-
PDF
- Split View
-
Views
-
Cite
Cite
Amanda Marie Egeskov-Cavling, Caroline Klint Johannesen, Birgitte Lindegaard, Thea Kølsen Fischer, PROMISE Investigators , Underreporting and Misclassification of Respiratory Syncytial Virus–Coded Hospitalization Among Adults in Denmark Between 2015–2016 and 2017–2018, The Journal of Infectious Diseases, Volume 229, Issue Supplement_1, 15 March 2024, Pages S78–S83, https://doi.org/10.1093/infdis/jiad415
Close - Share Icon Share
Abstract
Low awareness and lack of routine testing for respiratory syncytial virus (RSV) infections among adults has led to underreporting in hospital records. This study aimed to assess the underreporting and misclassification of RSV infections among adults hospitalized with an respiratory tract infection (RTI)-coded hospitalization.
This study is an observational cohort study of RSV-associated hospitalizations among Danish adults (≥18 years old) conducted, between 2015 to 2018. Data were extracted from the Danish National Patient Registry (DNPR) and the Danish Microbiology Database. We identified RSV-positive hospitalizations by linking RTI-coded hospitalizations with a positive RSV test.
Using hospital admission registries, we identified 440 RSV-coded hospitalizations, of whom 420 (95%) had a positive RSV test registered. By linking patients with RTI-coded hospital admissions to RSV test result, we found 570 additional episodes of RSV-positive hospitalizations without an RSV-coded diagnosis.
Our study of national register data showed that RSV is underreported among Danish adults. The study showed that the reliability of hospitalization data to estimate the burden of RSV among adults is questionable and are sensitive to changes in practice over time, even with complete nationwide healthcare data. Healthcare data can be useful to observe seasonality but to estimate the disease burden, prospective surveillance is recommended.
Respiratory syncytial virus (RSV) is a viral pathogen that causes respiratory tract infections (RTIs) throughout life [1]. RSV is the leading cause of hospitalization with severe bronchiolitis and pneumonia among infants and young children, and severe annual RSV epidemics can lead to overwhelmed hospital capacity during the winter season [2, 3]. Furthermore, RSV infections among elderly (65 years and older) and high-risk adults worldwide have recently been demonstrated to constitute a major burden of disease [4, 5]. However, recent meta-analyses highlight a lack of reliable high-quality population-level data on the burden of RSV disease among adults and elderly persons [4, 5]. As in many other countries, there is low awareness of RSV in adults and the elderly in Denmark. This could be due to the absence of effective vaccines and antiviral treatment, as well as missing guidelines for routine testing [6, 7]. Considering that the first RSV vaccines for adults recently have been approved by the US Food and Drug Administration and European Medicines Agency and more are expected to be available in the coming years [8, 9], it is essential to establish valid disease burden estimates and identify the most vulnerable age groups and other risk groups as well as the RSV seasonality for correct and optimal timing of a potential annual vaccination schedule.
Estimating the burden of RSV infections based on International Classification of Diseases, Tenth Revision (ICD-10) codes is found to underestimate the actual number of RSV infections. Linking hospital admission data based on ICD-10 codes with laboratory-confirmed RSV tests can be a way to explore the accuracy of RSV-specific ICD-10 diagnosis codes for the identification of true RSV infections. A German study by Cai et al is one of the few studies to examine this, and the authors found that using only RSV-specific codes underestimated the RSV burden, but by combining RSV-specific codes and acute lower respiratory infection ICD-10 codes, the estimates become more reliable [10].
In Denmark, the availability of unique social security identification numbers allows us to link individual-level information on the Danish population from national registries, including all hospital contacts, to laboratory data. The aim of this study is to assess the underreporting and misclassification of RSV infections among adults >18 years in Denmark hospitalized with an RTI diagnosis by linking laboratory RSV tests and RTI-coded hospitalizations.
