maturation of spores; disappearance of clumps; cell lysis; decreasing growth curve; minimum oxygen uptake rates |
| Phase of cellular growth . | Main characteristics . |
|---|---|
| Vegetative growth (Phase I) | Fast decrease of pH values; occurrence of exponential growth phase, with higher μ X,m, followed by a period of non-exponential growth; the cell growth curve presents a linear profile; long and uniform rods; isolated, double rods and chains; high motility; high oxygen uptake rates |
| Transition to sporulation (Phase II) | Rise of pH; shorter and isolated cells; no motility; beginning of the flocculation; reduction on growth rates; drastic reduction on oxygen uptake rates |
| Sporulation (Phase III) | Spore formation; intense flocculation; decreasing growth curve; increasing dissolved oxygen concentrations; decreasing oxygen uptake rates |
| Cell lysis (Phase IV) | Complete maturation of spores; disappearance of clumps; cell lysis; decreasing growth curve; minimum oxygen uptake rates |
Main morphological and physiological characteristics of Bacillus thuringiensis var. israelensis cultures carried out under different initial glucose concentrations (S 0)
| Phase of cellular growth . | Main characteristics . |
|---|---|
| Vegetative growth (Phase I) | Fast decrease of pH values; occurrence of exponential growth phase, with higher μ X,m, followed by a period of non-exponential growth; the cell growth curve presents a linear profile; long and uniform rods; isolated, double rods and chains; high motility; high oxygen uptake rates |
| Transition to sporulation (Phase II) | Rise of pH; shorter and isolated cells; no motility; beginning of the flocculation; reduction on growth rates; drastic reduction on oxygen uptake rates |
| Sporulation (Phase III) | Spore formation; intense flocculation; decreasing growth curve; increasing dissolved oxygen concentrations; decreasing oxygen uptake rates |
| Cell lysis (Phase IV) | Complete maturation of spores; disappearance of clumps; cell lysis; decreasing growth curve; minimum oxygen uptake rates |
| Phase of cellular growth . | Main characteristics . |
|---|---|
| Vegetative growth (Phase I) | Fast decrease of pH values; occurrence of exponential growth phase, with higher μ X,m, followed by a period of non-exponential growth; the cell growth curve presents a linear profile; long and uniform rods; isolated, double rods and chains; high motility; high oxygen uptake rates |
| Transition to sporulation (Phase II) | Rise of pH; shorter and isolated cells; no motility; beginning of the flocculation; reduction on growth rates; drastic reduction on oxygen uptake rates |
| Sporulation (Phase III) | Spore formation; intense flocculation; decreasing growth curve; increasing dissolved oxygen concentrations; decreasing oxygen uptake rates |
| Cell lysis (Phase IV) | Complete maturation of spores; disappearance of clumps; cell lysis; decreasing growth curve; minimum oxygen uptake rates |
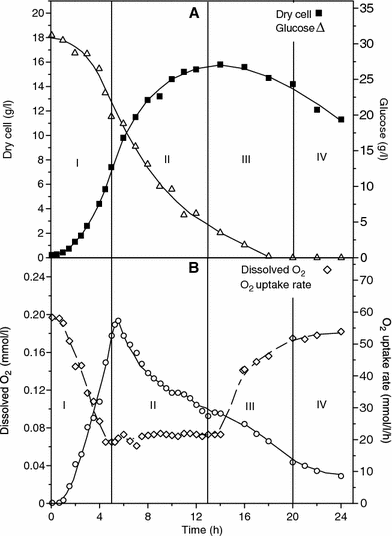
Dry cell and glucose concentrations (a), dissolved oxygen concentration and oxygen uptake rate (OUR) (b), as a function of time in batch run with S 0 = 30.8 g/l
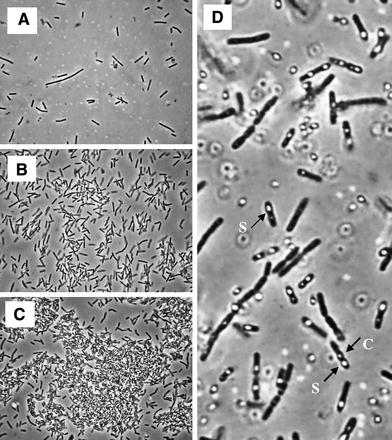
Phase contrast micrographs (×400) of Bacillus thuringiensis var. israelensis in batch run with S 0 = 30.8 g/l, showing main morphological characteristics of the four growth phases. a Phase I, at 2 h of fermentation: only vegetative cells. b Phase II, at 10 h of fermentation: the beginning of flocculation. c Phase III, at 18 h of fermentation: large clumps. d Phase IV, at 21 h of fermentation: complete maturation of spores, disappearance of clumps and cell lysis (S spores, C crystals of δ-endotoxins)
Phase I
The beginning of the fermentation process was characterized by a rapid period of adaptation of the cells to the culture medium, resulting in a shorter lag phase, because of the use of an active inoculum, with vegetative cells only (Fig. 2a). Long bacilli with uniform dimensions were predominant. From the second hour onwards, they assembled as double rods and as small chains. Most of the population presented high motility. An exponential phase of about 3 h was observed, with a maximum specific growth rate (μ X,m) of 1.1 per hour, this phase being followed by a longer period of non-exponential growth. During this period, the cell growth curve presented a linear profile with a constant rate of ca. 2.5 g/l per hour. Decreasing μ X (specific growth rate) values (data not shown) were then calculated, and this trend remained until the end of the fermentation time. The pH fell rapidly and reached values of around 5.6 in the fourth hour, which indicates an intense production of organic acids, mainly acetic acid, as already described for Bti [12] and other Bt strains [20]. The pattern of evolution of the dissolved oxygen concentrations (DO) and the oxygen uptake rate (OUR) pointed out a strong respiratory metabolism from the end of the lag phase onwards. Increasing values of OUR were observed up to 5 h of cultivation, when the maximum OUR value (57 mmol/l per hour) was attained.
