-
PDF
- Split View
-
Views
-
Cite
Cite
Jinnan Chen, Yixian Guo, Yu Huang, Zhaohui Ding, Jing Wang, Xiao Liang, Ping Xu, Yaohua Han, Hong Lu, Rifabutin-Containing Triple Therapy Versus Bismuth Quadruple Therapy for Helicobacter pylori Rescue Treatment: A Multicenter, Randomized Controlled Trial, The Journal of Infectious Diseases, Volume 228, Issue 5, 1 September 2023, Pages 511–518, https://doi.org/10.1093/infdis/jiad114
Close - Share Icon Share
Abstract
We compared the efficacy and safety of rifabutin-containing triple therapy with bismuth quadruple therapy for rescue treatment of Helicobacter pylori.
This was a noninferiority study trial of H. pylori treatment for subjects who had failed at least 2 prior treatments. Subjects were randomly assigned to receive rifabutin triple therapy with 14-day esomeprazole (20 mg), amoxicillin (1.0 g), and rifabutin (150 mg) twice a day; or bismuth quadruple therapy with esomeprazole (20 mg) and bismuth (220 mg) twice a day, plus metronidazole (400 mg) and tetracycline (500 mg) 4 times a day. Antimicrobial susceptibility was assessed by agar dilution and E-test.
From May 2021 to October 2022, a total of 364 subjects were randomized. The eradication rates by intention-to-treat, per-protocol, and modified intention-to-treat were 89.0% (162/182; 95% confidence interval [CI], 83.6%–92.8%), 94.0% (157/167; 95% CI, 89.3%–96.7%), and 93.6% (162/173; 95% CI, 89.0%–96.4%) for rifabutin triple group. For bismuth quadruple group, they were 89.6% (163/182; 95% CI, 84.3%–93.2%), 95.3% (143/150; 95% CI, 90.7%–97.7%), and 93.7% (163/174; 95% CI, 89.0%–96.4%).
The rifabutin triple therapy is an alternative to classical bismuth quadruple therapy for the rescue treatment of H. pylori with fewer side effects and higher compliance.
NCT04879992.
The prevalence of antibiotic-resistant Helicobacter pylori in China has increased due to the prior use of antibiotics, resulting in a decline in cure rates of empiric clarithromycin-, levofloxacin-, and metronidazole-containing triple therapy, even with the addition of bismuth [1–4]. Presently, classical bismuth-quadruple therapy, which includes a proton pump inhibitor (PPI), metronidazole, tetracycline, and a bismuth salt, remains an effective rescue therapy for H. pylori infections in China, and its safety and efficacy have been confirmed [5, 6]. However, due to the lack of coverage of tetracycline by public health insurance, its high incidence of side effects, poor compliance, and contraindications, it has not been universally accepted in China [7].
Rifabutin, a rifamycin S derivative, is commonly used to treat Mycobacterium avium and Mycobacterium intracellular infections by blocking the β subunit of microbial DNA-dependent RNA polymerase. Due to its low antibiotic resistance, superior antibacterial activity in vitro, and stability in the gastric acid environment, rifabutin, when used in combination with amoxicillin and PPIs, has the potential to be an effective treatment option for H. pylori infections [8–11]. Several studies have confirmed the efficacy of rifabutin-containing triple therapy as a first-line or rescue treatment for H. pylori [12, 13].
This study aimed to conduct a randomized controlled trial to assess the effectiveness, tolerability, and safety of a 14-day rifabutin-containing triple rescue therapy for subjects who were unsuccessful in eradicating H. pylori after at least 2 treatment courses. We used bismuth quadruple therapy as a comparator, as it has been demonstrated to be effective in our previous research [6].
METHOD
Ethics
We obtained informed consent from all participating institutions, and the study protocol was approved by the Ethics Committee of the 3 hospitals involved. This trial has been registered with ClinicalTrials.gov under the identifier NCT04879992. We adhered to the CONSORT statement's guidelines for reporting randomized controlled trials, as outlined in the Supplementary Material.
Design and Participants
This noninferiority, open-label, randomized trial was conducted in 3 hospitals between May 2021 and October 2022. The study included consecutive patients who tested positive for H. pylori and had previously failed 2 or more eradication regimens. All patients in our study had previously received clarithromycin, metronidazole, levofloxacin, and/or amoxicillin (if not allergic) in their previous treatments. None of the subjects had been treated with bismuth quadruple therapy, as tetracycline is not widely available in China. To confirm H. pylori infections, both 13C-urea breath test (UBT) and rapid urease testing (using 2 biopsies, 1 from the antrum and 1 from the corpus) were performed. Additionally, all patients underwent gastroscopy and gastric biopsy for H. pylori culture at baseline. Exclusion criteria were patients younger than 18 years, those who were H. pylori treatment naive, those with previous tuberculosis infection, pregnant or lactating women, those who had undergone previous stomach surgery, those infected with coronavirus disease 2019 (COVID-19), those with severe clinical diseases (respiratory, cardiovascular, renal, or hematological conditions), those who had used bismuth, antibiotics, PPI, or Chinese herbal medicine in the past 12 weeks, and those who had allergies to any of the medications.
Randomization and Masking
The randomization process was conducted using computer-generated random codes with permuted blocks, and the corresponding assignments were stored in sealed envelopes. A total of 364 eligible subjects were randomly allocated in a 1:1 ratio to receive one of the two 14-day rescue therapies: rifabutin triple therapy or bismuth quadruple therapy, with 182 subjects in each group. Because this was an open-label study, the subjects were aware of which regimen they received. However, the technicians who performed the UBT were blinded to the regimen allocation.
