-
PDF
- Split View
-
Views
-
Cite
Cite
Carter Merenstein, Ayannah S Fitzgerald, Layla A Khatib, Jevon Graham-Wooten, Frederic D Bushman, Ronald G Collman, Effects of Mask Reuse on the Oropharyngeal, Skin, and Mask Microbiome, The Journal of Infectious Diseases, Volume 228, Issue 4, 15 August 2023, Pages 479–486, https://doi.org/10.1093/infdis/jiad167
Close - Share Icon Share
Abstract
Face masks have been critical in the coronavirus disease 2019 (COVID-19) pandemic, but supplies were sometimes limited and disposable masks contribute greatly to environmental waste. Studies suggest that filtration capacity is retained with repeated use, and surveys indicate many people reuse surgical masks. However, the impact of mask reuse on the host is understudied.
We applied 16S rRNA gene sequencing to investigate the bacterial microbiome of the facial skin and oropharynx of individuals randomized to wearing fresh surgical masks daily versus masks reused for 1 week.
Compared to daily fresh masks, reuse was associated with increased richness (number of taxa) of the skin microbiome and trend towards greater diversity, but no difference in the oropharyngeal microbiome. Used masks had either skin-dominant or oropharynx-dominant bacterial sequences, and reused masks had >100-fold higher bacterial content but no change in composition compared to those used for 1 day.
One week of mask reuse increased the number of low-abundance taxa on the face but did not impact the upper respiratory microbiome. Thus, face mask reuse has little impact on the host microbiome, although whether minor changes to the skin microbiome might relate to reported skin sequelae of masking (maskne) remains to be determined.
Face masks are effective in preventing the spread of infectious respiratory diseases and have been critical in managing the coronavirus disease 2019 (COVID-19) pandemic [1]. Epidemiological studies and randomized controlled trials repeatedly found reduced COVID-19 spread when masks are consistently worn [2]. For example, a study in elementary schools found 37% fewer COVID-19 infections when masking was required [3], and a trial involving 600 villages in Bangladesh saw an 11% reduction when mask use was encouraged [4]. A modeling study using data from the United States during peak COVID-19 transmission estimated that masking could reduce mortality by 24%–65% [5]. Because of this evidence, mask wearing has been recommended or mandated across the world and billions of individuals have worn face masks over the last 3 years [6].
A variety of mask types have been used, primarily either reusable cloth facemasks or disposable masks such as surgical masks and respirators. Disposable surgical masks show greater filtration effectiveness compared to cloth masks and greater efficacy in preventing spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [7–9]. Therefore, organizations such as the Centers for Disease Control and Prevention recommend disposable surgical masks.
Widespread mask use has resulted in 2 concerns—first that supplies have sometimes been unable to meet demand, and second, that disposable masks have created a new source of pollution. Early supply shortages led to rationing of masks [10–12], and modeling studies suggest mask rationing can have a significant impact on COVID-19 mortality [13]. Importantly, disposable face masks are made of nonrecyclable polymers (typically polypropylene), which creates an environmental hazard in the form of microplastic pollution [14]. Estimates of the number of masks disposed of each day vary, but studies from Peru and the Arabian Peninsula each suggest thousands of tons of facemask plastic trash annually [15, 16]. Furthermore, 0.15 to 0.39 million tons of facemask microplastic are believed to have entered the oceans each year of the COVID-19 pandemic [17]. These supply and pollution issues have resulted in increased pressure to reuse disposable masks.
A study of adults in Brazil found 55% of individuals reused disposable surgical masks [18], and 2 studies in Hong Kong reported 54% and 35% of individuals reused masks, respectively [19, 20]. While reuse of disposable masks is not recommended, Alcaraz et al reported that surgical masks retained filtration capability even after several forms of decontamination [21]. Furthermore, Varanges and colleagues simulated mask wearing by soaking disposable masks in saliva for 8 hours, and observed only minimal decreases in filtration [22]. These studies suggest that reused disposable masks may remain effective, if mask reuse is determined to be safe.
