-
PDF
- Split View
-
Views
-
Cite
Cite
Anne H Rowley, David Arrollo, Stanford T Shulman, Abigail Torres, Amornrat O’Brien, Kristine Wylie, Kwang-Youn A Kim, Susan C Baker, Analysis of Plasmablasts From Children With Kawasaki Disease Reveals Evidence of a Convergent Antibody Response to a Specific Protein Epitope, The Journal of Infectious Diseases, Volume 228, Issue 4, 15 August 2023, Pages 412–421, https://doi.org/10.1093/infdis/jiad048
Close - Share Icon Share
Abstract
Kawasaki disease (KD) is a febrile illness of young childhood that can result in coronary artery aneurysms and death. Coronavirus disease 2019 (COVID-19) mitigation strategies resulted in a marked decrease in KD cases worldwide, supporting a transmissible respiratory agent as the cause. We previously reported a peptide epitope recognized by monoclonal antibodies (MAbs) derived from clonally expanded peripheral blood plasmablasts from 3 of 11 KD children, suggesting a common disease trigger in a subset of patients with KD.
We performed amino acid substitution scans to develop modified peptides with improved recognition by KD MAbs. We prepared additional MAbs from KD peripheral blood plasmablasts and assessed MAb characteristics that were associated with binding to the modified peptides.
We report a modified peptide epitope that is recognized by 20 MAbs from 11 of 12 KD patients. These MAbs predominantly use heavy chain VH3-74; two-thirds of VH3-74 plasmablasts from these patients recognize the epitope. The MAbs were nonidentical between patients but share a common complementarity-determining region 3 (CDR3) motif.
These results demonstrate a convergent VH3-74 plasmablast response to a specific protein antigen in children with KD, supporting one predominant causative agent in the etiopathogenesis of the illness.
Kawasaki disease (KD) is an acute febrile illness of childhood with the clinical features of oral and conjunctival erythema, rash, cervical adenopathy, and redness and swelling of the hands and feet. The disorder is distinctive in its potential to cause severe and persisting coronary artery aneurysms that can lead to myocardial infarction, aneurysm rupture, and sudden death [1–3]. However, no diagnostic test exists, and diagnosis can be difficult because many infectious and inflammatory conditions of childhood can mimic KD. This recently led to confusion between KD and multisystem inflammatory syndrome in children (MIS-C), which share some clinical features [4].
We previously reported an oligoclonal immunoglobulin A (IgA) response in acute KD coronary arteries [5], and others have reported an oligoclonal B-cell response in peripheral blood mononuclear cells from children with KD [6]. We recently identified a protein epitope targeted by monoclonal antibodies (MAbs) derived from clonally expanded peripheral blood plasmablasts from 3/11 children with recently diagnosed KD that bind to intracytoplasmic inclusion bodies in KD tissues [7]. Incubation of KD MAbs with human protein arrays that display full-length or near full-length proteins of 80% of the canonical proteome did not yield a human target of the antibodies, and a microbial, likely viral, target seems most likely, especially in view of the binding of the MAbs to RNA-containing inclusion bodies in KD tissues that we observed to have virus-like particles in close proximity [7–9]. Recently, coronavirus disease 2019 (COVID-19) mitigation strategies such as masking and social distancing resulted in a significant reduction in KD cases worldwide, providing a real-world experiment supporting a presently unknown respiratory virus as the most likely causative agent [10–15].
In this work, we report our discovery of a convergent plasmablast response to a specific protein epitope in 11/12 children with KD. We identified this epitope using amino acid substitution matrix analyses of the epitope we previously reported, with the goal of identifying whether a modified protein sequence would result in improved binding to KD MAbs [7]. We discovered that MAbs that bind to the epitope were predominantly of the variable heavy 3-74 (VH3-74) family, characteristic of a convergent response, and that these antibodies shared a common complementarity-determining region 3 (CDR3) motif. Because convergent antibody responses are typical of the B-cell response to distinct antigens of specific pathogens, the refined epitope likely represents either a linear epitope or a mimotope of an antigen derived from the triggering agent of KD [16, 17]. These results strongly favor one predominant cause of KD and provide significant new insight into its pathogenesis.
METHODS
Patients
This study was approved by the Institutional Review Board of the Ann and Robert H. Lurie Children's Hospital of Chicago. Clinical and plasmablast data on the 12 patients are described in Table 1. We deliberately focused the patient dataset on children who presented with coronary artery aneurysms, as the diagnosis of KD is most certain in such patients.
| Patient . | Age . | Sex . | Race/Ethnicity . | Day of Illness of Sample . | Day of Illness of IVIG Given . | Incomplete KD . | Zmax RCA/LAD . | Total No. of Plasmablasts (Isotype) . |
|---|---|---|---|---|---|---|---|---|