Intervention
In this study, all the regimens were used as at least third-line therapies. The 14-day rifabutin triple therapy group received esomeprazole (AstraZeneca) 20 mg, amoxicillin (Ruiyang Pharmaceutical) 1.0 g, and rifabutin (Med-Shine Pharmaceutical) 150 mg twice a day. The bismuth quadruple therapy group received esomeprazole 20 mg and bismuth potassium citrate (Dawnrays Pharmaceutical) 600 mg twice a day, in addition to metronidazole (Xinyi Wanxiang Pharmaceutical Industry) 400 mg and tetracycline (Chengdu Jinhua Pharmaceutical) 500 mg 4 times a day. In our study, bismuth and esomeprazole were administered 30 minutes before morning and evening meals, amoxicillin and rifabutin were given 30 minutes after morning and evening meals, and metronidazole and tetracycline were given 30 minutes after each meal and at bedtime.
All subjects were informed about potential adverse events and drug administration times to improve compliance. Moreover, they were instructed to record any discomfort or side effects in a diary during the treatment period. The severity of side effects was graded based on their impact on daily activities, categorized as mild (transient and well tolerated), moderate (causing discomfort and partially interfering with daily activities), or severe (causing significant interference with daily activities). Blood tests were performed for patients who experienced fever or other signs of systemic toxicity during their treatment [14]. The investigators who evaluated the side effects were blinded to treatment allocation. Compliance was deemed poor if the total medication taken by the subjects was less than 80% of the study drugs. At least 6 weeks after treatment, subjects returned for a UBT without taking PPIs, bismuth, or antibiotics. Successful eradication was defined as negative results (<4‰, with 4‰ as the cutoff value).
Culture of H. pylori Strains
During endoscopy, 1 gastric antrum and 1 gastric corpus biopsy specimen were collected and transported to Renji Hospital to isolate H. pylori. The isolates were cultured and maintained on brain heart infusion agar medium (OXOID) containing 5% defibrinated sheep blood under microaerophilic conditions (85% N2, 10% CO2, 5% O2) at 37°C. Isolates were stored in brain-heart infusion (Difco Laboratory) supplemented with 30% glycerol at −80°C. The culture was considered successful when isolates were positive for urease, oxidase, catalase, or Giemsa staining.
Antimicrobial Susceptibility Testing
We determined the minimal inhibitory concentrations (MIC) of amoxicillin, levofloxacin, clarithromycin, metronidazole, and tetracycline using the E-test method (Liofilchem). One hundred microliters of H. pylori suspension (3 McFarland) were plated onto agar plates, and E-test strips containing different antibiotic concentrations were placed at the center of the agar plate. After 3 days of microaerophilic incubation, the lowest drug concentration that prevented bacterial growth was considered the MIC.
The MIC of rifabutin was determined using the agar dilution method. We prepared bacterial suspensions (0.5 McFarland) with saline and plated them with an inoculator (Sakuma Seisaku) onto agar plates containing different concentrations of rifabutin. After 3 days of incubation, the lowest concentration of a drug that prevented the visible growth of bacteria was defined as the MIC.
We used H. pylori ATCC43504 as the quality control. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) has determined the breakpoint of each antibiotic as follows: MIC > 8 mg/L for metronidazole, MIC > 0.5 mg/L for clarithromycin, MIC > 1 mg/L for levofloxacin, MIC > 0.125 mg/L for amoxicillin, MIC > 1 mg/L for tetracycline, and MIC > 0.125 mg/L for rifabutin [10].
Statistics
This study was designed as a noninferiority trial. Based on our previous research [6, 15], we assumed a success rate of 88% for both the classical bismuth quadruple regimen and rifabutin triple therapy, an α level of .025 (1-sided), a power of 80%, and a noninferiority margin of −10%. To enroll at least 166 subjects per treatment arm, we planned to recruit approximately 182 patients for each treatment group, assuming a dropout rate of 10%.
We conducted 3 types of analyses to assess the H. pylori eradication rate of each group: intention-to-treat (ITT), per-protocol (PP), and modified intention-to-treat (MITT). For the MITT analysis, we included subjects who received at least 1 dose of medication and completed the follow-up UBT. In the ITT analysis, subjects lost to follow-up UBT were considered treatment failures. Subjects with a poor compliance or lost to follow-up UBT were excluded from the PP analysis. The comparative noninferiority of the 2 groups were assessed by calculating 95% confidence intervals (CI) on 2-sided through hypothesis testing (1-sided µ test). If the P value of the trial is less than .025 and the lower bound of 95% CI of the difference is higher than 10%, noninferiority of rifabutin triple therapy versus classical bismuth quadruple therapy can be concluded. Subgroup analysis was also performed based on the results of antibiotic susceptibility. Student t test for continuous variables and Pearson χ2 or Fisher exact test for categorical variables were performed for evaluating between-group differences. The statistical significance level was set at P < .05.
RESULTS
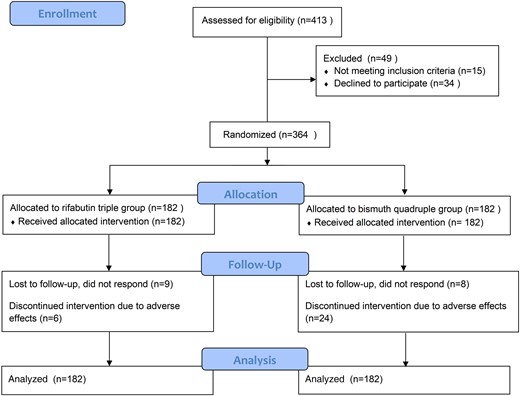
The flow of study subject recruitment and screening is shown in Figure 1. A total of 364 individuals were enrolled and randomly assigned to either the rifabutin triple therapy group or bismuth quadruple therapy group. Baseline demographic and clinical characteristics are provided in Table 1, and no significant differences were found in age or sex between the 2 groups. All patients had failed to achieve eradication after at least 2 previous treatments. Eight subjects in each group did not return for the UBT, and 1 subject in the rifabutin triple therapy group did not undergo testing despite having taken at least 80% of the medication. These 17 individuals were considered treatment failures in the ITT analysis. In addition, 30 subjects (6 in the rifabutin triple therapy group and 24 in the bismuth quadruple therapy group) did not take at least 80% of their medication and were therefore excluded from the PP analysis.