Discussion of mask reuse risks have focused primarily on decontamination, as SARS-CoV-2 can persist on masks for multiple days [23]. Other risks of mask reuse have not yet been explored in detail. Given that a mask is in contact with an individual's face for hours each day, we hypothesized that mask reuse may affect the hygiene of the wearer. Therefore, we investigated the bacterial microbiome of the skin and the upper respiratory tract (oropharynx) to determine whether mask reuse over the course of a week affected the host microbiome compared to using daily fresh masks. We also investigated the microbiome of masks, comparing fresh masks, masks worn only once, and masks worn for 7 consecutive days.
METHODS
Study Participants
Subjects (n = 20; Supplementary Table 1) were recruited from local community members and trainees or employees of the University of Pennsylvania. Inclusion criteria were age ≥18 years, and routine wearing of a mask for ≥6 hours each day, ≥ 5 days per week. Exclusion criteria were any respiratory illness within 1 month, use of antibiotics within 3 months, or application of topical skincare products containing antibacterials. Participants were instructed not to change their routine skin or oral care during the study. Participants provided written informed consent under protocol No. 850394 approved by the University of Pennsylvania Institutional Review Board.
Sample Collection and Processing
Study protocols are outlined in Figure 1. Participants were given their own box of surgical style 3-layered disposable masks (Tianchang Dongan Protective Equipment). For the first 14 study days, participants wore a new mask each day. On day 15, participants were randomized to 1 of 2 groups. The “reuse” group wore the same mask for the next 7 days. The “daily” group continued to wear a new mask each day for the next 7 days. Participants were instructed to change masks at the same time each day, corresponding to the time of their sampling visits. This ensured that when masks were collected the number of hours of masking were roughly consistent across participants within each group. Therefore, all samples from the daily group, and visit 1 (V1) and V2 samples from the reuse group represent participants having worn the same mask for 1 day, while V3 samples from the reuse group came from individuals having worn the same mask for 7 days.
![Outline of study design and sample collection. All subjects underwent a 14-day wash-in period in which they changed masks daily. At day 15, subjects were randomized to either daily (new mask each day) or reuse (1 mask for 7 days) groups. Samples were collected on day 15 (visit 1 [V1]), 16 (visit 2 [V2]), and 21 (visit 3 [V3]) as indicated. All samples from the daily group, and V1 and V2 samples from the reuse group represent participants having worn the same mask for 1 day, while V3 samples from the reuse group reflect wearing the same mask for 7 days.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/228/4/10.1093_infdis_jiad167/1/m_jiad167f1.jpeg?Expires=1750486088&Signature=JSD~AnoYk-0ciTDPAvnLxw5IZiITMg73MzzsTHTz~R6M9mbINcbwT-gcnMd9Cc52IjKt33bofsNg1HVDBtsXNmK7hvd~kUtBtp2u6hA6HJbKA4ZeP-YGJNAZAdfp0BrW1OIDMzS8G71vVwOkYg3lWM6iekknWn~hFzU9-ydTqid5cPjJDX~6zB8rtSmRsyO0uedPEhkbm8hR4k8ZZtWuuq-1KjwAqta0mrfKPZ2oUThXjnLDaj8b77HjyGKqnXA2UIq6kZC12DMiNK10Urx5-2Z8~iYkIe7wM2a105WZuzJmDDFqpM0qf3OpM5BpVzNmgFwTtP2lMUwK49AaohbrTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Outline of study design and sample collection. All subjects underwent a 14-day wash-in period in which they changed masks daily. At day 15, subjects were randomized to either daily (new mask each day) or reuse (1 mask for 7 days) groups. Samples were collected on day 15 (visit 1 [V1]), 16 (visit 2 [V2]), and 21 (visit 3 [V3]) as indicated. All samples from the daily group, and V1 and V2 samples from the reuse group represent participants having worn the same mask for 1 day, while V3 samples from the reuse group reflect wearing the same mask for 7 days.