METHODS
Data Sources
We extracted data from the Danish Civil Registration System (CRS), the Danish National Patient Registry (DNPR), and the Danish Microbiology Database (MiBa) [11–13] . The CRS holds civil and vital information on all inhabitants of Denmark, and the data are made available for research by Statistics Denmark [12, 13]. The CRS was used to link data from the relevant healthcare registries using the unique social security identification number provided to all Danish citizens upon birth or immigration into Denmark. It was therefore also possible to fully link the data from the registers at an individual level. The DNPR contains nationwide longitudinal registration of administrative and clinical data from all hospitals in Denmark on an individual level [11]. The DNPR was used to collect information on all RTI-coded hospital admissions and diagnoses. The hospital admissions and diagnoses are coded using ICD-10. The laboratory data for RSV testing were obtained from MiBa, where electronic copies of results from all microbiology laboratories testing human samples in Denmark are transferred [14]. Data from MiBa were used to describe the trends in RSV testing and linked to the DNPR data to explore the association between RSV diagnosis, RTI hospitalizations, and tests positive for RSV at an individual level. The data sources enabled us to link the hospital admission data and laboratory data from the registers to explore the underreporting and misclassification of RSV infections among hospitalized adults.
Study Design
This study is an observational cohort study using national healthcare registers conducted from week 40, 2015 to week 40, 2018. The national registries provide a unique opportunity to identify all patients hospitalized with an RTI diagnosis based on ICD-10–coded hospital admissions. The following ICD-10 codes were applied for the RTI: RSV (B97.4, J12.1, J20.5, J21.0), acute upper respiratory infection (J00–J06), influenza and pneumonia (J09–J18), bronchitis and bronchiolitis (J20–J21, J40) and unspecified acute lower respiratory infection (J22). If a patient were hospitalized with RSV more than once within 30 days, we considered the hospitalizations to belong to the same event. The first admission date marked the start of the event. RTI hospitalizations were linked to positive laboratory tests for RSV conducted 30 days before/after admission.
Study Population
The study population included all Danish residents 18 years or older living in Denmark at any point between 2015 and 2018. Using the DNPR, all RTI hospitalizations were identified in this population using the ICD-10 codes mentioned above. Cases were defined as admitted patients with any mention of any one of the above ICD-10 codes on any diagnosis.
Safety Considerations
This registry-based study did not involve participants. According to Danish law, ethics approval is exempt for this research. The results in this study are aggregated and do not contain personal data. The study is thus not covered by the European General Data Protection Regulation.
Data Linkage and Analysis
All data linkage and descriptive analyses were carried out in R statistical software [15]. Our analysis was conducted by initially linking the unique personal identification number from the CRS with the ICD-10-coded hospitalization data from DNPR. This analysis enables us to estimate the numbers of RSV-coded hospitalizations among adults during the study period. Based on the linked data from CRS, MiBa, and DNPR, it was possible to analyze the trend in the use of RSV laboratory tests over time.
The sensitivity of RSV-coded diagnosis was calculated as the proportion of RSV-coded hospitalizations with a positive test among all RTI-coded hospitalizations with a positive RSV test. The positive predictive value (PPV) was calculated as the proportion of RSV-coded hospitalization with a confirmed positive test among all RSV-coded hospitalizations. The sensitivity and PPV were calculated with 95% confidence intervals (CIs) applying the Clopper–Pearson exact method. The age distribution of the attributable proportion of RSV-coded hospitalizations among RTI-coded hospitalizations with a positive test was also analyzed.
Laboratory data from MiBa were applied to identify all conducted polymerase chain reaction (PCR) tests for RSV, including both positive and negative results in the period week 40, 2015 to week 40, 2018 to analyze the periodical trends in RSV testing. The laboratory-confirmed cases were individually linked to any hospitalization with an RTI diagnosis within ±30 days. The number of RSV tests conducted in each season was also compared to the number of RSV-diagnosed hospitalizations. We conducted a linear regression to estimate the correlation between the monthly number of RSV-coded hospitalized patients and the monthly number of conducted tests. Last, the actual number of positive RSV tests among the different RTI-coded diagnoses was estimated.
RESULTS
Trends in Hospitalizations
During the 3-year study period from week 40, 2015 to week 40, 2018, we identified a total of 440 RSV-coded hospitalizations among individuals 18 years and older in Denmark (Supplementary Table 1). Through data linkage, a total of 990 RTI-diagnosed hospitalizations with a positive RSV test within ±30 days of admission were identified (the specific ICD-10–coded diagnoses are listed in Supplementary Table 2). Among the RTI-diagnosed hospitalizations with a positive RSV test, 57.6% (570) were not hospitalized with an ICD-10–coded RSV diagnosis (Supplementary Table 1). Thus, we identified an additional 570 episodes of RSV-associated hospitalizations through data linkage. The overall sensitivity of RSV-coded hospitalizations was calculated to be 42.4% (95% CI, 39.3%–45.6%).
The proportions of patients with a positive RSV test among RTI diagnostic groups are shown in Supplementary Table 2. More patients were identified with a positive RSV test among patients hospitalized with influenza and pneumonia (n = 517) than patients hospitalized with an RSV-coded diagnosis (n = 420) (Supplementary Table 2). Moreover, among the 440 RSV-coded hospitalizations, 95.5% (n = 420) of patients had a positive PCR result for RSV tests registered (Supplementary Table 1). The PPV for true RSV-coded hospitalization was thereby 95.5% (95% CI, 93.1%–97.2%). The observed RSV-coded hospitalization rates among RTI-coded hospitalizations were 39.4%, 44.4%, 42.7%, and 42.1% in the age groups 18–45, 46–65, 66–75, and ≥75 years, respectively (Supplementary Table 3).
Trends in RSV Laboratory Testing
During the study period, a total of 59 364 PCR RSV tests were conducted among adults in Denmark. Among all conducted PCR tests, we identified 2530 RSV-positive tests, a positivity rate of 4.3% (Supplementary Table 4). With 990 RTI-diagnosed hospitalizations with a positive test, 39.0% of these adults were coded with an RSV diagnosis (Supplementary Table 1).
We explored the weekly trend of adults tested for RSV from week 40, 2015 to week 40, 2018 (Figure 1). We analyzed the trend to explore whether the increased incidence of RSV-diagnosed hospitalizations could be explained by an increased number of conducted PCR tests. First, we found a peak in PCR tests conducted in 2017–2018, and second, an increased peak in positive percentage in all winter seasons, indicating a seasonal variation in the transmission of RSV infections (Figure 1). The weekly number of positive tests is presented in Figure 1, which illustrates the seasonality and trend of RSV infections among Danish adults.
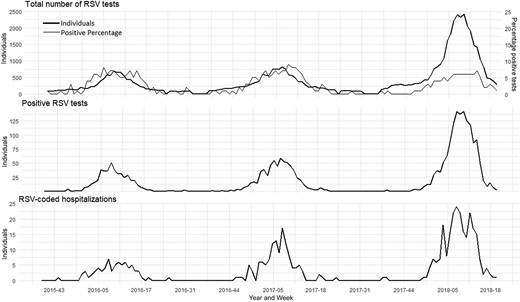
Documented respiratory syncytial virus (RSV) in Danish adults from week 40, 2015 to week 40, 2018. Weekly number of conducted RSV tests and positive percentage of RSV tests (top), weekly number of RSV-positive tests (middle), and weekly number of RSV-coded hospitalizations (bottom).
As illustrated in Figure 1, the increased number of RSV-coded hospitalized patients correlates with the increased number of conducted tests (see Supplementary Table 4 for more details). Using a linear regression analysis, the correlation between the monthly number of RSV-coded hospitalized patients and the monthly number of conducted tests was assessed and found to be significant (P = 1.32 × 10–14 [P < .05] and adjusted R2 = 0.85).
DISCUSSION
Underreporting
We identified a total of 440 RSV-diagnosed hospitalizations among adults. To accommodate challenges due to potential underdiagnosis and misclassification and provide a more comprehensive estimate of the burden of RSV infections only using health administration data, we linked all RTI hospital admissions with information on laboratory-confirmed RSV tests to assess the underestimation of RSV-associated hospitalizations among adults. By combining laboratory test results with nationwide hospital admission data (DNPR), we found that among patients hospitalized with an RTI diagnosis and tested positive for RSV, 57.6% (570) were not registered with an ICD-10 diagnosis for RSV during their admission. The additional 570 episodes of RSV-associated hospitalizations were, therefore, identified among hospital admission for other RTIs. These results indicate a large misclassification and underreporting of RSV-coded hospitalizations among adults in DNPR. This is considerably higher than the compatible underreporting documented among Danish children <5 years of age hospitalized with RSV infections [16]. In a recent Danish study, 12% underreporting among childhood RSV hospital admissions was documented, when comparing the proportion of positive tests for RSV with the number of children hospitalized with an ICD-10 diagnosis code for RSV [16].
