-
PDF
- Split View
-
Views
-
Cite
Cite
Seyed Ahmad Ataei, Ebrahim Vasheghani-Farahani, In situ separation of lactic acid from fermentation broth using ion exchange resins, Journal of Industrial Microbiology and Biotechnology, Volume 35, Issue 11, 1 November 2008, Page 1229, https://doi.org/10.1007/s10295-008-0418-6
Close - Share Icon Share
Abstract
Lactic acid fermentation is an end product inhibited reaction. In situ separation of lactic acid from fermentation broth using ion exchange resins was investigated and compared with conventional fermentation system. Amberlite resin (IRA-400, Cl−) was used to separate lactic acid from fermentation broth and pH was controlled online with an automatic pH controller. The effect of process variables on lactic acid production by Lactobacillus casei in whey permeate was studied. The maximum productivity was obtained at pH = 6.1, T = 37 °C and impeller speed = 200 rpm. The maximum concentration of lactic acid at optimum condition was found to be 37.4 g/L after 38 h of fermentation using in situ separation system. The productivity of in situ separation system was five times increased in comparison with conventional system.
Introduction
Whey is a by-product of cheese production with high COD (chemical oxygen demand) content (50 Kg O2 per ton permeate) and cannot be processed by sewage treatment without expense [10]. Making dairy powder from whey is possible, but it needs special equipment that makes it economically unfavorable [1].
Whey contains all the lactose of milk (4–5%), proteins, vitamins and mineral salts. It is generally a low-priced source of nutrient for the fermentation processes [1, 17]. One possibility for reducing the COD is the fermentative conversion of the lactose to lactic acid [10]. Lactic acid is commercially produced by fermentation of whey with lactic acid bacteria [5]. It is used widely in numerous fields of application such as food, medicine, and pharmaceutical, and in other industrial applications and its demand could rise rapidly in near future [14]. l(+) lactic acid has been considered a potential feedstock for biodegradable lactide polymers (poly lactic acid), e.g., for use in packaging [2, 3, 12]. Poly l(+) lactic acid is a biodegradable and biocompatible polymer and used in medicine especially for controlled release drug delivery and tissue engineering [11].
The end product inhibition by lactic acid causes several problems, among which low lactate formation rate and its recovery from fermentation broth are the most important ones [15, 16]. Therefore, an integrated approach with separation of product has gained a lot of interest in this fermentation [23].
Solvent extraction of lactic acid from fermentation broth is one way for reducing the concentration of lactic acid [6]. The solvent extraction technique is not preferred because it causes several physical, chemical and biochemical problems on the catalytic activity of the cells, e.g., interference with the aseptic condition and contamination of fermentation system [24]. The membrane extraction method has advantages over conventional solvent extraction and may be applied as the mechanism in separation, purification, pollutant removal and recovery processes [18]. A cellulose triacetate (CTA) membrane containing quaternary ammonium salt was used for lactate separation from fermentation broth [13]. A satisfactory recovery of lactic acid from both aqueous solution and actually fermented broth was accomplished; signifying the great potential of integrating the membrane extraction with fermentation process [21]. Continuous extraction of lactic acid from a simulated aqueous stream was achieved using Alamine 336 in 2-octanol, contained in a hollow-fiber membrane extractor. In this process, the extractant was simultaneously regenerated by stripping with NaOH in a second membrane extractor, and the final product is a concentrated lactate salt solution [8].
The restriction imposed by lactic acid on its fermentation has been avoided by an extractive fermentation technique employing ion exchange resins with automatic control of pH [22].
Lactobacillus casei subsp. casei is a homo-fermentative lactic acid bacterium which yielded l(+) lactic acid exclusively [24]. This type of lactic acid isomer has some special medical uses, e.g., production of biodegradable polymers that have applications in making surgery threads and currently costs ten times more than d(−) form [7].
The objective of the present work was to study the effect of process variables on production of l(+) lactic acid using in situ separation technique.
Materials and methods
Bacterial strain and media
Lactobacillus casei sbsps. casei PTCC 1293, provided by “Razi Institute, Tehran, Iran” was used for lactic acid production.
The medium for seed culture contained per liter: peptone 10 g, meat extract 5 g, yeast extract 5 g, Glucose 20 g, potasium hydrogen phosphate 2 g, Tween80 1 g, Di-ammonium hydrogen citrate 2 g, sodium acetate 5 g, magnesium sulfate 0.1 g, manganese sulfate 0.01 g [19].