Phase II
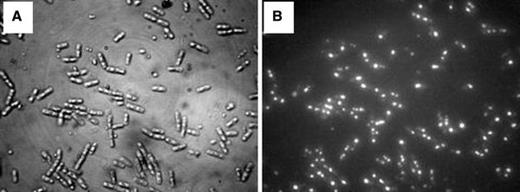
Light (a) and epifluorescent micrograph (b) of Bacillus thuringiensis var. israelensis cells at 14 h of fermentation (S 0 = 30.8 g/l), showing the intracellular granules of PHB stained with Nile blue. The Nile blue A-stained cells were photographed with simultaneous fluorescent and visible light
During Phase II, besides the reduction in μ X values, μ S (specific substrate consumption rate) values also diminished continuously, even though the biomass production has occurred up to 13 h, which was the event that characterized the end of this phase. Because of the reduction in μ X values, a progressive and pronounced decrease in OUR values was verified along this phase, indicating smaller oxygen requirements by the culture.
Phase III
The main feature of this phase was the intense sporulation of the culture. Although most of the cells were within large clumps (Fig. 2c), it was possible to visualize some free sporangia around of flocks. While the sporulation process advanced, the growth curve presented a decreasing profile, due to the reduction in cellular mass. At the same time, increased dissolved oxygen concentrations and decreased OUR values were observed, which means that oxygen demand was smaller than oxygen supply, at that moment. The end of this phase (20 h) was characterized by a sporulation rate of 90% and a slight decrease in clump size.
Phase IV
The last phase of the cellular cycle was defined as the period for the complete maturation of spores and partial lysis of sporulated cells population. The clump size diminished progressively, and disappeared completely 4 h after the beginning, while cell lysis increased continuously. The formation of δ-endotoxin crystals was detected by means of microscopic analysis (Fig. 2d). The fermentation finished when almost 50% of the sporulated cells had released their spores.
Other authors proposed the division of cellular cycle of Bt fermentations carried out under varied conditions. Rodríguez and de la Torre [18] observed three different metabolic states in continuous culture of B. thuringiensis var. kurstaki, according to the dilution rate employed, which, in turn, influenced the biomass yield based on glucose and the cell morphology. At dilution rates (D) between 0.18 and 0.31 per hour (State I) coexisted vegetative cells, sporulating cells and free spores. At D values between 0.42 and 0.47 per hour (State II), only vegetative cells were observed. At D = 0.50 per hour (State III) there were also only vegetative cells, but they showed intracellular refractive granules of PHB. Sarrafzadeh et al. [21] distinguished two situations over the fermentation time course of B. thuringiensis var. israelensis in fed-batch culture and different biomass quantification techniques. In the first one, based on the on-line dielectric permittivity measurements with a capacitance analyzer, three phases were observed, characterized by the authors as: (1) a rapid growth phase, when only vegetative cells were present; (2) a slow growth phase, when the concentration of vegetative cells remained nearly constant and the sporulated cells began to appear; and (3), a decline phase, representing the true sporulation phase, with cell lysis and liberation of free spores. In the other situation, observed after the plotting of the optical density and the biomass dry weight values along time, they observed only two phases, growth and decline. Kraemer-Schafhalter and Moser [12] had also described some general characteristics of Bti fermentations, carried out with S 0 = 10 g/l in batch mode, but they did not divide the process into phases. The descriptions were made hour by hour up to 9 h of fermentation and then for the 16th hour, when the spore formation has been completed and cell lysis began. The final cultivation time was marked by almost complete liberation of spores, on the 26th hour.
General fermentation parameters under different S 0
The main fermentation data obtained in the experiments are shown in Table 2. As can be seen, with S 0 up to 75.0 g/l, the maximum specific growth rates were similar (1.0–1.1 per hour). However, the use of S 0 values over 124.0 g/l led to decreasing μ X,m values. As μ X,m is normally measured at the beginning of process, when no nutritional and/or environmental limitations for cell growth are expected, one can assume that the reduction of μ X,m was due to inhibition by the large substrate concentration. Total glucose consumption was not observed during Phases I and II, except in the assay with S 0 = 10.0 g/l. Very similar final cell concentrations (X m = 15.2–15.9 g/l) were achieved for S 0 between 30.8 and 124.0 g/l. With S 0 = 152.0 g/l, a slight reduction on X m (14.6 g/l) was observed, indicating that the cell growth began to be affected by such a high substrate concentration. The interruption on cell multiplication, which characterizes the final of Phase II, can be explained by the lack of one or more nutrients in fermentation media.
Main fermentation results obtained in Bacillus thuringiensis var. israelensis batch fermentations carried out under different initial glucose concentrations (S 0)
| . | S 0 (g/l) . | |||||
|---|---|---|---|---|---|---|
| 10.0 . | 30.8 . | 57.7 . | 75.0 . | 124.0 . | 152.0 . | |
| t X,m (h)a | 6.0 | 13.0 | 16.0 | 17.0 | 18.0 | 22.0 |
| t f (h)b | 22 | 24 | 26 | 30 | 38 | 43 |
| ΔS (g/l)c | 10.0 | 26.7 | 30.5 | 35.7 | 37.3 | 39.6 |
| ΔS t (g/l)d | 10.0 | 30.8 | 37.0 | 37.0 | 51.0 | 54.0 |
| X m (g/l)e | 8.5 | 15.6 | 15.9 | 15.2 | 15.4 | 14.6 |
| Y X/S (g/g)f | 0.83 | 0.58 | 0.51 | 0.42 | 0.41 | 0.36 |
| p X (g/l.h)g | 1.4 | 1.2 | 0.98 | 0.88 | 0.84 | 0.65 |
| μ X,m (per hour)h | 1.0 | 1.1 | 1.1 | 1.0 | 0.91 | 0.79 |
| OURm (mmol/l per hour)i | 47.0 | 57.0 | 56.0 | 55.0 | 48.0 | 50.0 |
| . | S 0 (g/l) . | |||||
|---|---|---|---|---|---|---|
| 10.0 . | 30.8 . | 57.7 . | 75.0 . | 124.0 . | 152.0 . | |
| t X,m (h)a | 6.0 | 13.0 | 16.0 | 17.0 | 18.0 | 22.0 |
| t f (h)b | 22 | 24 | 26 | 30 | 38 | 43 |
| ΔS (g/l)c | 10.0 | 26.7 | 30.5 | 35.7 | 37.3 | 39.6 |
| ΔS t (g/l)d | 10.0 | 30.8 | 37.0 | 37.0 | 51.0 | 54.0 |
| X m (g/l)e | 8.5 | 15.6 | 15.9 | 15.2 | 15.4 | 14.6 |
| Y X/S (g/g)f | 0.83 | 0.58 | 0.51 | 0.42 | 0.41 | 0.36 |
| p X (g/l.h)g | 1.4 | 1.2 | 0.98 | 0.88 | 0.84 | 0.65 |
| μ X,m (per hour)h | 1.0 | 1.1 | 1.1 | 1.0 | 0.91 | 0.79 |
| OURm (mmol/l per hour)i | 47.0 | 57.0 | 56.0 | 55.0 | 48.0 | 50.0 |
aTime to achieve the maximum cell concentration
bFermentation time (lysis = 50%)
cGlucose consumption until t X,m
dTotal glucose consumption
eMaximum cell concentration
fBiomass yield based on glucose, calculated at t X,m
gCell productivity, calculated at t X,m
hMaximum specific growth rate
iMaximum oxygen uptake rate
Main fermentation results obtained in Bacillus thuringiensis var. israelensis batch fermentations carried out under different initial glucose concentrations (S 0)
| . | S 0 (g/l) . | |||||
|---|---|---|---|---|---|---|
| 10.0 . | 30.8 . | 57.7 . | 75.0 . | 124.0 . | 152.0 . | |
| t X,m (h)a | 6.0 | 13.0 | 16.0 | 17.0 | 18.0 | 22.0 |
| t f (h)b | 22 | 24 | 26 | 30 | 38 | 43 |
| ΔS (g/l)c | 10.0 | 26.7 | 30.5 | 35.7 | 37.3 | 39.6 |
| ΔS t (g/l)d | 10.0 | 30.8 | 37.0 | 37.0 | 51.0 | 54.0 |
| X m (g/l)e | 8.5 | 15.6 | 15.9 | 15.2 | 15.4 | 14.6 |
| Y X/S (g/g)f | 0.83 | 0.58 | 0.51 | 0.42 | 0.41 | 0.36 |
| p X (g/l.h)g | 1.4 | 1.2 | 0.98 | 0.88 | 0.84 | 0.65 |
| μ X,m (per hour)h | 1.0 | 1.1 | 1.1 | 1.0 | 0.91 | 0.79 |
| OURm (mmol/l per hour)i | 47.0 | 57.0 | 56.0 | 55.0 | 48.0 | 50.0 |
| . | S 0 (g/l) . | |||||
|---|---|---|---|---|---|---|
| 10.0 . | 30.8 . | 57.7 . | 75.0 . | 124.0 . | 152.0 . | |
| t X,m (h)a | 6.0 | 13.0 | 16.0 | 17.0 | 18.0 | 22.0 |
| t f (h)b | 22 | 24 | 26 | 30 | 38 | 43 |
| ΔS (g/l)c | 10.0 | 26.7 | 30.5 | 35.7 | 37.3 | 39.6 |
| ΔS t (g/l)d | 10.0 | 30.8 | 37.0 | 37.0 | 51.0 | 54.0 |
| X m (g/l)e | 8.5 | 15.6 | 15.9 | 15.2 | 15.4 | 14.6 |
| Y X/S (g/g)f | 0.83 | 0.58 | 0.51 | 0.42 | 0.41 | 0.36 |
| p X (g/l.h)g | 1.4 | 1.2 | 0.98 | 0.88 | 0.84 | 0.65 |
| μ X,m (per hour)h | 1.0 | 1.1 | 1.1 | 1.0 | 0.91 | 0.79 |
| OURm (mmol/l per hour)i | 47.0 | 57.0 | 56.0 | 55.0 | 48.0 | 50.0 |
aTime to achieve the maximum cell concentration
bFermentation time (lysis = 50%)
cGlucose consumption until t X,m
dTotal glucose consumption
eMaximum cell concentration
fBiomass yield based on glucose, calculated at t X,m
gCell productivity, calculated at t X,m
hMaximum specific growth rate
iMaximum oxygen uptake rate
In assays with S 0 varying from 30.8 to 152.0 g/l, a gradual increase in sugar utilization was observed, since a larger amount of glucose was consumed at the beginning of the process. With S 0 = 124.0 g/l, for example, total glucose consumption (ΔS) value was 40% larger than the one verified with S 0 = 30.8 g/l. Considering the similar X m values obtained in these two assays, it seems to be clear that part of glucose consumed was used for the formation of other metabolic products, like organic acids. In fact, increasing volumes of KOH were necessary for pH control with the rise in S 0 values, demonstrating the increment in acid production.
During all cellular growth (Phases I and II), a reduction in cell yield from glucose (Y X/S) was noticed as increasing S 0 values were used. In the assays with S 0 from 30.8 to 124.0 g/l, it happened due to the increasing glucose consumption. Due to the process conditions, S 0 = 152.0 g/l caused the increase in ΔS and the decrease in X m values. With S 0 = 10 g/l, a much higher Y X/S value (0.83 g/g) was obtained.
The comparative analysis between the assays with S 0 = 10.0 g/l and S 0 = 30.8 g/l showed that the cellular concentration obtained in the second case was almost twice as high as the one achieved in the first case. However, only in the medium with S 0 = 10 g/l, the interruption in growth corresponded to the total glucose depletion, revealing that, in this case, glucose concentration was a limiting factor. In contrast, with S 0 = 30.8 g/l, the interruption in growth cannot be assigned to the same kind of limitation, since a residual glucose concentration in t X,m was verified. In this case, depletion of one or more components caused the interruption of cell growth.