Based on our results, we contend that estimating the burden of RSV hospitalizations based on only ICD-10 codes is not adequate to estimate the true burden of RSV, even in a country with nationwide hospital registers. We identify more patients with a positive RSV test among patients hospitalized with influenza and pneumonia (n = 517) than patients hospitalized with an RSV-coded diagnosis (n = 440). Furthermore, by using the laboratory test data, it was possible to validate whether all the RSV-coded hospitalizations in DNPR also had a positive RSV test. We found that 95.5% of all patients with RSV-coded hospitalizations tested positive within ±30 days of their admission. With that, we contend that although the PPV of the RSV-coded hospitalizations in the DNPR is high, the sensitivity is not as remarkable. The PPV among RSV point-of-care tests (POCTs) varies highly among various tests [17]. Compared to a Canadian study linking RSV-coded hospitalization to laboratory data, the study found a sensitivity of 69% and a PPV of 91% among patients of all ages [18]. Though we also show that more than a third of all positive tests were related to an RTI hospitalization, we do not argue that RSV infection leads to severe disease in one-third of adult patients.
It has been shown in many studies that the burden of RSV infections is highly underestimated in studies based on ICD-10 codes compared to modeling studies and prospective surveillance [10, 18]. Another limitation of using national laboratory data is the lack of systematic routine testing for RSV among adults, which means that some RTI patients may not even be tested for RSV. RSV studies based on routinely collected healthcare data can be useful to identify general trends and observe seasonality but will only be able to discover severe to moderate RSV cases [10, 18].
Instead, as registry-based studies cannot stand alone as documentation for RSV disease burden among adults, prospective studies with systematic RSV screening of adult patients admitted to hospitals for respiratory infections should lead the way to ascertainment of more reliable estimates for severe RSV disease burden. As RSV is a ubiquitous virus, studies generated at 1 or 2 hospitals in any country during at least 1 annual season, but preferably 2 annual seasons due to documented interseasonal variations, can be considered representative [19] and the estimates used to generate national burden estimates. The ideal prospective screening of admitted patients would be extended to other respiratory pathogens (eg, influenza virus and severe acute respiratory syndrome coronavirus 2). Moreover, studies have found that diagnostic testing using only nasopharyngeal swabs and nasal swabs for RSV detection is less sensitive among adults compared to younger children [20, 21]. This is due to low viral titers and shorter duration of viral shedding among adults compared to younger children [20]. This influences RSV detection, and many RSV infections in adults may be missed through test-based classification error, which again affects the estimation of the true disease burden of RSV among adults.
Trends in Laboratory Testing
Since 2016, use of PCR POCTs that include testing for influenza A and B and RSV, as described by Broberg et al [22], has increased in popularity at Danish hospitals and particularly in emergency rooms. As the test results from POCTs do not undergo mandatory reporting as do all other laboratory tests, data on the overall use and the outcome on Danish hospitals are not complete in the MiBa. The test is mainly used for patients presenting with influenza-like-illness (ILI) to provide a rapid indication of influenza virus and need for isolation of the patient and RSV will often be unsuspected when found. This can cause bias in estimating the disease burden and seasonality of RSV, as the number of detected cases and conducted tests will likely depend on the influenza season and the variability of how many patients present with ILI symptoms rather than the RSV season. Thus, if RSV transmission occurs outside of the annual influenza seasonal weeks, it is less likely to be picked up in the hospital laboratories and rather would be detected by the national year-round sentinel surveillance of ILI patients at general practitioners’ offices.