Sample collection occurred at study days 15 (V1), 16 (V2), and 21 (V3). At each time point, the subject removed their mask and a skin swab was taken from the nasal sidewall to the alar crease with a sterile foam swab (Puritan Medical), The oropharynx was then sampled using a sterile flocked nylon swab (Copan Diagnostics). Samples were immediately placed on ice and then frozen (−80°C). Additionally, at V1 (day 15) and V3 (day 21) we collected the masks subjects were were wearing at the time of the study visit. For the daily group, masks were also collected at V2 (day 16) (V2 mask could not be collected from the reuse group because this group needed to keep wearing that mask). Masks were folded in half (inside in), placed in individual plastic bags, and stored at −80°C.
DNA was extracted from swabs (PowerSoil Pro; Qiagen) as described [24]. Masks were dissected in a laminar flow hood using flame-sterilized scissors and forceps to remove the inner ply in a sterile manner. A 2-cm2 section of the central region of the inner ply was used for DNA extraction. The bacterial 16S rRNA gene was amplified from extracted DNA using primers spanning the hypervariable V1/V2 region and sequenced on an Illumina MiSeq (Supplementary Table 2). Unused masks and unused swabs were processed in parallel to serve as background controls. Bacterial biomass was measured on mask samples by 16S rRNA gene quantitative polymerase chain reaction (qPCR; Supplementary Table 3) as described [25]. For unknown reasons, V3 masks from the daily group also showed lower cycle threshold (Ct) values, despite only being worn for 1 day; positive and negative controls suggest this was not a batch effect. Primers, reagents and other details are shown in Supplementary Table 4.
Analysis
Raw reads were processed using the QIIME2 pipeline (version 2022.08). DADA2 was used for error processing and to define amplicon sequence variants. After quality filtering, samples had a median depth of 27 622 reads. The SILVA database was used to assign taxonomy using a naive Bayesian classifier (version 138_99). All statistical analysis was conducted in R (version 4.0.2). For comparisons between sample types (Figure 2), no filtering of contaminant taxa was conducted and a minimum of 100 reads was required.
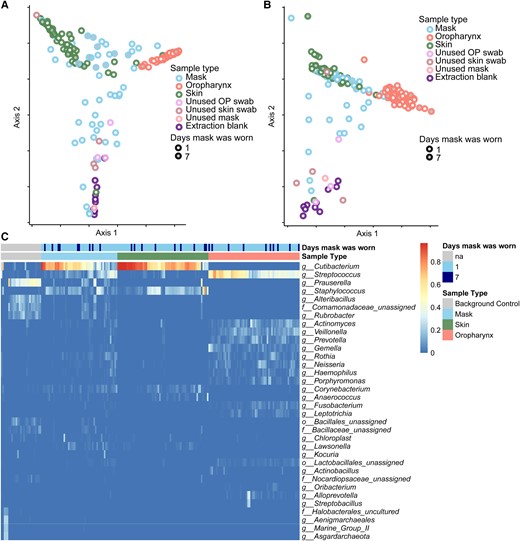
Microbiome composition of skin, mask, oropharyngeal, and background communities. A, Principal coordinate analysis (PCoA) using unweighted UniFrac distances of bacterial communities of all samples with minimum of 100 reads, colored by sample type. B, PCoA using weighted UniFrac distances. C, Relative abundance of common taxa (relative abundance >5% in at least 1 sample), identified at the genus or lowest level possible. Samples are shown in vertical columns and taxa are in rows; color represents the relative abundance in the sample. The top 2 rows indicate the sample type and duration of mask use associated with each sample. Abbreviations: OP, oropharynx; na, not applicable; f, family; g, genus; o, order.
For subsequent analysis, contaminant taxa were identified using extraction blanks and unused swabs, with taxa significantly more abundant in these controls (Wilcoxon P < .01) considered contaminants and removed [26]. A threshold of 20 reads was used to avoid spurious taxa detection, and samples with <1000 reads after contaminant removal were excluded. UniFrac analysis was conducted after rarefaction to 1000 reads.
Data Availability
Data has been deposited in the NCBI Sequence Read Archive (PRJNA926513). Code is available at https://github.com/cmerenstein/2023_mask_study.