| Variables . | Rifabutin Triple Group (n = 182) . | Bismuth Quadruple Group (n = 182) . | P Value . |
|---|---|---|---|
| Age, median ± SD (range), y | 47.4 ± 11 (23–74) | 49.5 ± 12 (18–76) | .087 |
| Sex, male/female | 82/100 | 77/105 | .597 |
| Diagnosis, n/N (%) | .024 | ||
| Functional dyspepsia | 167/182 (91.8) | 153/182 (84.1) | |
| Peptic ulcer | 15/182 (8.2) | 29/182 (15.9) | |
| Antibiotic resistance, n (%) | |||
| Cultured | 142/182 (78.0) | 144/182 (79.1) | .798 |
| Clarithromycin | 130/142 (91.5) | 140/144 (97.2) | .037 |
| Levofloxacin | 120/142 (84.5) | 124/144 (86.1) | .702 |
| Metronidazole | 135/142 (95.1) | 138/144 (95.8) | .757 |
| Amoxicillin | 13/142 (9.2) | 17/144 (11.8) | .465 |
| Tetracycline | 1/142 (0.7) | 1/144 (0.7) | 1.000 |
| Rifabutin | 0/142 (0) | 0/144 (0) | 1.000 |
| Variables . | Rifabutin Triple Group (n = 182) . | Bismuth Quadruple Group (n = 182) . | P Value . |
|---|---|---|---|
| Age, median ± SD (range), y | 47.4 ± 11 (23–74) | 49.5 ± 12 (18–76) | .087 |
| Sex, male/female | 82/100 | 77/105 | .597 |
| Diagnosis, n/N (%) | .024 | ||
| Functional dyspepsia | 167/182 (91.8) | 153/182 (84.1) | |
| Peptic ulcer | 15/182 (8.2) | 29/182 (15.9) | |
| Antibiotic resistance, n (%) | |||
| Cultured | 142/182 (78.0) | 144/182 (79.1) | .798 |
| Clarithromycin | 130/142 (91.5) | 140/144 (97.2) | .037 |
| Levofloxacin | 120/142 (84.5) | 124/144 (86.1) | .702 |
| Metronidazole | 135/142 (95.1) | 138/144 (95.8) | .757 |
| Amoxicillin | 13/142 (9.2) | 17/144 (11.8) | .465 |
| Tetracycline | 1/142 (0.7) | 1/144 (0.7) | 1.000 |
| Rifabutin | 0/142 (0) | 0/144 (0) | 1.000 |
| Variables . | Rifabutin Triple Group (n = 182) . | Bismuth Quadruple Group (n = 182) . | P Value . |
|---|---|---|---|
| Age, median ± SD (range), y | 47.4 ± 11 (23–74) | 49.5 ± 12 (18–76) | .087 |
| Sex, male/female | 82/100 | 77/105 | .597 |
| Diagnosis, n/N (%) | .024 | ||
| Functional dyspepsia | 167/182 (91.8) | 153/182 (84.1) | |
| Peptic ulcer | 15/182 (8.2) | 29/182 (15.9) | |
| Antibiotic resistance, n (%) | |||
| Cultured | 142/182 (78.0) | 144/182 (79.1) | .798 |
| Clarithromycin | 130/142 (91.5) | 140/144 (97.2) | .037 |
| Levofloxacin | 120/142 (84.5) | 124/144 (86.1) | .702 |
| Metronidazole | 135/142 (95.1) | 138/144 (95.8) | .757 |
| Amoxicillin | 13/142 (9.2) | 17/144 (11.8) | .465 |
| Tetracycline | 1/142 (0.7) | 1/144 (0.7) | 1.000 |
| Rifabutin | 0/142 (0) | 0/144 (0) | 1.000 |
| Variables . | Rifabutin Triple Group (n = 182) . | Bismuth Quadruple Group (n = 182) . | P Value . |
|---|---|---|---|
| Age, median ± SD (range), y | 47.4 ± 11 (23–74) | 49.5 ± 12 (18–76) | .087 |
| Sex, male/female | 82/100 | 77/105 | .597 |
| Diagnosis, n/N (%) | .024 | ||
| Functional dyspepsia | 167/182 (91.8) | 153/182 (84.1) | |
| Peptic ulcer | 15/182 (8.2) | 29/182 (15.9) | |
| Antibiotic resistance, n (%) | |||
| Cultured | 142/182 (78.0) | 144/182 (79.1) | .798 |
| Clarithromycin | 130/142 (91.5) | 140/144 (97.2) | .037 |
| Levofloxacin | 120/142 (84.5) | 124/144 (86.1) | .702 |
| Metronidazole | 135/142 (95.1) | 138/144 (95.8) | .757 |
| Amoxicillin | 13/142 (9.2) | 17/144 (11.8) | .465 |
| Tetracycline | 1/142 (0.7) | 1/144 (0.7) | 1.000 |
| Rifabutin | 0/142 (0) | 0/144 (0) | 1.000 |
H. pylori Eradication Rates
Table 2 illustrates the results of the ITT analysis, PP analysis, and mITT analysis, where the H. pylori eradication rates were 89.0% (162/182; 95% CI, 83.6%–92.8%), 94.0% (157/167; 95% CI, 89.3%–96.7%), and 93.6% (162/173; 95% CI, 89.0%–96.4%) in the rifabutin triple group, respectively. The corresponding eradication rates in the bismuth quadruple group were 89.6% (163/182; 95% CI, 84.3%–93.2%), 95.3% (143/150; 95% CI, 90.7%–97.7%), and 93.7% (163/174; 95% CI, 89.0%–96.4%), respectively.