| KD1 | 4 y | F | Hispanic | 17 | 13 | No | RCA 4.7 | 119 (49A, 28G, 42 M) |
| KD2 | 4 y | F | White | 24 | 8 | No | Normal | 115 (64A, 22G, 29 M) |
| KD3 | 5 y | M | Black | 20 | 19 | Yes | Normal | 67 (33A, 31G, 3 M) |
| KD4 | 3 mo | M | Hispanic | 8 | 6 | Yes | RCA 3.0, LAD 3.5 | 153 (102G, 30A, 21 M) |
| KD5 | 11 mo | F | Caucasian, Asian (Vietnamese) | 14 | 10 | Yes | Normal | 115 (63G, 39A, 13 M) |
| KD6 | 4 y | F | Black | 13 | 5 and 8 | No | RCA 3.0 | 80 (43A, 23G, 14 M) |
| KD7 | 17 mo | M | Caucasian | 13 | 9 | No | RCA 9.8, LAD 5.7 | 121 (56A, 50G, 15 M) |
| KD8 | 17 mo | M | Caucasian | 14 | 12 | Yes | LAD 3.5 | 85 (41A, 34G, 10 M) |
| KD9 | 3 y | M | Asian (Indian) | 9 | 6 | No | Normal | 81 (30A, 38G, 13 M) |
| KD10 | 5 y | F | Asian (1/2 Japanese, 1/2 Korean) | 11 | 7 | No | RCA 5.4, LAD 7.4, distal large aneurysms | 88 (41A, 41G, 6 M) |
| KD11 | 3 y | F | Caucasian | 15 | 5 | No | RCA 2.55, distal L main 5.6 | 132 (36A, 50G, 46 M) |
| KD12 | 7 mo | M | Caucasian | 22 | 18 | No | RCA 4.3, LAD 11.6 | 159 (69A, 34G, 56 M) |
| Patient . | Age . | Sex . | Race/Ethnicity . | Day of Illness of Sample . | Day of Illness of IVIG Given . | Incomplete KD . | Zmax RCA/LAD . | Total No. of Plasmablasts (Isotype) . |
|---|---|---|---|---|---|---|---|---|
| KD1 | 4 y | F | Hispanic | 17 | 13 | No | RCA 4.7 | 119 (49A, 28G, 42 M) |
| KD2 | 4 y | F | White | 24 | 8 | No | Normal | 115 (64A, 22G, 29 M) |
| KD3 | 5 y | M | Black | 20 | 19 | Yes | Normal | 67 (33A, 31G, 3 M) |
| KD4 | 3 mo | M | Hispanic | 8 | 6 | Yes | RCA 3.0, LAD 3.5 | 153 (102G, 30A, 21 M) |
| KD5 | 11 mo | F | Caucasian, Asian (Vietnamese) | 14 | 10 | Yes | Normal | 115 (63G, 39A, 13 M) |
| KD6 | 4 y | F | Black | 13 | 5 and 8 | No | RCA 3.0 | 80 (43A, 23G, 14 M) |
| KD7 | 17 mo | M | Caucasian | 13 | 9 | No | RCA 9.8, LAD 5.7 | 121 (56A, 50G, 15 M) |
| KD8 | 17 mo | M | Caucasian | 14 | 12 | Yes | LAD 3.5 | 85 (41A, 34G, 10 M) |
| KD9 | 3 y | M | Asian (Indian) | 9 | 6 | No | Normal | 81 (30A, 38G, 13 M) |
| KD10 | 5 y | F | Asian (1/2 Japanese, 1/2 Korean) | 11 | 7 | No | RCA 5.4, LAD 7.4, distal large aneurysms | 88 (41A, 41G, 6 M) |
| KD11 | 3 y | F | Caucasian | 15 | 5 | No | RCA 2.55, distal L main 5.6 | 132 (36A, 50G, 46 M) |
| KD12 | 7 mo | M | Caucasian | 22 | 18 | No | RCA 4.3, LAD 11.6 | 159 (69A, 34G, 56 M) |
Abbreviations: IVIG, intravenous gammaglobulin; KD, Kawasaki disease; LAD, left anterior descending coronary artery; RCA, right coronary artery; Zmax , maximal Z score.
Patients 1–11 were previously reported in Rowley et al [7].
| Patient . | Age . | Sex . | Race/Ethnicity . | Day of Illness of Sample . | Day of Illness of IVIG Given . | Incomplete KD . | Zmax RCA/LAD . | Total No. of Plasmablasts (Isotype) . |
|---|---|---|---|---|---|---|---|---|
| KD1 | 4 y | F | Hispanic | 17 | 13 | No | RCA 4.7 | 119 (49A, 28G, 42 M) |
| KD2 | 4 y | F | White | 24 | 8 | No | Normal | 115 (64A, 22G, 29 M) |
| KD3 | 5 y | M | Black | 20 | 19 | Yes | Normal | 67 (33A, 31G, 3 M) |
| KD4 | 3 mo | M | Hispanic | 8 | 6 | Yes | RCA 3.0, LAD 3.5 | 153 (102G, 30A, 21 M) |
| KD5 | 11 mo | F | Caucasian, Asian (Vietnamese) | 14 | 10 | Yes | Normal | 115 (63G, 39A, 13 M) |
| KD6 | 4 y | F | Black | 13 | 5 and 8 | No | RCA 3.0 | 80 (43A, 23G, 14 M) |
| KD7 | 17 mo | M | Caucasian | 13 | 9 | No | RCA 9.8, LAD 5.7 | 121 (56A, 50G, 15 M) |
| KD8 | 17 mo | M | Caucasian | 14 | 12 | Yes | LAD 3.5 | 85 (41A, 34G, 10 M) |
| KD9 | 3 y | M | Asian (Indian) | 9 | 6 | No | Normal | 81 (30A, 38G, 13 M) |
| KD10 | 5 y | F | Asian (1/2 Japanese, 1/2 Korean) | 11 | 7 | No | RCA 5.4, LAD 7.4, distal large aneurysms | 88 (41A, 41G, 6 M) |
| KD11 | 3 y | F | Caucasian | 15 | 5 | No | RCA 2.55, distal L main 5.6 | 132 (36A, 50G, 46 M) |
| KD12 | 7 mo | M | Caucasian | 22 | 18 | No | RCA 4.3, LAD 11.6 | 159 (69A, 34G, 56 M) |
| Patient . | Age . | Sex . | Race/Ethnicity . | Day of Illness of Sample . | Day of Illness of IVIG Given . | Incomplete KD . | Zmax RCA/LAD . | Total No. of Plasmablasts (Isotype) . |
|---|---|---|---|---|---|---|---|---|
| KD1 | 4 y | F | Hispanic | 17 | 13 | No | RCA 4.7 | 119 (49A, 28G, 42 M) |
| KD2 | 4 y | F | White | 24 | 8 | No | Normal | 115 (64A, 22G, 29 M) |
| KD3 | 5 y | M | Black | 20 | 19 | Yes | Normal | 67 (33A, 31G, 3 M) |
| KD4 | 3 mo | M | Hispanic | 8 | 6 | Yes | RCA 3.0, LAD 3.5 | 153 (102G, 30A, 21 M) |
| KD5 | 11 mo | F | Caucasian, Asian (Vietnamese) | 14 | 10 | Yes | Normal | 115 (63G, 39A, 13 M) |
| KD6 | 4 y | F | Black | 13 | 5 and 8 | No | RCA 3.0 | 80 (43A, 23G, 14 M) |
| KD7 | 17 mo | M | Caucasian | 13 | 9 | No | RCA 9.8, LAD 5.7 | 121 (56A, 50G, 15 M) |
| KD8 | 17 mo | M | Caucasian | 14 | 12 | Yes | LAD 3.5 | 85 (41A, 34G, 10 M) |
| KD9 | 3 y | M | Asian (Indian) | 9 | 6 | No | Normal | 81 (30A, 38G, 13 M) |
| KD10 | 5 y | F | Asian (1/2 Japanese, 1/2 Korean) | 11 | 7 | No | RCA 5.4, LAD 7.4, distal large aneurysms | 88 (41A, 41G, 6 M) |
| KD11 | 3 y | F | Caucasian | 15 | 5 | No | RCA 2.55, distal L main 5.6 | 132 (36A, 50G, 46 M) |
| KD12 | 7 mo | M | Caucasian | 22 | 18 | No | RCA 4.3, LAD 11.6 | 159 (69A, 34G, 56 M) |
Abbreviations: IVIG, intravenous gammaglobulin; KD, Kawasaki disease; LAD, left anterior descending coronary artery; RCA, right coronary artery; Zmax , maximal Z score.