RESULTS
Sampling the Microbiome Associated With Mask Reuse
To assess the consequences of long-term mask reuse, we sampled the skin, oropharynx, and the mask itself at 3 time points in 20 healthy volunteers (Supplementary Table 1) who were randomized to either change masks daily or reuse the same mask for 7 days (Figure 1).
Mask and Host Microbiome Composition
We first examined microbiome composition amongst all samples using the UniFrac distance metric, which compares the phylogenic relatedness of communities; unweighted UniFrac separates samples based on presence/absence of taxa; weighted UniFrac also takes abundance into account. We observed clear separation between the microbiome of the skin, oropharynx, and background controls using both unweighted and weighted UniFrac (Figure 2A and 2B ; P < 1e-5 unweighted, P = .002 weighted; PERMANOVA).
Bacterial lineages inferred from 16S rRNA gene sequencing are summarized in the heat map in Figure 2C . Skin communities were dominated by Cutibacterium and Staphylocccus, while oropharyngeal communities were comprised of Streptococcus and lower abundance oral taxa such as Actinomyces, Prevotella, and Veillonella. Thus, these samples contained lineages expected for those sites from previous studies. Unused swabs, unused masks, extraction blanks and other background controls were dominated by Prasurella and other well-recognized contaminant taxa.
Bacterial communities on masks that had been worn appeared to span the space between all 3 distinct communities (skin, oropharynx, and background). This finding is consistent with an admixture of bacterial sequences derived from these sources. Masks reused for 7 days were closer to skin and oropharynx communities via both weighted and unweighted UniFrac distances (ANOVA P < .005) while single-day masks were closer to background (P < 2e-16 weighted and unweighted).
Effects of Mask Reuse on Mask Bacterial Load and Composition
Bacterial load on the masks was measured by qPCR targeting the 16S rRNA gene. Masks that had not been worn had similar Ct values as extraction blanks, indicating minimal background bacteria (Figure 3A and Supplementary Table 3). Masks that were used for 1 day had a significantly higher bacterial load than unused masks (P < .05). However, mask bacterial load was markedly greater after 7 days of use compared to those worn for just 1 day (P < .4e-5; Figure 3A). This difference (7 Ct) corresponds to a 128-fold greater bacterial biomass after 7 days compared with 1 day of use. We also found a significant correlation between bacterial loads on masks collected on different visits from the same participant (R2 = 0.4, P = .001; Supplementary Figure 1), suggesting that individual-specific factors also contribute to the bacterial load on facemasks.
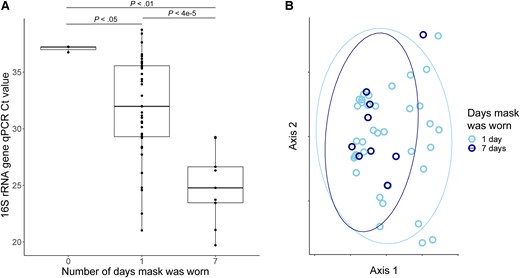
Bacterial communities on masks. A, Bacterial burden based on 16S rRNA gene abundance analyzed as a function of mask reuse. P values calculated by Wilcoxon rank-sum. B, Principal coordinate analysis of masks samples after removal of background contaminant sequences using unweighted UniFrac distances. Samples are colored by number of days that each mask was worn. No significant difference was observed between groups (PERMANOVA P > .05). Abbreviations: Ct, cycle threshold; qPCR, quantitative polymerase chain reaction. In panel A the line indicates median, box indicates interquartile range, whiskers indicate minimum and maximum values excluding outliers (1.5 x the interquartile range). In panel B the elipses indicate 95% confidence intervals.
After background (contaminant) sequences were removed, there was no compositional difference between the microbiome of masks worn 7 days and those worn only 1 day in either weighted or unweighted UniFrac (Figure 3B). This suggests that while masks increase in bacterial load with greater duration of use, there is no selection or diversification occurring on the masks over this time. Similarly, we identified no taxa that changed in relative abundance between masks worn 1 and 7 days, and no difference in alpha diversity. Overall, the mask microbiome contained taxa from both the skin and oropharyngeal microbiomes, but was closest in composition to the skin microbiome (P < 1e-7, weighted and unweighted UniFrac distances). Cutibacterium was highly abundant on masks, comprising >50% of reads in most mask samples. A minority of masks were more similar to oropharyngeal swabs than to skin swabs, dominated by Streptococcus and oral commensals, although there was no relation with mask reuse.