| Analysis . | Rifabutin Triple Group . | Bismuth Quadruple Group . | Treatment Difference, % . | P Value for Differencea . | P Value for Noninferiorityb . |
|---|---|---|---|---|---|
| ITT | |||||
| Eradication rate, (%) | 162/182 (89.0) | 163/182 (89.6) | −0.6 | .866 | .001 |
| 95% CI, % | 83.6–92.8 | 84.3–93.2 | −7.1 to 5.9 | ||
| PP | |||||
| Eradication rate, (%) | 157/167 (94.0) | 143/150 (95.3) | −1.3 | .602 | .001 |
| 95% CI, % | 89.3–96.7 | 90.7–97.7 | −6.6 to 4.1 | ||
| MITT | |||||
| Eradication rate, (%) | 162/173 (93.6) | 163/174 (93.7) | −0.04 | .989 | <.001 |
| 95% CI, % | 89.0–96.4 | 89.0–96.4 | −5.5 to 5.4 |
| Analysis . | Rifabutin Triple Group . | Bismuth Quadruple Group . | Treatment Difference, % . | P Value for Differencea . | P Value for Noninferiorityb . |
|---|---|---|---|---|---|
| ITT | |||||
| Eradication rate, (%) | 162/182 (89.0) | 163/182 (89.6) | −0.6 | .866 | .001 |
| 95% CI, % | 83.6–92.8 | 84.3–93.2 | −7.1 to 5.9 | ||
| PP | |||||
| Eradication rate, (%) | 157/167 (94.0) | 143/150 (95.3) | −1.3 | .602 | .001 |
| 95% CI, % | 89.3–96.7 | 90.7–97.7 | −6.6 to 4.1 | ||
| MITT | |||||
| Eradication rate, (%) | 162/173 (93.6) | 163/174 (93.7) | −0.04 | .989 | <.001 |
| 95% CI, % | 89.0–96.4 | 89.0–96.4 | −5.5 to 5.4 |
Abbreviations: CI, confidence interval; ITT, intention-to-treat; MITT, modified intention-to-treat; PP, per-protocol.
P values are 2-sided for comparing the difference of rifabutin triple group and bismuth quadruple group.
P values are 1-sided for comparing the noninferiority of rifabutin triple group and bismuth quadruple group.
| Analysis . | Rifabutin Triple Group . | Bismuth Quadruple Group . | Treatment Difference, % . | P Value for Differencea . | P Value for Noninferiorityb . |
|---|---|---|---|---|---|
| ITT | |||||
| Eradication rate, (%) | 162/182 (89.0) | 163/182 (89.6) | −0.6 | .866 | .001 |
| 95% CI, % | 83.6–92.8 | 84.3–93.2 | −7.1 to 5.9 | ||
| PP | |||||
| Eradication rate, (%) | 157/167 (94.0) | 143/150 (95.3) | −1.3 | .602 | .001 |
| 95% CI, % | 89.3–96.7 | 90.7–97.7 | −6.6 to 4.1 | ||
| MITT | |||||
| Eradication rate, (%) | 162/173 (93.6) | 163/174 (93.7) | −0.04 | .989 | <.001 |
| 95% CI, % | 89.0–96.4 | 89.0–96.4 | −5.5 to 5.4 |
| Analysis . | Rifabutin Triple Group . | Bismuth Quadruple Group . | Treatment Difference, % . | P Value for Differencea . | P Value for Noninferiorityb . |
|---|---|---|---|---|---|
| ITT | |||||
| Eradication rate, (%) | 162/182 (89.0) | 163/182 (89.6) | −0.6 | .866 | .001 |
| 95% CI, % | 83.6–92.8 | 84.3–93.2 | −7.1 to 5.9 | ||
| PP | |||||
| Eradication rate, (%) | 157/167 (94.0) | 143/150 (95.3) | −1.3 | .602 | .001 |
| 95% CI, % | 89.3–96.7 | 90.7–97.7 | −6.6 to 4.1 | ||
| MITT | |||||
| Eradication rate, (%) | 162/173 (93.6) | 163/174 (93.7) | −0.04 | .989 | <.001 |
| 95% CI, % | 89.0–96.4 | 89.0–96.4 | −5.5 to 5.4 |
Abbreviations: CI, confidence interval; ITT, intention-to-treat; MITT, modified intention-to-treat; PP, per-protocol.
P values are 2-sided for comparing the difference of rifabutin triple group and bismuth quadruple group.
P values are 1-sided for comparing the noninferiority of rifabutin triple group and bismuth quadruple group.
The differences in eradication rates between rifabutin triple and bismuth quadruple therapies were in the ITT, PP, and MITT analyses −0.6%, −1.3%, and −0.04%, respectively, and there were no statistically significant differences in the ITT, PP, and MITT analyses (P = .866, .602, and .989, respectively). Moreover, the lower boundaries of the 95% CI for the difference in the eradication rates between the 2 therapies in ITT (−7.1% to 5.9%), PP (−6.6% to 4.1%), and MITT (−5.5% to 5.4%) analyses were greater than the prespecified noninferiority margin of −10%. Therefore, rifabutin triple therapy satisfied the criteria for noninferiority to bismuth quadruple therapy in the ITT, MITT, and PP analyses.
Susceptibility Testing
In samples from 364 participants, 286 strains (78.6%) were successfully isolated and cultured. The resistance rates of H. pylori were 94.4% (270/286) for clarithromycin, 85.3% (244/286) for levofloxacin, 95.5% (273/286) for metronidazole, 10.5% (30/286) for amoxicillin, 0.7% (2/286) for tetracycline, and 0% (0/286) for rifabutin (Table 1).