A volume of 100 mL of the sterile medium was inoculated with the microorganism and after 24 h of incubation at 37 °C, it was used as seed culture for the fermentation system. The microbial density of seed culture at the time of inoculation was about 1.8 g/L. Whey from Pegah milk factory (Tehran, Iran) was used as fermentation medium. Lactose content of the whey was 44 g/L. Since the whey contains vitamins, protein and minerals, it considered as a self sufficient medium for fermentation [4].
Fermentor
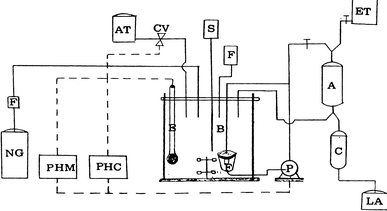
Schematic diagram of extractive lactic acid fermentation. A anion exchanger column, B fermentor, C cation exchanger column, E electrode of pH meter, F filter, P pump, S site for sampling, AT alkali solution tank, CV control valve, ET eluant solution, LA Lactic acid (product), NG N2 gas, PHC pH controller equipment, PHM pH meter
Columns of ion-exchange resins
Amberlit resin (IRA-400, Cl−, Rohmand Haas Company, USA) was used to separate lactic acid from the fermentation broth. As shown in Fig. 1, fermentation broth passed through an ion-exchange column that had been hydroxylated previously with a solution of sodium hydroxide (2.5 N). Lactate ions exchanged with hydroxide ions and were separated from the fermentation broth. After saturation, the column was eluted with a solution of sodium hydroxide (1.5 N). Sodium lactate, resulted from the elution, entered the second column packed with Amberlite (IR 120, H+). In the second column, sodium ions exchanged with hydrogen ions and lactic acid exited from the column. The second column after saturation was regenerated with a solution of hydrochloric acid (1.5 N).
Preparation of proteolytic solution
A liquid medium containing 2 g/L yeast extract, 5 g/L peptone and 5 g/L sodium chloride was inoculated with the species Bacillus subtillis PTCC 1204 and incubated in a shaker incubator at a speed of 60 rpm at 30 °C for 24 h. This solution was used for hydrolysis of whey proteins.
Fermentation and product separation
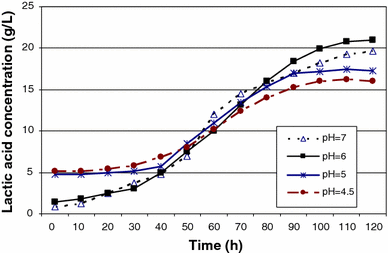
For each experiment, 1 L of whey was poured into the fermentor and supplemented with the proteolytic solution (1% V/V) while pH of the solution was adjusted to 5.2. After 5 h of stirring at 100 rpm (for protein hydrolysis), the fermentor content was sterilized at 121 °C for 15 min. After cooling, the fermentor was inoculated with the seed culture of Lactobacillus casei subsp. casei (10%V/V). The extractive fermentation was carried out at different pH, temperatures and impeller revolutions to determine the optimum conditions at which the rest of experiment was conducted.
The fermentor was connected to the columns of ion exchange resins (anionic and cationic) and incubated at 37 °C. Constant stirring (200 rpm) was maintained during the fermentation.
As fermentation proceeded, the pH of medium decreased gradually due to lactic acid production. When it reached below 6.0, the controller switched on the recycle pump and allowed the broth to pass through the anion exchange resin at a flow rate of three bed volumes per hour (3 BV/h). The anion exchanger resin selectively removes the lactate ions. The effluent of the column was recycled back to the fermentor and pH of the medium was increased. The pump stopped when the pH reached 6.2. Therefore the pH of fermentation medium was kept constant at 6.1 ± 0.1 using an automatic pH controller. The fermentation process was terminated after 38 h. Sodium lactate was eluted by NaOH (1.5 N) from the anion-exchange column with a rate of three bed volume per hour (3 BV/h). Sodium lactate entered into the cation-exchange column where lactic acid was produced and was then collected for analysis. This latter column was regenerated with (1.5 N) HCl (1.5 BV/h).
Analytical methods
Lactic acid concentration was determined by an HPLC equipped with ODS-PLO column (Waters-associates, USA). A mixture of water-methanol (1:1) with the flow rate of 1 mL/min was used as the carrier phase. The system was equipped with a UV detector. Concentration of lactic acid was detected at 275 nm.
Concentration of lactose was determined using the method of Somogi–Nelson [20].