Altered Richness in the Skin Microbiome With Mask Reuse
The skin microbiome showed an increase in species richness in individuals reusing the same mask for 7 days, compared to participants changing their masks daily, when comparing the baseline visit (V1) and visit 1 week later (V3) (P < .05; Figure 4A). There was also a nonsignificant trend toward a greater increase in diversity with mask reuse (Shannon P = .055, Simpson P = .15) in the skin microbiome in the same comparison. In contrast, there were no significant differences in UniFrac distances between the V1 and V3 samples in subjects wearing the same mask for 7 days versus those wearing a new mask each day (Supplementary Figure 2A and 2B). Furthermore, there were no significant differences overall in either weighted or unweighted UniFrac distances between skin samples after 1 versus 7 days of mask use (Supplementary Figure 2C and 2D). Together, these results indicate that repeated mask reuse has no major effect on the skin microbiome, although it may cause a small increase in low-abundance taxa, compared to daily fresh mask use.
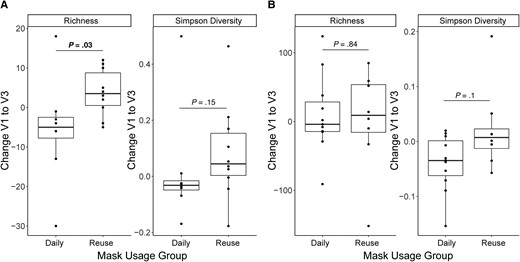
Effect of mask reuse on the skin and oropharyngeal microbiome. Change in species richness and Simpson diversity in (A) skin samples and (B) oropharyngeal samples from visit 1 (V1) to visit 3 (V3), by mask use group. P values from Wilcoxon rank-sum test. Boxplots indicate values as described in the legend to Figure 3.
Lack of Changes in the Upper Respiratory Track Microbiome With Mask Reuse
The oropharyngeal microbiome showed no change in richness from V1 to V3 and no difference with masking habits (Figure 4B). There was a small nonsignificant trend towards decreased alpha diversity in daily mask participants, with no change in mask reuse participants (Shannon and Simpson both P = .11). There were no differences in UniFrac distance from V1 to V3 in the oropharyngeal microbiome based on masking habits (Supplementary Figure 3A and 3B). Similarly, there was no difference between oropharyngeal microbiomes based on number of days since changing masks (Supplementary Figure 3C and 3D). These findings indicate that mask reuse for 1 week did not cause greater changes in the oropharyngeal microbiome than daily mask switching.
DISCUSSION
Repeated use of disposable masks became commonplace during the COVID-19 pandemic [18, 20, 21]. Masks are an essential tool for preventing spread of SARS-CoV-2 [4, 7, 9], but the impact of mask reuse has not been fully explored. Reusing disposable masks can reduce supply issues, making more masks available for those with the greatest need [13], and can lessen their impact on the environment by reducing microplastic pollution [17]. However, masks have shown considerable bacterial colonization after just 4 hours of use [27], suggesting that there may be hygiene concerns with prolonged reuse. Here we examined the impact of mask wearing habits on the facial skin and upper respiratory tract microbiome, comparing individuals reusing the same mask for 7 days to individuals changing masks daily. We found an approximately 100-fold increase in the total bacteria on masks after 7 days of reuse, and a modest change in richness in skin bacteria, but no changes in the oropharyngeal microbiome.
The skin microbiome is particularly interesting given case reports of mask-associated acne, with some studies suggesting up to 25% of mask wearers experiencing new or worsening acne [28–30]. Additionally, instances of dermatitis in conjunction with face mask use have been reported [31, 32]. Both of these conditions can be influenced by changes in the skin microbiome [33, 34].
Our study identified increased skin species richness after 7 days of mask reuse, and a trend towards increased alpha diversity. This indicates that reusing the same mask for a week may result in more low-abundance organisms on the skin. We did not observe a corresponding increase in microbial richness on the mask itself with 7 days of reuse (although we sampled the central region rather than that likely to contact facial skin), despite much greater total bacterial burden. One possible explanation is that microbiome differences may result from physiochemical changes on the skin, such as accumulation of oils, rather than seeding of new microbes from the mask. The skin microbiome in mask-associated acne has not been investigated to our knowledge, and thus it remains to be determined whether changes seen in reuse but not daily mask participants might be related to skin changes linked to mask-associated acne.
In contrast, we found no differences with mask reuse in skin microbiome overall composition as measured by weighted and unweighted UniFrac. A recent study of the skin microbiome underneath face masks of patients with mild acne vulgaris found no differences based on daily duration of mask wearing [35]. Previous studies have shown that the skin microbiome is generally highly stable even over prolonged time periods and resistant to changes in composition [36]. We selected 1 week to reflect the extreme of reported mask reuse duration [18, 19, 20]. Our findings suggest that reused of a mask even over 1 week of wearing does not disrupt the composition of the skin microbiome.
The upper respiratory tract (oropharyngeal) microbiome is functionally related to and seeds the microbiome of the lower airways and lung [37]. The oropharyngeal microbiome is also implicated in seeding the microbiome of the mask, given that droplets from the mouth will be captured by the mask [38]. Indeed, we saw a minority of masks that appeared more similar to the oropharyngeal microbiome than to the skin. However, we did not observe any significant compositional changes in the oropharyngeal microbiome with mask reuse and, unlike the skin microbiome, there was no change in species richness over the study period in either group.
Masks worn for 7 days had >100-fold greater bacterial load than masks worn for 1 day. Previous results in this area have been mixed. One study of surgeons found increasing bacterial load with increasing time up to 6 hours of mask use [39], while another study of healthy volunteers found no difference in bacterial count on masks used for 1, 2, or 3 days [40]. Our work indicates that over longer time periods, bacterial load increases markedly with repeated use.
Unused masks had bacterial loads similar to other negative controls, suggesting that surgical masks do not carry a background microbiome even though they are not indicated as sterile, which is in keeping with other reports [27]. Thus, our data suggest that masks start with very low microbial biomass and accumulate bacteria primarily from the skin, and to a lesser extent the oral or upper respiratory tract. A strong correlation between the bacterial load of different masks worn by the same participant suggests individual factors influence bacterial contamination of face masks. These determinants might be biological factors, such as oil or sweat production, or behavioral factors, such as coughing or activities done with a mask on, which could be further explored to determine personalized guidance for facemask hygiene.
While our data shed light on the microbiome consequences of mask reuse, more work is needed to determine the efficacy of masks after multiple days of reuse. Some reports suggest disposable masks can be used more than once, but no studies have followed mask reuse out to 7 days [21, 22]. In this ongoing pandemic, the ability for a mask to filter out viral particles is paramount and must be established before mask reuse can be safely recommended, despite the fact that it is widely practiced.
A limitation of our study is reliance on 16S rRNA gene sequencing, which does not allow fine-scale resolution of bacterial strains nor recover information about fungi or viruses. While our results show little effect of mask reuse on the microbiome, we could not look specifically for pathological strains that may have an outsized impact on human health. We also sampled only one location on the face, albeit one that is located within the mask coverage area. Along with that, any potential inefficiency in DNA extraction limits the ability to make conclusions about absolute bacterial content, although comparisons between mask groups remain valid. Finally, the modest number of individuals in each group may have limited our ability to detect small microbiome differences, although whether such small differences might be clinically important is uncertain.
In summary, the present study indicates that the widespread practice of reusing disposable masks over multiple days does not have a major impact on the microbiome of the skin or upper respiratory tract.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to volunteers who participated in this study; Elizabeth Grice for advice on skin sampling; and members of the Bushman and Collman laboratories for helpful suggestions.
Financial support. This work was supported by National Institutes of Health (grant number R33-HL137063); the Penn Center for Coronaviruses and Other Emerging Pathogens; and the Penn-CHOP Microbiome Program.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