Subgroup analysis was conducted to evaluate the effects of rifabutin triple therapy and bismuth quadruple therapy on the PP population with antibiotic resistance (Table 3). No significant difference in eradication rates was found between the 2 therapies for clarithromycin (92.2% vs 96.5%, P = .158), levofloxacin (90.8% vs 95.0%, P = .243), metronidazole (91.7% vs 95.6%, P = .224), tetracycline (100% vs 100%, P = 1.000), and amoxicillin (66.7% vs 92.3%, P = .160) resistance. However, amoxicillin resistance significantly affected the efficacy of rifabutin triple therapy, with 94.8% efficacy in sensitive strains and 66.7% efficacy in resistant strains (P = .007). For triple-resistant infections, both therapies achieved similar eradication rates (90.6% vs 95.7%, P = .168).
Eradication Rates of Rifabutin and Tetracycline Therapy in the Presence of Antibiotic Resistance in the PP Analysis
| PP Population . | Rifabutin Triple Group (n = 127) . | Bismuth Quadruple Group (n = 118) . | P Valuea . | ||
|---|---|---|---|---|---|
| n/N (%) | 95% CI, % | n/N (%) | 95% CI, % | ||
| CLA-R | 106/115 (92.2) | 85.8–95.8 | 110/114 (96.5) | 91.3–98.6 | .158 |
| LEV-R | 99/109 (90.8) | 83.9–94.9 | 95/100 (95.0) | 88.8-97.9 | .243 |
| MET-R | 110/120 (91.7) | 85.3–95.4 | 108/113 (95.6) | 90.1–98.1 | .224 |
| AMO-R | 8/12 (66.7) | 39.1–86.2 | 12/13 (92.3) | 66.7–98.7 | .160 |
| RIF-R | No resistance | NA | No resistance | NA | NA |
| TET-R | 1/1 (100) | 100–100 | 1/1 (100) | 100–100 | 1.000 |
| Resistance pattern (clarithromycin-metronidazole-levofloxacin) | |||||
| R-R-S | 13/13 (100) | 100–100 | 17/17 (100) | 100–100 | 1.000 |
| R-S-R | 5/5 (100) | 100–100 | 4/4 (100) | 100–100 | 1.000 |
| S-R-R | 6/7 (85.7) | 48.7–97.4 | 2/3 (66.7) | 20.8–93.9 | 1.000 |
| R-R-R | 87/96 (90.6) | 83.1–95.0 | 89/93 (95.7) | 89.5–98.3 | .168 |
| PP Population . | Rifabutin Triple Group (n = 127) . | Bismuth Quadruple Group (n = 118) . | P Valuea . | ||
|---|---|---|---|---|---|
| n/N (%) | 95% CI, % | n/N (%) | 95% CI, % | ||
| CLA-R | 106/115 (92.2) | 85.8–95.8 | 110/114 (96.5) | 91.3–98.6 | .158 |
| LEV-R | 99/109 (90.8) | 83.9–94.9 | 95/100 (95.0) | 88.8-97.9 | .243 |
| MET-R | 110/120 (91.7) | 85.3–95.4 | 108/113 (95.6) | 90.1–98.1 | .224 |
| AMO-R | 8/12 (66.7) | 39.1–86.2 | 12/13 (92.3) | 66.7–98.7 | .160 |
| RIF-R | No resistance | NA | No resistance | NA | NA |
| TET-R | 1/1 (100) | 100–100 | 1/1 (100) | 100–100 | 1.000 |
| Resistance pattern (clarithromycin-metronidazole-levofloxacin) | |||||
| R-R-S | 13/13 (100) | 100–100 | 17/17 (100) | 100–100 | 1.000 |
| R-S-R | 5/5 (100) | 100–100 | 4/4 (100) | 100–100 | 1.000 |
| S-R-R | 6/7 (85.7) | 48.7–97.4 | 2/3 (66.7) | 20.8–93.9 | 1.000 |
| R-R-R | 87/96 (90.6) | 83.1–95.0 | 89/93 (95.7) | 89.5–98.3 | .168 |
Abbreviations: AMO, amoxicillin; CI, confidence interval; CLA, clarithromycin; LEV, levofloxacin; MET, metronidazole; PP, per protocol; R, resistance; RIF, rifabutin; S, sensitive; TET, tetracycline; NA, not applicable.
P values are 2-sided for comparing the difference of rifabutin triple group and bismuth quadruple group in the presence of antibiotic resistance.