Patients 1–11 were previously reported in Rowley et al [7].
Flow Cytometry
CD3−CD19+CD38++CD27++ peripheral blood mononuclear cells were gated and single cells sorted into individual wells of 96-well plates, as previously described [7].
Amplification, Sequencing, and Cloning of Immunoglobulin Variable Region Genes
Reverse transcription, polymerase chain reaction amplification of heavy and light chain variable genes, and cloning into immunoglobulin expression vectors were performed as previously described [7, 18]. Heavy and light chains of the MAbs produced for this study have been submitted to GenBank with accession numbers OP207904 through OP207952.
Antibody Production and Analysis
Antibodies were produced by transfection of 293F suspension cells with immunoglobulin expression vectors as described in Supplementary Methods.
Substitution Analysis
Substitution analysis was performed on peptides recognized by KD MAbs by creating a peptide array that includes stepwise substitution of all amino acid positions of the peptide with all 20 amino acids, to determine the amino acids that yielded optimal antibody binding (PEPperPRINT, www.pepperprint.com).
ELISA for Binding of Peptides to KD Monoclonal Antibodies
Immulon 2 HB 96-well plates (ThermoFisher) were coated with synthetic peptides and incubated with KD MAbs as described in Supplementary Methods. We used 2-sample t tests to compare the difference in MAb binding to KDpeptide1 and KDpeptide3 for each monoclonal antibody.
ELISA for Reactivity of KD MAbs With SARS-CoV-2 Proteins
Immulon 2 HB plates were coated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike HexaPro or full-length nucleocapsid protein followed by KD MAbs as described in Supplementary Methods.
Western Blot Assay Using Mouse Fc-Concatemerized KD Peptide Fusion Proteins
We optimized the nucleotide sequence that codes for KD peptide sequences for expression in 293F cells and prepared mouse Fc fusion proteins containing multimers of KD peptide sequences linked by short spacers for use in western blot assays as described in Supplementary Methods.
RESULTS
To characterize the antibody response in KD patients, we generated MAbs from clonally expanded peripheral blood plasmablasts obtained by single-cell sorting from 12 children with KD (Figure 1A and Table 1). In our prior work, we reported that KD MAbs from 3 of 11 patients, designated KD4-2H4, KD6-2B2, and KD8-1D4, bind to intracytoplasmic inclusion bodies in tissues from fatal KD cases and recognize a specific epitope designated as KDpeptide1. These MAbs did not recognize a human protein by protein array analyses [7]. In this work, we prepared additional KD MAbs from a total of 12 KD patients and performed amino acid substitution arrays to determine whether the specific epitope could be refined to optimize binding to KD MAbs. We also analyzed the KD MAbs that bound to the refined epitope, to determine if MAbs from different KD patients that bind to the epitope share sequence similarity (Figure 1B).
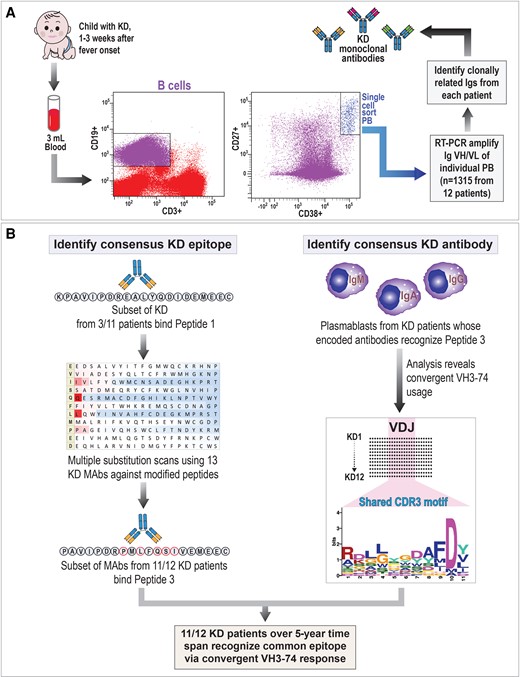
Approach to identify antigen-antibody response in KD patients. A, Scheme for analysis of plasmablast response in patients with KD. B, Diagram of the 2 approaches used to identify a consensus KD epitope and the convergent antibody response in plasmablasts from patients with KD. Abbreviations: CDR3, complementarity-determining region 3; Ig, immunoglobulin; KD, Kawasaki disease; MAb, monoclonal antibody; PB, plasmablast; RT-PCR, reverse transcription polymerase chain reaction; VH, variable heavy; VL, variable light.
Optimizing the Epitope Recognized by KD MAbs Using Amino Acid Substitution Scans
In our earlier studies, we performed substitution matrix analysis using MAbs recognizing the initial KD epitope N′-KPAVIPDREALYQDIDEMEEC-C′ (KDpeptide1) [7]. Each position in the peptide was substituted with all other amino acids and binding of KD MAbs was evaluated by peptide array. From these initial substitution matrix arrays, we made amino acid substitutions in KDpeptide1 to determine if they enhanced binding to KD MAbs. Based on results of substitution scans using individual KD MAbs, we used slightly modified peptides as the base peptide in substitution analyses using a total of 13 KD MAbs, with representative results using 6 KD MAbs shown in Figure 2. The results are depicted in a heat map with red indicating strong binding and blue indicating decreased or no binding compared to the base peptide. The sequential use of the substitution scans led to development of a second KD peptide KPAVIPDREMLIQSIVEMEEC (KDpeptide2) and then to a third peptide PAVIPDRPMLFQSIVEMEEC (KDpeptide3). Multiple substitution scans demonstrated the essential or near-essential nature of P8, L10, Q12, S13, and I14 of KDpeptide3 for binding to the MAbs, with M9 and V15 highly favored and F11 less essential, allowing for substitution by V, T, or I at that position (amino acid numbering is based on KDpeptide3, where position 1 = P and position 20 = C; Figure 2).