We found a significant increase in the number of conducted tests in the 2017–2018 season, which can be related to POCT units running influenza and RSV testing together in major Danish acute hospitals in combination with a major influenza epidemic (influenza B). Thus, the increased number of RSV-coded hospitalizations in the 2017–2018 season can be expected to be attributed to the increased number of conducted tests (Figure 1). Importantly, the percentage positive of the conducted RSV tests in 2017–2018 was lower than in the 2 previous seasons (Figure 1), which can indicate an undetected burden of RSV among adults in previous seasons.
Limitations
As this study shows, the reliability of only using healthcare data to estimate the burden of RSV can be questioned based on our findings related to the underreporting of RSV among adults. Based on ICD-10 codes alone, our estimates are likely to be underestimated. However, the estimates are based on the Danish national healthcare data and national microbiology database, which are of high quality covering the entire population on an individual level, which is ideal when estimating the disease burden in a population. By crosslinking the registers, we were able to validate the use of the healthcare data in DNPR and observe the general trends and seasonality of RSV among adults. On the other hand, the study cannot account for some of the identified hospitalized patients with an RTI and a positive RSV test, not necessarily sick due to RSV but just infected and tested during hospitalization. Overall, the use of data from the national register (not developed for research) is to some extent limited and should be studied with vigilance. Moreover, we used ±30 days as the time period between a positive RSV test and an RTI-coded hospitalization, which might overestimate our results, since the patient can, in the meantime, have been infected and sick due to another pathogen. In addition, the patients identified with an RTI diagnosis and a positive test may be hospitalized with RSV and not because of RSV, which might also overestimate the results. This study did not investigate coinfections among RSV-positive hospitalizations but this is recommended for future studies among adults.
This study is based on hospitalizations and laboratory data before the coronavirus disease 2019 (COVID-19) pandemic; thus, these results may not reflect the current disease burden of RSV in adults. The testing practices and hospitalizations due to RTI may have been influenced by the COVID-19 pandemic. Recent studies have shown a change in the seasonality of RSV due to COVID-19 restrictions [7, 23, 24].
CONCLUSIONS
Our study demonstrates how a combination of routinely collected data can be beneficial to explore the burden of RSV in hospitalized patients. Our findings showed that RSV-coded hospitalizations are underdiagnosed among adults in Denmark and that surveillance of RSV among adults is affected by the testing strategy for influenza. It is, therefore, very important to promote better RSV surveillance with more systematic testing for a given case definition, increase surveillance in the communities, and increase awareness of RSV in adults among health professionals. Future studies should estimate the disease burden of RSV in prospective hospital cohort studies, limiting the risk of underestimation and misclassification and enabling reliable estimates to be used to evaluate the need and cost-effectiveness of the new adult RSV vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
PROMISE investigators. Harish Nair and Harry Campbell (University of Edinburgh), Hanna Nohynek (Finish Institute for health and welfare), Anne Teirlinck (Rijksinstituut voor Volkgezondheid en Milieu), Louis Bont (University Medical Center Utrecht), Peter Openshaw (Imperial College, London), Andrew Pollard (University of Oxford), Philipe Beutels (University of Antwerp), Veena Kumar (Novavax), Tin Tin Htar (Pfizer), Charlotte Vernhes and Rolf Kramer (Sanofi Pasteur), Gael Dos Santos (GlaxoSmithKline), Jeroen Aerssens (Janssen), and Nuria Manchin (TEAM IT Research, S.L.).
Disclaimer. This article reflects only the authors’ view. The funding agency is not responsible for any use that may be made of the information contained herein.
Financial support. This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement number 101034339. The JU receives support from the European Union's Horizon 2020 research and innovation program and European Federation of Pharmaceutial Industries Associations.
Supplement sponsorship. This article appears as part of the supplement “Preparing Europe for Introduction of Immunization Against RSV: Bridging the Evidence and Policy Gap.”
References
Author notes
Potential conflicts of interest. T. K. F. declared participating as a principal investigator (unpaid) in a Pfizer RSV vaccine trial on maternal vaccination, outside the submitted work. A. M. E.-C. is funded by the Independent Research Fund Denmark for her PhD project (grant number 10.46540/2096-00046B). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