Eradication Rates of Rifabutin and Tetracycline Therapy in the Presence of Antibiotic Resistance in the PP Analysis
| PP Population . | Rifabutin Triple Group (n = 127) . | Bismuth Quadruple Group (n = 118) . | P Valuea . | ||
|---|---|---|---|---|---|
| n/N (%) | 95% CI, % | n/N (%) | 95% CI, % | ||
| CLA-R | 106/115 (92.2) | 85.8–95.8 | 110/114 (96.5) | 91.3–98.6 | .158 |
| LEV-R | 99/109 (90.8) | 83.9–94.9 | 95/100 (95.0) | 88.8-97.9 | .243 |
| MET-R | 110/120 (91.7) | 85.3–95.4 | 108/113 (95.6) | 90.1–98.1 | .224 |
| AMO-R | 8/12 (66.7) | 39.1–86.2 | 12/13 (92.3) | 66.7–98.7 | .160 |
| RIF-R | No resistance | NA | No resistance | NA | NA |
| TET-R | 1/1 (100) | 100–100 | 1/1 (100) | 100–100 | 1.000 |
| Resistance pattern (clarithromycin-metronidazole-levofloxacin) | |||||
| R-R-S | 13/13 (100) | 100–100 | 17/17 (100) | 100–100 | 1.000 |
| R-S-R | 5/5 (100) | 100–100 | 4/4 (100) | 100–100 | 1.000 |
| S-R-R | 6/7 (85.7) | 48.7–97.4 | 2/3 (66.7) | 20.8–93.9 | 1.000 |
| R-R-R | 87/96 (90.6) | 83.1–95.0 | 89/93 (95.7) | 89.5–98.3 | .168 |
| PP Population . | Rifabutin Triple Group (n = 127) . | Bismuth Quadruple Group (n = 118) . | P Valuea . | ||
|---|---|---|---|---|---|
| n/N (%) | 95% CI, % | n/N (%) | 95% CI, % | ||
| CLA-R | 106/115 (92.2) | 85.8–95.8 | 110/114 (96.5) | 91.3–98.6 | .158 |
| LEV-R | 99/109 (90.8) | 83.9–94.9 | 95/100 (95.0) | 88.8-97.9 | .243 |
| MET-R | 110/120 (91.7) | 85.3–95.4 | 108/113 (95.6) | 90.1–98.1 | .224 |
| AMO-R | 8/12 (66.7) | 39.1–86.2 | 12/13 (92.3) | 66.7–98.7 | .160 |
| RIF-R | No resistance | NA | No resistance | NA | NA |
| TET-R | 1/1 (100) | 100–100 | 1/1 (100) | 100–100 | 1.000 |
| Resistance pattern (clarithromycin-metronidazole-levofloxacin) | |||||
| R-R-S | 13/13 (100) | 100–100 | 17/17 (100) | 100–100 | 1.000 |
| R-S-R | 5/5 (100) | 100–100 | 4/4 (100) | 100–100 | 1.000 |
| S-R-R | 6/7 (85.7) | 48.7–97.4 | 2/3 (66.7) | 20.8–93.9 | 1.000 |
| R-R-R | 87/96 (90.6) | 83.1–95.0 | 89/93 (95.7) | 89.5–98.3 | .168 |
Abbreviations: AMO, amoxicillin; CI, confidence interval; CLA, clarithromycin; LEV, levofloxacin; MET, metronidazole; PP, per protocol; R, resistance; RIF, rifabutin; S, sensitive; TET, tetracycline; NA, not applicable.
P values are 2-sided for comparing the difference of rifabutin triple group and bismuth quadruple group in the presence of antibiotic resistance.
Adverse Effects and Compliance
Table 4 demonstrates that the incidence of adverse effects was significantly lower in the rifabutin triple therapy group compared to the bismuth quadruple therapy group (26.4% vs 54.4%, P < .001), and this difference also applied to moderate and severe adverse effects (14.3% vs 28.6%, P < .001). The most frequently reported adverse effects in the rifabutin triple therapy group were fever (12.6%, 23/182), skin rash (8.2%, 15/182), myalgia (4.9%, 9/182), and fatigue (3.3%, 6/182). In contrast, the most commonly reported adverse effects in the bismuth quadruple therapy group were nausea (32.4%, 59/182), dizziness (13.7%, 25/182), fatigue (8.2%, 15/182), bloating (6.6%, 12/182), diarrhea (5.5%, 10/182), and taste distortion (5.5%, 10/182). All adverse events in the 147 subjects resolved after discontinuation of the medications. The rifabutin triple therapy group exhibited better compliance than the bismuth quadruple therapy group (96.2% vs 85.4%, P = .001). Among subjects with poor compliance, 7 in the rifabutin triple therapy group and 24 in the bismuth quadruple therapy group discontinued medication due to adverse effects. Poor compliance in both groups was linked to severe adverse effects (all P values <.001).
| . | Rifabutin Triple Group . | Bismuth Quadruple Group . | P Value . |
|---|---|---|---|
| Total, n/N (%) | 48/182 (26.4) | 99/182 (54.4) | <.001 |
| AE grade | |||
| Mild | 22 | 47 | |
| Moderate | 7 | 28 | |
| Severe | 19 | 24 | |
| Moderate + severe | 26 | 52 | .001 |
| AE | |||
| Taste distortion | 0 | 10 | |
| Dyspepsia | 1 | 5 | |
| Nausea | 0 | 59 | |
| Vomiting | 1 | 5 | |
| Acid reflux | 2 | 2 | |
| Abdominal pain | 1 | 7 | |
| Bloating | 2 | 12 | |
| Diarrhea | 3 | 10 | |
| Skin rash | 15 | 3 | |
| Fatigue | 6 | 15 | |
| Fever | 23 | 3 | |
| Dizziness | 2 | 25 | |
| Myalgia | 9 | 0 | |
| Discontinued due to AEs | 7 | 24 | |
| Compliance, n/N (%) | 175/182 (96.2) | 158/182 (85.4) | .001 |
| Compliance according to AE grade, n/N (%) | |||
| None | 134/134 (100) | 81/83 (97.6) | |
| Mild | 22/22 (100) | 45/47 (95.7) | |
| Moderate | 7/7 (100.0) | 27/28 (96.4) | |
| Severe | 12/19 (64.7)a | 5/24 (20.8)b |
| . | Rifabutin Triple Group . | Bismuth Quadruple Group . | P Value . |
|---|---|---|---|
| Total, n/N (%) | 48/182 (26.4) | 99/182 (54.4) | <.001 |
| AE grade | |||
| Mild | 22 | 47 | |
| Moderate | 7 | 28 | |
| Severe | 19 | 24 | |
| Moderate + severe | 26 | 52 | .001 |
| AE | |||
| Taste distortion | 0 | 10 | |
| Dyspepsia | 1 | 5 | |
| Nausea | 0 | 59 | |
| Vomiting | 1 | 5 | |
| Acid reflux | 2 | 2 | |
| Abdominal pain | 1 | 7 | |
| Bloating | 2 | 12 | |
| Diarrhea | 3 | 10 | |
| Skin rash | 15 | 3 | |
| Fatigue | 6 | 15 | |
| Fever | 23 | 3 | |
| Dizziness | 2 | 25 | |
| Myalgia | 9 | 0 | |
| Discontinued due to AEs | 7 | 24 | |
| Compliance, n/N (%) | 175/182 (96.2) | 158/182 (85.4) | .001 |
| Compliance according to AE grade, n/N (%) | |||
| None | 134/134 (100) | 81/83 (97.6) | |
| Mild | 22/22 (100) | 45/47 (95.7) | |
| Moderate | 7/7 (100.0) | 27/28 (96.4) | |
| Severe | 12/19 (64.7)a | 5/24 (20.8)b |
Abbreviation: AE, adverse effect. n, number in subgroup; N, total number in group. aThe P values were two-sided and were for comparing the difference of rifabutin triple group and bismuth quadruple group. bThe P values were one-sided and were for comparing the non-inferiority of rifabutin triple group and bismuth quadruple group.