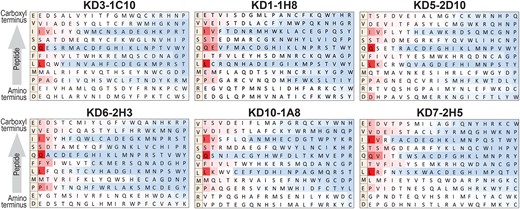
Amino acid substitution scans using monoclonal antibodies (MAbs) from multiple Kawasaki disease (KD) patients support a consensus PMLFQSIV epitope. The base peptide used is shown on the far left of each scan. Each amino acid of the base peptide was replaced by each of 20 amino acids and the relative binding of the peptide with each amino acid replacement is shown. Red indicates strong binding while blue indicates inhibition of binding relative to the base peptide. The base peptide differs slightly in individual scans. Scans using MAbs from KD patients 1, 3, 5, 6, 7, and 10 are shown; additional scans performed using MAbs from KD patients 2, 4, and 8 gave similar results.
MAbs From Children With KD Show Preferential Binding to KDpeptide3
We directly compared binding of KD MAbs to KDpeptide1, KDpeptide2, and KDpeptide3, as shown in Figure 3A and Supplementary Figure 1. Serial dilutions of MAb reactivity against KDpeptide3 are shown in Supplementary Figure 2. The 3 previously identified KD MAbs that bind to KDpeptide1 and 13 newly generated KD MAbs from multiple patients preferentially recognized KDpeptide3 over the other peptides. A subset of antibodies demonstrated binding to KDpeptide3 and KDpeptide2, but did not bind to KD peptide 1 (KD1-2B1, KD1-1H8, KD2-1D10, KD5-2D7, KD6-2H3, KD7-2H5, KD7-1B5, KD10-1G3, KD11-2A12, and KD11-2E4; Figure 3A and Supplementary Figure 1). Of note, substitution of P8 in KDpeptide3 for E at the corresponding position in KDpeptide1 and KDpeptide2 resulted in a significant enhancement of binding for multiple KD MAbs. BLAST analyses of the core sequence PMLF(V, T)QSIV did not yield human protein hits. Because we have previously demonstrated that KD inclusion bodies identified by KD MAbs contain RNA and not DNA [9], we also performed BLASTp analyses restricted to RNA viruses, yielding remote hits to diverse RNA viruses. These analyses were largely uninformative because of the limitations of the short core sequence length of about 8 amino acids, similar to the length of other linear epitopes bound by antibodies [19], and because the KD agent is likely one whose sequence is not present in established databases. Moreover, acute plasmablast responses to viral infection are often targeted at viral surface proteins, which are less conserved between viral families and can even be divergent between different viral genotypes of the same virus, making it less likely that homology to a known virus could be readily identified [20–22].
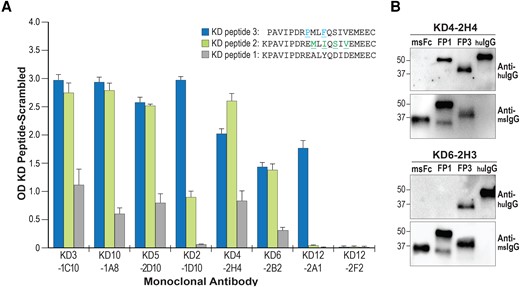
KD MAbs show preferential binding to KD peptide 3 over KD peptide 1. A, ELISA of a subset MAbs to the 3 peptides. For all antibodies recognizing KD peptide 3, binding to KD peptide 3 was significantly greater than KD peptide 1 in triplicate assays, P < .01. B, Western blots of KD peptide 3 (FP3) and KD peptide 1 (FP1) mouse Fc fusion proteins probed with KD MAbs KD4-2H4 and KD6-2H3. Human IgG (huIgG) was used as a positive control for the secondary anti-human IgG antibody, and stripped blots were reprobed with anti-mouse IgG (msIgG) to assess protein loading. Abbreviations: ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; KD, Kawasaki disease; MAb, monoclonal antibody; OD, optical density.
We also prepared mouse-Fc fusion proteins containing 3 copies of KDpeptide1 and KDpeptide3 for use in western blot assays with KD MAbs to assess binding of the MAbs to these fusion proteins. Western blot results using the MAbs were comparable to enzyme-linked immunosorbent assay (ELISA) using the peptides themselves. KD MAbs demonstrated enhanced binding to fusion proteins containing KDpeptide3 compared with those containing KDpeptide1 (KD4-2H4; Figure 3A and 3B). Some MAbs recognized fusion proteins containing KDpeptide3 but did not recognize those containing KDpeptide1, similar to the ELISA results (KD6-2H3; Figure 3B and Supplementary Figure 1).
Importantly, all KD MAbs that were initially discovered to bind to KDpeptide1 showed stronger binding to KDpeptide3 (P value <.01 for all MAbs; Figure 3A), indicating that KDpeptide3 is a common epitope in the KD response. These results suggest that the KDpeptide3 sequence more closely resembles the sequence of the antigen in the KD triggering agent (either as a linear sequence or as a mimotope) than does the KDpeptide1 sequence.
KD MAbs Binding to KDpeptide3 From 10 of 11 KD Patients Use Heavy Chain VH3-74 or its Paralogs
KD4-2H4, KD6-2B2, and KD8-1D4, the MAbs originally identified as binding to KDpeptide1, are members of the VH3-74, VH3-33, and VH3-72 families. Of the 3 MAbs, KD4-2H4 showed strongest binding to KDpeptide1 and is a member of the VH3-74 family. To determine if plasmablasts encoding antibodies from the VH3-74 family preferentially bind to the epitope, in this study we prepared additional MAbs from 8 KD patients. This yielded VH3-74 antibodies that recognized KDpeptide1 by ELISA from KD3 (KD3-1C10), KD5 (KD5-2D10), and KD10 (KD10-1A8) (Table 2 [VH3-74 MAbs shown in bold] and Supplementary Table 1). These results suggested that VH3-74 antibodies and their paralogs might be preferentially used by children with KD to respond to the protein epitope, presumably because the antibody structure of this family allows for binding to this KD antigen. With modification of KDpeptide1 to KDpeptide2 and KDpeptide3, we found that KD MAbs showed optimal binding to KDpeptide3, and using this peptide, we identified additional KD MAbs that recognized KDpeptide3, some of which did not bind to KDpeptide1, as noted above. All MAbs binding to KDpeptide3 derived from VH3-74 or other VH3 MAbs with >80% similarity to VH3-74 [23] (Table 2 and Supplementary Table 1). These MAbs were identified in 10 of 11 children with KD, including all of 8 children who developed coronary aneurysms (Table 1 and Table 2). The single child (KD9) from whom we did not identify a MAb that binds to any of the 3 peptides did not have plasmablasts encoding VH3-74 or paralogs VH3-33 or VH3-72 among the 81 single cells sequenced. This patient fulfilled clinical diagnostic criteria for KD but did not develop coronary artery abnormalities, so the diagnosis could not be confirmed.