Results and discussions
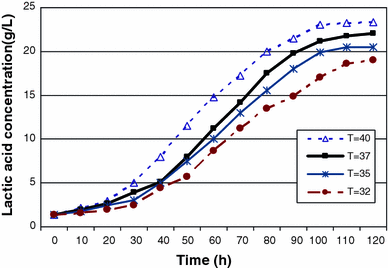
The effect of temperature on lactic acid production by batch fermentation
Despite the high production of lactic acid at 40 °C, the productivity of extractive fermentation decreased due to the low capacity of resin to adsorb lactate ions at high temperature. Therefore the extractive fermentation is very much dependent on the temperature of operation and 37 °C is the optimum temperature of extractive fermentation.
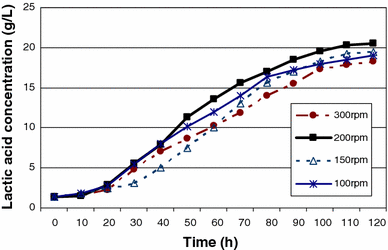
The effect of impeller speed on lactic acid production by batch fermentation
Comparison of batch and extractive fermentation for lactic acid production
| Parameter . | Bach fermentation . | Extractive fermentation . |
|---|---|---|
| Time (h) | 120 | 38 |
| Lactose conversion% | 76 | 99.3 |
| Yield (Pa/Sb) | 0.5 | 0.85 |
| Productivity (g/L per hour) | 0.183 | 0.984 |
| Parameter . | Bach fermentation . | Extractive fermentation . |
|---|---|---|
| Time (h) | 120 | 38 |
| Lactose conversion% | 76 | 99.3 |
| Yield (Pa/Sb) | 0.5 | 0.85 |
| Productivity (g/L per hour) | 0.183 | 0.984 |
aProduct (lactic acid)
bSubstrate (lactose)
Comparison of batch and extractive fermentation for lactic acid production
| Parameter . | Bach fermentation . | Extractive fermentation . |
|---|---|---|
| Time (h) | 120 | 38 |
| Lactose conversion% | 76 | 99.3 |
| Yield (Pa/Sb) | 0.5 | 0.85 |
| Productivity (g/L per hour) | 0.183 | 0.984 |
| Parameter . | Bach fermentation . | Extractive fermentation . |
|---|---|---|
| Time (h) | 120 | 38 |
| Lactose conversion% | 76 | 99.3 |
| Yield (Pa/Sb) | 0.5 | 0.85 |
| Productivity (g/L per hour) | 0.183 | 0.984 |
aProduct (lactic acid)
bSubstrate (lactose)
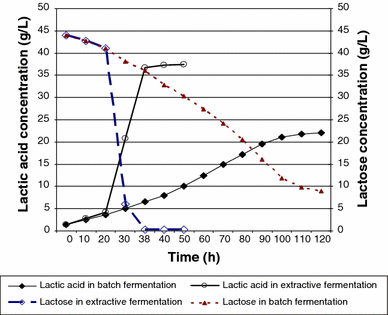
Lactic acid production and lactose consumption in batch an extractive fermentation
It can be seen from this figure that the concentration of lactic acid in the conventional fermentation system (Batch) after 38 h was only 6 g/L, and after 5 days, the concentration increased to the maximum value of 22 g/L. But in extractive fermentation (in situ separation), this maximum value was 37.4 g/L within 38 h. It means that the yield of lactic acid production increased by 60% in extractive fermentation compared to conventional fermentation, while the fermentation time decreased considerably.
The measurement of COD (chemical oxygen demand) of whey before and after the extractive fermentation showed a 65% decrease in the COD content of the whey. Extractive fermentation of whey reduced its COD content considerably and produced lactic acid with high productivity, which is of prime importance from environmental and economical points of view. Polarimetric experiments gave the value of +2.6 for the product. This value is for the l(+) isomer of lactic acid (Sigma, 1997; Merck Index, 1998).
Conclusions
Due to sterile-conditions requirement and product inhibitory effects, in situ separation of lactic acid from fermentation broth using ion exchange resin at controlled pH is a useful technique. l(+) lactic acid yield by extractive fermentation in comparison with non-extractive process was considerably increased while the time of maximum yield decreased. As a result productivity of the extractive fermentation process was about 4.3 times higher than that of the conventional process for l(+) lactic acid production from cheese whey.
Acknowledgment
We thank Razi institute for providing Lactobacillus casei and Bacillus subtillis strains.
References
Nolan-Itu Pty Ltd (2002) Prepared in association with Excel Pals Australia. Biodegradable plastic Developments and Environmental