| . | Rifabutin Triple Group . | Bismuth Quadruple Group . | P Value . |
|---|---|---|---|
| Total, n/N (%) | 48/182 (26.4) | 99/182 (54.4) | <.001 |
| AE grade | |||
| Mild | 22 | 47 | |
| Moderate | 7 | 28 | |
| Severe | 19 | 24 | |
| Moderate + severe | 26 | 52 | .001 |
| AE | |||
| Taste distortion | 0 | 10 | |
| Dyspepsia | 1 | 5 | |
| Nausea | 0 | 59 | |
| Vomiting | 1 | 5 | |
| Acid reflux | 2 | 2 | |
| Abdominal pain | 1 | 7 | |
| Bloating | 2 | 12 | |
| Diarrhea | 3 | 10 | |
| Skin rash | 15 | 3 | |
| Fatigue | 6 | 15 | |
| Fever | 23 | 3 | |
| Dizziness | 2 | 25 | |
| Myalgia | 9 | 0 | |
| Discontinued due to AEs | 7 | 24 | |
| Compliance, n/N (%) | 175/182 (96.2) | 158/182 (85.4) | .001 |
| Compliance according to AE grade, n/N (%) | |||
| None | 134/134 (100) | 81/83 (97.6) | |
| Mild | 22/22 (100) | 45/47 (95.7) | |
| Moderate | 7/7 (100.0) | 27/28 (96.4) | |
| Severe | 12/19 (64.7)a | 5/24 (20.8)b |
| . | Rifabutin Triple Group . | Bismuth Quadruple Group . | P Value . |
|---|---|---|---|
| Total, n/N (%) | 48/182 (26.4) | 99/182 (54.4) | <.001 |
| AE grade | |||
| Mild | 22 | 47 | |
| Moderate | 7 | 28 | |
| Severe | 19 | 24 | |
| Moderate + severe | 26 | 52 | .001 |
| AE | |||
| Taste distortion | 0 | 10 | |
| Dyspepsia | 1 | 5 | |
| Nausea | 0 | 59 | |
| Vomiting | 1 | 5 | |
| Acid reflux | 2 | 2 | |
| Abdominal pain | 1 | 7 | |
| Bloating | 2 | 12 | |
| Diarrhea | 3 | 10 | |
| Skin rash | 15 | 3 | |
| Fatigue | 6 | 15 | |
| Fever | 23 | 3 | |
| Dizziness | 2 | 25 | |
| Myalgia | 9 | 0 | |
| Discontinued due to AEs | 7 | 24 | |
| Compliance, n/N (%) | 175/182 (96.2) | 158/182 (85.4) | .001 |
| Compliance according to AE grade, n/N (%) | |||
| None | 134/134 (100) | 81/83 (97.6) | |
| Mild | 22/22 (100) | 45/47 (95.7) | |
| Moderate | 7/7 (100.0) | 27/28 (96.4) | |
| Severe | 12/19 (64.7)a | 5/24 (20.8)b |
Abbreviation: AE, adverse effect. n, number in subgroup; N, total number in group. aThe P values were two-sided and were for comparing the difference of rifabutin triple group and bismuth quadruple group. bThe P values were one-sided and were for comparing the non-inferiority of rifabutin triple group and bismuth quadruple group.
DISCUSSION
This study is the first clinical trial conducted in China to evaluate the efficacy and safety of rifabutin for H. pylori treatment, as its use has been limited to respiratory physicians and rarely employed for H. pylori infections. The results of this trial demonstrated that rifabutin triple therapy is highly effective and comparable to bismuth quadruple therapy with fewer adverse effects and greater compliance.
Prior research has demonstrated favorable outcomes with rifabutin for H. pylori rescue therapy. A systematic review reported that rifabutin triple rescue therapy resulted in an eradication success rate of 71.3%, with cure rates of 73.3% for second-line therapy and 64.3% for third-line therapy, meanwhile, increasing the dosage of amoxicillin or PPI was shown to improve the efficacy of rifabutin triple therapy [16]. Recently, the Food and Drug Administration (FDA) authorized a 3-in-1 capsule (Talicia; RedHill Biopharma) containing rifabutin, amoxicillin, and omeprazole for the treatment of H. pylori infection. A randomized controlled trial confirmed the efficacy of a 14-day course of Talicia (amoxicillin, 3 g; rifabutin, 150 mg; omeprazole 120 mg) as first-line H. pylori treatment with a good eradication rate of 90.3% in the confirmed adherent population [17].
In our study, the successful eradication rate was found to be higher than past reports on rifabutin-amoxicillin-PPI therapy, which is in line with previous findings that higher eradication rates are observed in Asian populations compared to non-Asian populations. This difference might be partly attributed to genetic factors, as poor PPI metabolism is more prevalent in Asian populations due to the CYP2C19 polymorphism. This leads to a greater reduction in gastric acid levels with PPIs, thereby enhancing the bactericidal activity of amoxicillin [18].