MAbs Generated From Plasmablasts of Patients With KD That Bind to KD Peptide
| Patient . | MAb . | IGHV . | JH . | HCDR3 Length . | IGHV Amino Acid Mutations From GL . | IGK(L)V . | K(L) CDR3 Length . | IGK(L)V Amino Acid Mutation From GL . | PB Isotype . |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| KD4 | KD4-2H4 | 3-74*01 | 2 | 14 | 4 | L2-11*01 | 10 | 0 | IgG |
| KD6 | KD6-2B2 | 3-33*01 | 3 | 12 | 15 | K1-5*01 | 10 | 9 | IgA |
| KD8 | KD8-1D4 | 3-72*01 | 3 | 12 | 1 | K1-6*01 | 9 | 4 | IgA |
| Group 2 | |||||||||
| KD1 | KD1-2B1 | 3-74*01 | 6 | 13 | 4 | L2-11*01 | 10 | 3 | IgA |
| KD1 | KD1-1H8 | 3-74*01 | 2 | 15 | 7 | K3-15*01 | 9 | 2 | IgA |
| KD2 | KD2-1D10 | 3-74*01 | 4 | 14 | 7 | L2-8*01 | 11 | 4 | IgG |
| KD3 | KD3-1C10 | 3-74*01 | 6 | 12 | 10 | K3-20*01 | 10 | 9 | IgA |
| KD5 | KD5-2D7 | 3-74*01 | 6 | 13 | 6 | L1-44*01 | 13 | 6 | IgG |
| KD5 | KD5-2D10 | 3-74*01 | 3 | 15 | 0 | K3-15*01 | 10 | 0 | IgG |
| KD6 | KD6-2H3 | 3-15*01 | 3 | 19 | 9 | K3-15*01 | 8 | 5 | IgG |
| KD7 | KD7-2H5 | 3-74*01 | 4 | 11 | 1 | L1-47*01 | 11 | 0 | IgA |
| KD7 | KD7-1B5 | 3-33*01 | 4 | 12 | 6 | L1-51*01 | 11 | 0 | IgA |
| KD10 | KD10-1G3 | 3-73*01 | 5 | 15 | 14 | L5-37*01 | 11 | 20 | IgA |
| KD10 | KD10-1A8 | 3-74*01 | 3 | 11 | 8 | K2-29*02 | 9 | 3 | IgG |
| KD11 | KD11-2E4 | 3-74*01 | 4 | 19 | 11 | K1-5*03 | 11 | 4 | IgA |
| KD11 | KD11-2A12 | 3-21*01 | 4 | 13 | 0 | K3-20*01 | 10 | 0 | IgA |
| Group 3 | |||||||||
| KD12 | KD12-2A1 | 3-74*01 | 4 | 16 | 1 | K3-20*01 | 9 | 1 | IgA |
| KD12 | KD12-1F10 | 3-21*01 | 4 | 8 | 0 | K1-39*01 | 9 | 0 | IgA |
| KD12 | KD12-2A10 | 3-15*07 | 4 | 12 | 7 | K3-20*01 | 9 | 7 | IgG |
| KD12 | KD12-1G7 | 3-66*01 | 4 | 11 | 10 | L1-44*01 | 11 | 6 | IgA |
| Patient . | MAb . | IGHV . | JH . | HCDR3 Length . | IGHV Amino Acid Mutations From GL . | IGK(L)V . | K(L) CDR3 Length . | IGK(L)V Amino Acid Mutation From GL . | PB Isotype . |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| KD4 | KD4-2H4 | 3-74*01 | 2 | 14 | 4 | L2-11*01 | 10 | 0 | IgG |
| KD6 | KD6-2B2 | 3-33*01 | 3 | 12 | 15 | K1-5*01 | 10 | 9 | IgA |
| KD8 | KD8-1D4 | 3-72*01 | 3 | 12 | 1 | K1-6*01 | 9 | 4 | IgA |
| Group 2 | |||||||||
| KD1 | KD1-2B1 | 3-74*01 | 6 | 13 | 4 | L2-11*01 | 10 | 3 | IgA |
| KD1 | KD1-1H8 | 3-74*01 | 2 | 15 | 7 | K3-15*01 | 9 | 2 | IgA |
| KD2 | KD2-1D10 | 3-74*01 | 4 | 14 | 7 | L2-8*01 | 11 | 4 | IgG |
| KD3 | KD3-1C10 | 3-74*01 | 6 | 12 | 10 | K3-20*01 | 10 | 9 | IgA |
| KD5 | KD5-2D7 | 3-74*01 | 6 | 13 | 6 | L1-44*01 | 13 | 6 | IgG |
| KD5 | KD5-2D10 | 3-74*01 | 3 | 15 | 0 | K3-15*01 | 10 | 0 | IgG |
| KD6 | KD6-2H3 | 3-15*01 | 3 | 19 | 9 | K3-15*01 | 8 | 5 | IgG |
| KD7 | KD7-2H5 | 3-74*01 | 4 | 11 | 1 | L1-47*01 | 11 | 0 | IgA |
| KD7 | KD7-1B5 | 3-33*01 | 4 | 12 | 6 | L1-51*01 | 11 | 0 | IgA |
| KD10 | KD10-1G3 | 3-73*01 | 5 | 15 | 14 | L5-37*01 | 11 | 20 | IgA |
| KD10 | KD10-1A8 | 3-74*01 | 3 | 11 | 8 | K2-29*02 | 9 | 3 | IgG |
| KD11 | KD11-2E4 | 3-74*01 | 4 | 19 | 11 | K1-5*03 | 11 | 4 | IgA |
| KD11 | KD11-2A12 | 3-21*01 | 4 | 13 | 0 | K3-20*01 | 10 | 0 | IgA |
| Group 3 | |||||||||
| KD12 | KD12-2A1 | 3-74*01 | 4 | 16 | 1 | K3-20*01 | 9 | 1 | IgA |
| KD12 | KD12-1F10 | 3-21*01 | 4 | 8 | 0 | K1-39*01 | 9 | 0 | IgA |
| KD12 | KD12-2A10 | 3-15*07 | 4 | 12 | 7 | K3-20*01 | 9 | 7 | IgG |
| KD12 | KD12-1G7 | 3-66*01 | 4 | 11 | 10 | L1-44*01 | 11 | 6 | IgA |
Group 1, KD MAbs previously described to bind KDpep117, shown in this report to have improved binding to KDpep3. Group 2, KD MAbs generated from 8 different KD patients in 2017–2018 that bind to KDpep3. Group 3, KD MAbs generated from 1 KD patient in 2022 that recognize KDpep3. Members of the IGHV 3–74 gene family are bolded. Abbreviations: GL, germline; HCDR3, heavy chain complementarIty-determining region 3 (CDR3); Ig, immunoglobulin; IGHV, heavy chain variable gene; IGK(L)V, light chain lambda (L) or kappa (K) variable gene; JH, heavy chain junctional gene; KD, Kawasaki disease; K(L) CDR3, light chain K or L CDR3; MAb, monoclonal antibody; PB, plasmablast.
MAbs Generated From Plasmablasts of Patients With KD That Bind to KD Peptide
| Patient . | MAb . | IGHV . | JH . | HCDR3 Length . | IGHV Amino Acid Mutations From GL . | IGK(L)V . | K(L) CDR3 Length . | IGK(L)V Amino Acid Mutation From GL . | PB Isotype . |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| KD4 | KD4-2H4 | 3-74*01 | 2 | 14 | 4 | L2-11*01 | 10 | 0 | IgG |
| KD6 | KD6-2B2 | 3-33*01 | 3 | 12 | 15 | K1-5*01 | 10 | 9 | IgA |
| KD8 | KD8-1D4 | 3-72*01 | 3 | 12 | 1 | K1-6*01 | 9 | 4 | IgA |
| Group 2 | |||||||||
| KD1 | KD1-2B1 | 3-74*01 | 6 | 13 | 4 | L2-11*01 | 10 | 3 | IgA |
| KD1 | KD1-1H8 | 3-74*01 | 2 | 15 | 7 | K3-15*01 | 9 | 2 | IgA |
| KD2 | KD2-1D10 | 3-74*01 | 4 | 14 | 7 | L2-8*01 | 11 | 4 | IgG |
| KD3 | KD3-1C10 | 3-74*01 | 6 | 12 | 10 | K3-20*01 | 10 | 9 | IgA |
| KD5 | KD5-2D7 | 3-74*01 | 6 | 13 | 6 | L1-44*01 | 13 | 6 | IgG |
| KD5 | KD5-2D10 | 3-74*01 | 3 | 15 | 0 | K3-15*01 | 10 | 0 | IgG |
| KD6 | KD6-2H3 | 3-15*01 | 3 | 19 | 9 | K3-15*01 | 8 | 5 | IgG |
| KD7 | KD7-2H5 | 3-74*01 | 4 | 11 | 1 | L1-47*01 | 11 | 0 | IgA |
| KD7 | KD7-1B5 | 3-33*01 | 4 | 12 | 6 | L1-51*01 | 11 | 0 | IgA |
| KD10 | KD10-1G3 | 3-73*01 | 5 | 15 | 14 | L5-37*01 | 11 | 20 | IgA |
| KD10 | KD10-1A8 | 3-74*01 | 3 | 11 | 8 | K2-29*02 | 9 | 3 | IgG |
| KD11 | KD11-2E4 | 3-74*01 | 4 | 19 | 11 | K1-5*03 | 11 | 4 | IgA |
| KD11 | KD11-2A12 | 3-21*01 | 4 | 13 | 0 | K3-20*01 | 10 | 0 | IgA |
| Group 3 | |||||||||
| KD12 | KD12-2A1 | 3-74*01 | 4 | 16 | 1 | K3-20*01 | 9 | 1 | IgA |
| KD12 | KD12-1F10 | 3-21*01 | 4 | 8 | 0 | K1-39*01 | 9 | 0 | IgA |
| KD12 | KD12-2A10 | 3-15*07 | 4 | 12 | 7 | K3-20*01 | 9 | 7 | IgG |
| KD12 | KD12-1G7 | 3-66*01 | 4 | 11 | 10 | L1-44*01 | 11 | 6 | IgA |
| Patient . | MAb . | IGHV . | JH . | HCDR3 Length . | IGHV Amino Acid Mutations From GL . | IGK(L)V . | K(L) CDR3 Length . | IGK(L)V Amino Acid Mutation From GL . | PB Isotype . |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| KD4 | KD4-2H4 | 3-74*01 | 2 | 14 | 4 | L2-11*01 | 10 | 0 | IgG |
| KD6 | KD6-2B2 | 3-33*01 | 3 | 12 | 15 | K1-5*01 | 10 | 9 | IgA |
| KD8 | KD8-1D4 | 3-72*01 | 3 | 12 | 1 | K1-6*01 | 9 | 4 | IgA |
| Group 2 | |||||||||
| KD1 | KD1-2B1 | 3-74*01 | 6 | 13 | 4 | L2-11*01 | 10 | 3 | IgA |
| KD1 | KD1-1H8 | 3-74*01 | 2 | 15 | 7 | K3-15*01 | 9 | 2 | IgA |
| KD2 | KD2-1D10 | 3-74*01 | 4 | 14 | 7 | L2-8*01 | 11 | 4 | IgG |
| KD3 | KD3-1C10 | 3-74*01 | 6 | 12 | 10 | K3-20*01 | 10 | 9 | IgA |
| KD5 | KD5-2D7 | 3-74*01 | 6 | 13 | 6 | L1-44*01 | 13 | 6 | IgG |
| KD5 | KD5-2D10 | 3-74*01 | 3 | 15 | 0 | K3-15*01 | 10 | 0 | IgG |
| KD6 | KD6-2H3 | 3-15*01 | 3 | 19 | 9 | K3-15*01 | 8 | 5 | IgG |
| KD7 | KD7-2H5 | 3-74*01 | 4 | 11 | 1 | L1-47*01 | 11 | 0 | IgA |
| KD7 | KD7-1B5 | 3-33*01 | 4 | 12 | 6 | L1-51*01 | 11 | 0 | IgA |
| KD10 | KD10-1G3 | 3-73*01 | 5 | 15 | 14 | L5-37*01 | 11 | 20 | IgA |
| KD10 | KD10-1A8 | 3-74*01 | 3 | 11 | 8 | K2-29*02 | 9 | 3 | IgG |
| KD11 | KD11-2E4 | 3-74*01 | 4 | 19 | 11 | K1-5*03 | 11 | 4 | IgA |
| KD11 | KD11-2A12 | 3-21*01 | 4 | 13 | 0 | K3-20*01 | 10 | 0 | IgA |
| Group 3 | |||||||||
| KD12 | KD12-2A1 | 3-74*01 | 4 | 16 | 1 | K3-20*01 | 9 | 1 | IgA |
| KD12 | KD12-1F10 | 3-21*01 | 4 | 8 | 0 | K1-39*01 | 9 | 0 | IgA |
| KD12 | KD12-2A10 | 3-15*07 | 4 | 12 | 7 | K3-20*01 | 9 | 7 | IgG |
| KD12 | KD12-1G7 | 3-66*01 | 4 | 11 | 10 | L1-44*01 | 11 | 6 | IgA |
Group 1, KD MAbs previously described to bind KDpep117, shown in this report to have improved binding to KDpep3. Group 2, KD MAbs generated from 8 different KD patients in 2017–2018 that bind to KDpep3. Group 3, KD MAbs generated from 1 KD patient in 2022 that recognize KDpep3. Members of the IGHV 3–74 gene family are bolded. Abbreviations: GL, germline; HCDR3, heavy chain complementarIty-determining region 3 (CDR3); Ig, immunoglobulin; IGHV, heavy chain variable gene; IGK(L)V, light chain lambda (L) or kappa (K) variable gene; JH, heavy chain junctional gene; KD, Kawasaki disease; K(L) CDR3, light chain K or L CDR3; MAb, monoclonal antibody; PB, plasmablast.
The genetic features of the MAbs prepared for this report are detailed in Supplementary Table 1. MAbs recognizing KDpeptide3 differed in CDR3 sequence, with lengths varying from 11 to 19 amino acids, and with 0 to 15 amino acid mutations from germline. D genes D4-17*01, D3-9*01, D6-13*01, and D6-19*01, and light chains L2-11*01, L1-44*01, K3-15*01, K3-20*01, and K1-5*03 were used by 2 or more MAbs in the dataset (Supplementary Table 1). Overall, 10 of 14 (71%) VH3-74 MAbs and 2 of 3 (67%) VH3-33 MAbs that we produced from plasmablasts from 11 children with KD recognized KDpeptide3 (Table 2 and Supplementary Table 1). We note that neither 3 VH1 nor 4 VH4 antibodies from clonally expanded plasmablasts from the original 11 KD patients recognized KDpeptide1, 2, or 3, nor did 5 VH3-74 MAbs from these patients, suggesting that the response to KD likely includes additional epitopes that we have not yet identified and/or that some VH3-74 MAbs circulating in the plasmablast pool were not responding to KD (Supplementary Table 2). The prevalence of VH3-74 MAbs from children with KD that bind to KDpeptide3 define a convergent plasmablast response to a specific protein epitope in KD.
Plasmablast Analysis of KD Patient 12 Also Yields MAbs Binding to KDpeptide3
Our initial study of KD plasmablasts included children with KD diagnosed in 2017–2018 [7]. To determine if the antigen that includes the KDpeptide3 sequence was also recognized by a KD patient presenting 5 years following our initial study, we sequenced 159 single plasmablasts from KD12, an infant with classic KD who developed a giant coronary artery aneurysm in 2022. Nine sets of clonally expanded plasmablasts were identified in this child's peripheral blood (Supplementary Table 1). These included 3 sets of clonally expanded VH3-74 plasmablasts, 2 of which included plasmablasts of more than 1 isotype within the set, compatible with isotype switching during an acute response to infection. One of these VH3-74 plasmablasts, KD12-2A1, recognized KDpeptide3 but not KDpeptide1 or KDpeptide2 (Table 2 and Figure 3A). MAbs made from 3 additional clonally related sets of plasmablasts, KD12–1F10, KD12-1G7, and KD12-2A10, also recognized KDpeptide3 albeit with lower binding; these MAbs were of the VH3-21, VH3-66, and VH3-15 families, respectively [23] (Table 2 and Supplementary Table 1).
KD MAbs Binding to KDpeptide3 Share a Common CDR3 Epitope
We found that MAbs from KD patients 1–12 that recognize KDpeptide3 had different CDR3 sequences (Figure 4A). However, motif-based sequence analysis of the CDR3 amino acid sequences (https://meme-suite.org/meme/) revealed a statistically significant common motif at 2.3e-6 (Figure 4B). Of the 20 KD MAbs recognizing KDpeptide3 (Table 2), 19 share the motif, with KD12-1F10 the only exception. These results provide further evidence of a convergent VH3-74 antibody response to the KDpeptide3 protein antigen in children with KD presenting over a 5-year time period.

Analysis of CDR3 sequences of KD MAbs supports a convergent antibody response in KD patients. A, Alignment of CDR3 amino acid sequences from KD MAbs that recognize KD peptide 3. B, Motif-based sequence analysis of CDR3 amino acid sequences of KD MAbs binding to KD peptide 3. Analysis was performed using the MEME suite (https://meme-suite.org/meme/), revealing a statistically significant consensus motif at 2.3e-6. Abbreviations: KD, Kawasaki disease; MAb, monoclonal antibody.
KD MAbs do not Demonstrate Cross-reactive Binding to SARS-CoV-2 Proteins by ELISA
Because of some clinical similarities between SARS-CoV-2–induced MIS-C and KD, some investigators have suggested that KD might be caused by a virus with homology to SARS-CoV-2. To determine if KD MAbs were cross-reactive with SARS-CoV-2 spike or nucleocapsid proteins, we performed ELISA of KD MAbs against these proteins. Control antibodies to these proteins gave positive results, while none of 13 KD MAbs reacted with these proteins, including 11 that react with KDpeptide3, and 2 others whose targets remain unknown (data not shown).
DISCUSSION
In the present study, we report a refined protein epitope targeted by a convergent VH3-74 antibody response with a common CDR3 motif in 11/12 children with KD. The one patient in whom this response was not identified had typical features of the illness but did not develop coronary artery abnormalities. The possibilities for a lack of detection of a similar response in this single patient include an insufficient number of plasmablasts sequenced, misdiagnosis of another childhood illness as KD, or a different KD triggering pathogen.
Because no specific infectious etiologic agent has been identified as the cause of KD to date, it has been speculated that it could be an autoimmune illness triggered by widely diverse infectious agents. We cannot completely exclude the possibility that the protein epitope that we have identified as a target of the KD immune response is a mimotope of a human protein, but protein arrays that display full-length or near full-length proteins of 80% of the canonical proteome did not yield a human target of KD MAbs [7]. Perhaps more importantly, the features of KD are difficult to reconcile with an autoimmune theory of pathogenesis. KD is a febrile illness with distinctive clinical features and an abrupt onset that resolves spontaneously in 1–2 weeks even without therapy in most cases, a very short time span for an autoimmune disease. Coronary artery disease in KD occurs in the first few weeks following fever onset but does not worsen over time even without treatment; it seems highly unexpected for autoimmune disease to cause enough vascular damage to potentially result in myocardial infarction but abruptly cease after a few weeks without recurrence of vascular injury. The illness affects a very restricted age group with peak incidence in infancy, which is a very unusual age for autoimmunity to arise. KD rarely recurs, but the autoimmune theory would predict that another infection could trigger another episode of KD. The worldwide reports of outbreaks and epidemics of KD should not be apparent if diverse triggering etiologies in a susceptible host cause KD by an autoimmune mechanism [24, 25]. In contrast, all of these features are consistent with a single causative infectious agent or group of closely related agents that usually results in asymptomatic infection but can cause KD in a subset of genetically predisposed children who generally develop lifelong immunity following the illness (Figure 5).
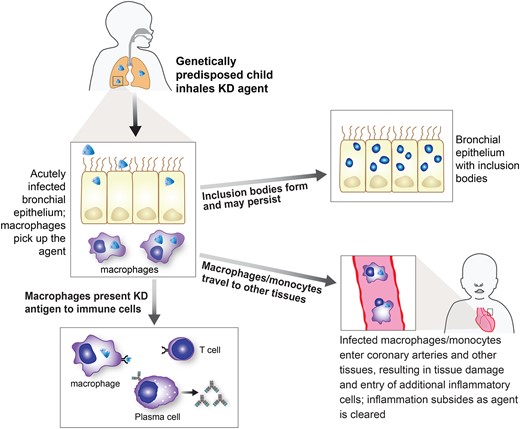
A proposed model of the pathogenesis of Kawasaki disease (KD). In this model, the KD agent is acquired via the respiratory route and infects ciliated bronchial epithelial cells, where inclusion bodies can form. It can be picked up by monocyte/macrophages and presented to the immune system, resulting in innate and acquired immune responses. Monocyte/macrophages travel through the circulation and enter coronary arteries and other tissues, with the most significant damage occurring in the coronary arteries. The immune system ultimately clears the infection, although inclusion bodies can persist.
Clinical features of KD supporting a viral etiology include the lack of response to antibiotic therapy, and its self-limited nature, even without treatment. Epidemiologic data supporting a respiratory transmissible etiologic agent include the significant decrease in KD cases worldwide that occurred when COVID-19 mitigation strategies were put in place [10–15]. Research data supporting a viral etiology include the discovery of intracytoplasmic inclusion bodies in KD tissues that are recognized by KD MAbs and have virus-like particles in close proximity [7, 8], and the immune signature of cytotoxic T cell and interferon response in KD coronary arteries [26].
Determining the nucleic acid sequence of the putative KD viral agent is a research priority. Identification of a novel virus in this young patient population is particularly challenging, especially because the target tissues of the disease, the coronary arteries, are unavailable to the researcher except in rare fatal cases, which generally occur months to years after the onset and could therefore contain very low quantities of the agent. We are presently focusing our efforts to identify the agent on preparation of proteins from translated unassigned nucleic acid sequences identified in laser-captured KD intracytoplasmic inclusion bodies from acute KD cases, to determine if they are recognized by sera from KD patients.
Because of some clinical similarities between KD and MIS-C and the observation that some children with MIS-C can have dilation of the coronary arteries during their acute illness, some investigators have postulated that a virus closely related to SARS-CoV-2 might be the cause of KD. However, coronary artery dilation arising from MIS-C is mild, short-lived, and peaks during the acute febrile illness, features that are distinct from KD and similar to what has been observed in other inflammatory conditions associated with marked cytokine release such as systemic onset juvenile idiopathic arthritis [27–29]. Moreover, autopsy studies on fatal MIS-C cases to date have not revealed coronary artery inflammation, which is the hallmark of KD [1, 30–32]. We tested KD MAbs to determine if they showed cross-reactivity with SARS-CoV-2 proteins, with negative results.
Our study has several limitations. Plasmablasts were obtained from children with KD in a single geographic area, because rapid processing of fresh blood is required for optimal recovery of these fragile cells. However, we included patients who presented over a 5-year timespan and obtained similar results regardless of the month or year of onset of KD. It seems doubtful that the triggering agent of KD would be consistent over time in Chicago yet differ from that in other parts of the world. Moreover, the MAbs we made from children with KD in our geographic area identified intracytoplasmic inclusion bodies in ciliated bronchial epithelium of children with KD from other geographic areas of the United States and from Japan [7], suggesting that children from different parts of the world had the same triggering agent for KD. We sequenced approximately 100 plasmablasts per patient, so could have missed additional VH3-74 or other plasmablasts that recognize the KD protein epitope.
CONCLUSION
These results identify a specific protein epitope targeted by VH3-74 plasmablasts encoding a shared CRD3 motif that is common to children with KD. Convergent antibody responses commonly occur to specific antigens of infectious agents [16, 17, 20–22, 33]. The findings support a research focus toward identification of a predominant etiologic agent for KD and provide insights into the pathogenesis of this potentially fatal illness of childhood.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. R. and S. B. designed the experiments. A. R. performed RT-PCR assays and sequencing of single plasmablasts, ELISA of MAbs against peptides, and gathered clinical data. A. T. cloned immunoglobulin sequences, prepared DNA for transfection, performed SARS-CoV-2 ELISA. D. A. performed transfections, produced MAbs, and performed western blot assays. K. K. performed statistical analyses. A. O. prepared figures. A. R. wrote the initial draft of the manuscript, and A. R., D. A., S. S., A. T., A. O., K. W., K. K., and S. B. revised the manuscript and approved the final version.
Financial support. This work was supported by the National Institutes of Health (NIH; grant number R01AI150719 to A. H. R.); the Feitler Family; the Max Goldenberg Foundation; the Ann and Robert H. Lurie Children’s Hospital of Chicago, Center for Kawasaki Disease; and the Northwestern University NUSeq Core Facility and the Northwestern University Flow Cytometry Core Facility supported by National Cancer Institute (grant number NCI CA060553). Flow cytometry cell sorting was performed on a BD FACSAria SORP system, purchased through the support of NIH (grant number 1S10OD011996-01).
References
Author notes
Presented in part: Pediatric Infectious Diseases Society IDWeek 2022, Caroline Breese Hall Lectureship, Washington, DC, 20 October 2022.
Potential conflicts of interest. A. H. R, S. C. B., and S. T. S. are coinventors on provisional patent applications related to this work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