Maximum Y X/S values obtained by Avignone-Rossa et al. [3] and Kraemer-Schafhalter and Moser [12] (0.68 and 0.73 g/g, respectively), in fermentations assays that were carried out under unlimited dissolved oxygen concentration and with glucose concentrations of 10 g/l, are lower when compared to the value achieved in this work, under the same assay conditions. Although these authors have employed a medium of similar composition, the yeast extract concentration used was four times lower than the one used in our assays. Yeast extract compounds are incorporated by cells without previous chemical modifications and are preferentially used for biomass production by B. thuringiensis var. israelensis [15]. This may explain the preferential use of yeast extract as nitrogen source in Bt fermentations [1, 17]. According to Mignone and Avignone-Rossa [15], glucose is exclusively used as energy source by Bt. If this hypothesis is accepted, and considering that the salts of GYS medium were present at adequate concentrations, the depletion of yeast extract would therefore be the determinant factor for the interruption of growth.
With S 0 = 10 g/l, the total glucose utilization allows to assure that there was a residual concentration of yeast extract in the medium. Therefore, the nutritional potential of the medium was not used. Under this energy limitation, it can be presumed that all glucose was used for growth and that no additional formation of other metabolic products occurred, as previously discussed. In this case, the Y X/S value achieved in this assay (0.83 g/g) would correspond to the maximum possible value to this culture, with the nitrogen source concentration employed (12 g/l). On the other hand, in the other assay, all yeast extract would have been used for biomass production, and the fraction of glucose that was not used as energy source would have been converted into other products of metabolism. Considering the assay with S 0 = 30.8 g/l, the final biomass concentration reached (15.4 g/l), for an Y X/S of 0.83 g/g, came from 18.6 g/l glucose, approximately. This allows estimating the “stoichiometric” relation glucose/yeast extract as being 1.55. For B. thuringiensis var. kurstaki, Anderson and Jayaraman [1] obtained a maximum cell concentration of 12.92 g/l from 27.85 g/l glucose and 19.7 g/l yeast extract, which resulted in a glucose/yeast extract ratio of 1.41.
When the effect of glucose concentration on cell productivity (p X) is evaluated, it is possible to verify a reduction on p X values with increasing S 0. The p X values obtained with S 0 from 30.8 to 124.0 g/l mainly resulted from the increasing duration time of Phases I and II. Such a behavior cannot be completely explained by the results of the present work. However, some aspects as the accumulation of toxic fermentation products and the depletion of some nutrient of GYS medium can be considered in further studies in this process. The highest productivity was achieved with S 0 = 10.0 g/l, in which no yeast extract limitation was seen, which suppressed the formation of extra inhibitory substances, favoring cell growth and reducing the time necessary to achieve the maximum cell concentration (t X,m).
The maximum values of OUR that were verified from the final of Phase I to the beginning of Phase II, varied from 47 to 57 mmol/l per hour and were achieved in very similar process times in the assays with S 0 from 10.8 to 75 g/l. With S 0 = 124.0 and 152.0 g/l, the reduction of growth rates led to the extension of Phase I.
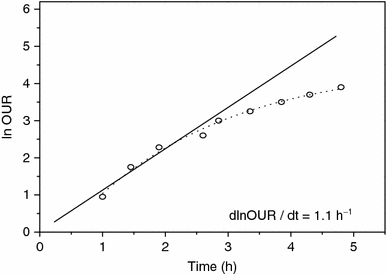
Variation of Neperian logarithms of the oxygen uptake rate values (ln OUR) as a function of time in batch run with S 0 = 30.8 g/l
Phases III and IV lasted longer duration in the assays with S 0 = 124.0 and 152.0 g/l, resulting in a rise in total time process (38 and 43 h, respectively). With S 0 from 10.0 to 75.0 g/l, the Phase IV had the same duration and the Phase III showed to be longer in the assay with S 0 = 10.0 g/l. In this assay, even though the sporulation process started earlier, due to a lower t X,m (6 h), the process finalization did not occur earlier. Sporulation rates of 90% (final of Phase III) and cellular lysis of 50% (final of Phase IV) were observed with 16 and 22 h of process, respectively. Higher S 0 values also influenced the physiological changes that characterize these two last process phases. With S 0 = 124.0 and 152.0 g/l, part of microbial population did not start the sporulation phase and at the end of fermentation vegetative cells with PHB granules and incomplete sporangia were seen. It has been previously observed that high organic nutrient concentrations reduced sporulation rates in Bt cultures [2].
Acknowledgments
This work was supported by the Centre of Biotechnology Development (CDB), Santa Catarina, and FAPERJ, Rio de Janeiro, Brazil.