Our study design showed that rifabutin triple therapy was well tolerated, with higher compliance and fewer adverse events than bismuth quadruple therapy. The most commonly reported adverse effects of rifabutin triple therapy were fever (12.6%) and skin rash (8.2%). The occurrence of rash on the extremities and neck was similar to that of amoxicillin allergy, and the late onset of the rash might have been due to a delayed hypersensitivity reaction associated with the development of amoxicillin allergy during therapy. However, we cannot entirely rule out a possible effect of rifabutin. More than half of the febrile subjects in our study had temperatures over 38.5°C, and the fever subsided within 48 hours of drug withdrawal, strongly suggesting a moderate-to-severe grade adverse event. Blood tests were performed in subjects with fever or other signs of systemic toxicity, but no underlying infections were found. However, 5 subjects with fever experienced transient leukopenia, which recovered in a few days after withdrawal of the medicine. This phenomenon might partly relate to the dosage of rifabutin, as previous studies reported that neutropenia was strongly correlated with rifabutin dosed at 5 mg/kg or greater [16, 19]. Red urine was common in the rifabutin triple group because of the red dye in the rifabutin preparation, and subjects were informed of this possibility before starting the regimen. In the bismuth quadruple group, nausea (32.4%) and dizziness (13.7%) were the most common adverse effects, which were consistent with our previous work [6].
However, the compliance of subjects who experienced severe adverse effects in the rifabutin triple group (63.2%) was better than that of the bismuth quadruple group (20.8%) due to the late onset of symptoms in the former group. Most subjects who experienced fever or rash in the rifabutin triple group did so between days 10 and 14 of treatment, and the reasons for this phenomenon might be interpreted partially by the cumulative dose of rifabutin with 300 mg/day, difference in racial or in pharmaceutical preparation. Conversely, the subjects in the bismuth quadruple group experienced symptoms at the beginning of treatment, mainly manifested as gastrointestinal disorders and dizziness, which severely interfered with their daily activities and led to poor compliance.
There is still controversy surrounding the optimal duration of rifabutin administration. Studies suggest that a 10-day course may be more effective than a 7-day course, but whether a 14-day course provides higher benefits remains debatable [13]. One study compared 10-day and 14-day eradication therapy and found that the latter had higher eradication rates but also more adverse effects [20]. Given the lack of studies on rifabutin triple therapy in China, we chose a 14-day duration of rifabutin to explore its efficacy in our study. Therefore, further studies on the efficacy of lower doses of rifabutin or shorter treatment courses are needed in China.
The previous treatment history of H. pylori can influence its antibiotic resistance. Although we recorded the antibiotics that subjects had used prior to treatment, we were unable to fully document previous antibiotic combination and the number of H. pylori eradications course for each patient due to fragmentary medical history and nonstandard antibiotic use, which made it difficult to observe difference in efficacy based on how many previous eradication therapies a patient had received.
In our study, the resistance of H. pylori to clarithromycin, levofloxacin, and metronidazole exceeded 80% in both groups with prior treatment failures. High resistance rates for levofloxacin and clarithromycin were mainly related to previous treatment and a high background prevalence exceeding 30% in China [21]. The resistance to amoxicillin was 10.4% in our study, similar to our previous work (8.3%) but significantly higher than in other studies [6]. This could be due to repeated use of amoxicillin in past treatments. More than half of the subjects reported receiving the same antibiotic in different courses, as in our previous report [22]. There was no significant difference in the cure rate between rifabutin triple therapy and bismuth quadruple therapy for subjects with amoxicillin resistance. However, due to the small sample size of the amoxicillin-resistance population, there might be a potential for a type II error. We did not observe a direct correlation between the MIC of amoxicillin-resistant strains and treatment success. Rifabutin triple therapy was successful in 5 strains with MICs greater than 0.5 µg/mL but failed in 4 strains with MICs of 0.25 µg/mL. This may be related to the effectiveness of rifabutin monotherapy and/or the dosage of amoxicillin, which was 2.0 g/day in our study. The bactericidal effect of amoxicillin is thought to depend, in part, on the percentage of time above the MIC rather than the maximum plasma concentration [23].
Although China carries a high burden of tuberculosis, there is no evidence of cross-resistance to rifabutin and other rifamycins in vitro [24]. Furthermore, based on the experience of treating mycobacterial infection treatment, cross-resistance is unlikely to occur in the short term and is only observed with long-term use of high doses of rifabutin [25, 26]. However, further research is necessary to determine whether prolonged usage of rifamycins could lead to the development of rifabutin-resistant H. pylori.
One limitation of our study is that we did not perform routine blood tests on all subjects before and after treatment as per the protocol. Instead, we opted for a more practical approach, which may have missed the opportunity to gain a better understanding of whether rifabutin at the dosages used resulted in hematologic changes that remained asymptomatic [27].
In conclusion, our findings indicate that triple therapy containing rifabutin can achieve acceptable eradication rates despite the high prevalence of antimicrobial resistance to traditional H. pylori antibiotics. This approach may be a potential alternative to bismuth quadruple therapy, with a lower incidence of side effects. The optimization of therapy for H. pylori in China should ideally result in a combination that offers the highest cure rate and the lowest incidence of side effects.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Guarantor of the article, H. L. conceived the study, obtained funding, and is responsible for data integrity. J. C. and Y. G. contributed to study design and methods. J. C., Y. H., Y. G., Z. D., J. W., P. X., X. L., and Y. H. recruited patients to the study. J. C., Y. H., and Y. G. collected data. J. C. did the statistical analysis and wrote the article, which was revised by H. L. All authors approved the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclaimer. The funder of the study had no role in the data collection, data analysis, data interpretation, or writing of the article.
Financial support. This work was supported by National Natural Science Foundation of China (grant number 82170578); and the Medical Engineering Cross Research Fund of Shanghai Jiaotong University (grant number YG2019ZDA11).
Data availability. The protocol of this study is available at https://www.clinicaltrials.gov/ NCT04879992. The individual deidentified participant data support the results of this article (text, tables, figures) are available from the corresponding author H. L. upon reasonable request.
References
Author notes
J. C., Y. G., and Y. H. contributed equally.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed




