-
PDF
- Split View
-
Views
-
Cite
Cite
Michelle C Sabo, Nguyen T T Thuong, Xuling Chang, Edwin Ardiansyah, Trinh T B Tram, Hoang T Hai, Ho D T Nghia, Nguyen D Bang, Sofiati Dian, A Rizal Ganiem, Shima Shaporifar, Vinod Kumar, Zheng Li, Martin Hibberd, Chiea Chuen Khor, Guy E Thwaites, Dorothee Heemskerk, Arjan van Laarhoven, Reinout van Crevel, Sarah J Dunstan, Javeed A Shah, MUC5AC Genetic Variation Is Associated With Tuberculous Meningitis Cerebral Spinal Fluid Cytokine Responses and Mortality, The Journal of Infectious Diseases, Volume 228, Issue 3, 1 August 2023, Pages 343–352, https://doi.org/10.1093/infdis/jiad050
Close - Share Icon Share
Abstract
The purpose of this study was to assess if single nucleotide polymorphisms (SNPs) in lung mucins MUC5B and MUC5AC are associated with Mycobacterium tuberculosis outcomes.
Independent SNPs in MUC5B and MUC5AC (genotyped by Illumina HumanOmniExpress array) were assessed for associations with tumor necrosis factor (TNF) concentrations (measured by immunoassay) in cerebral spinal fluid (CSF) from tuberculous meningitis (TBM) patients. SNPs associated with CSF TNF concentrations were carried forward for analyses of pulmonary and meningeal tuberculosis susceptibility and TBM mortality.
MUC5AC SNP rs28737416 T allele was associated with lower CSF concentrations of TNF (P = 1.8 × 10−8) and IFN-γ (P = 2.3 × 10−6). In an additive genetic model, rs28737416 T/T genotype was associated with higher susceptibility to TBM (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.03–1.49; P = .02), but not pulmonary tuberculosis (OR, 1.11, 95% CI, .98–1.25; P = .10). TBM mortality was higher among participants with the rs28737416 T/T and T/C genotypes (35/119, 30.4%) versus the C/C genotype (11/89, 12.4%; log-rank P = .005) in a Vietnam discovery cohort (n = 210), an independent Vietnam validation cohort (n = 87; 9/87, 19.1% vs 1/20, 2.5%; log-rank P = .02), and an Indonesia validation cohort (n = 468, 127/287, 44.3% vs 65/181, 35.9%; log-rank P = .06).
MUC5AC variants may contribute to immune changes that influence TBM outcomes.
Infection due to Mycobacterium tuberculosis leads to over 1 million deaths annually [1]. The clinical phenotype of M. tuberculosis disease ranges from asymptomatic to severe disseminated infection and death [1]. Tuberculous meningitis (TBM) is the deadliest form of M. tuberculosis disease and results in death or neurologic sequelae in over half of affected individuals [2].
Twin, linkage, and genetic studies demonstrate that host genetics influence M. tuberculosis acquisition risk and mortality [3]. Genome-wide association studies have established a link between genes involved in immune function and risk of pulmonary tuberculosis [4–6]. Less is known about host genetic factors that influence TBM susceptibility and severity. In candidate gene studies, variants in Toll-like receptor 2 (TLR2), TLR9, leukotriene A4 hydrolase (LTA4H), and toll interacting protein (TOLLIP) are associated with increased TBM susceptibility [7–10], and LTA4H and CD43 variants are associated with TBM mortality [8, 11–13]. How host genetic variation in nonimmune factors, such as lung mucins, influence M. tuberculosis pathogenesis is unknown.
The primary gel-forming mucins in the lungs, MUC5B and MUC5AC, are encoded by adjacent genes on chromosome 11p15.5 and are essential for lung health [14, 15]. Mice and humans lacking MUC5B develop severe pneumonia and upper airway obstruction [16, 17], suggesting that MUC5B plays an important role in airway clearance. In contrast, overexpression of MUC5B is associated with pulmonary fibrosis [18]. MUC5AC is induced by inflammation and influences pathogen clearance, asthma pathogenesis, and may contribute to M. tuberculosis immune escape during helminthic co-infection by altering inflammatory responses [19–23]. Together, these data suggest that lung mucins strongly influence local and systemic disease outcomes. However, the role of lung mucins on tuberculosis pathogenesis remains unknown.
Genetic studies provide a powerful tool for examining how novel genes may influence M. tuberculosis susceptibility and mortality. Studies of genetically regulated cytokine responses in Bacillus Calmette-Guerin–specific T cells, dendritic cells, and peripheral blood mononuclear cells have identified potential M. tuberculosis susceptibility factors [7, 24–26]. Because cerebral spinal fluid (CSF) tumor necrosis factor (TNF) concentrations have been linked with TBM outcomes and pathogenesis [11, 27], we evaluated variants in the mucin gene region for associations with TNF and other CSF cytokines, followed by evaluation of candidate susceptibility single nucleotide polymorphisms (SNPs) with M. tuberculosis outcomes, including pulmonary tuberculosis and TBM susceptibility, and TBM mortality.
METHODS
Study Subjects and Human Genetic Databases
Subjects were recruited from multiple independent studies (Supplementary Methods). In brief, SNP selection was performed using a genome-wide case-control SNP dataset that included 407 TBM cases [28, 29] and 1139 primary angle closure glaucoma controls (PACG) [30]. The same dataset was used to assess TBM susceptibility. A separate case-control dataset that included 1598 cases of pulmonary tuberculosis [31] and 1268 PACG controls was used to assess pulmonary tuberculosis susceptibility.
The association between SNPs of interest and TBM mortality was assessed in 3 independent cohorts. The Vietnam TBM discovery cohort consisted of 210 human immunodeficiency virus 1 (HIV-1)–seronegative participants enrolled in a study of intensified therapy (including dexamethasone) in Vietnam (ISRCTN clinical trial registry No. ISRCTN6164929) [28]. The Vietnam TBM validation cohort included 87 HIV-1–seronegative participants enrolled in a randomized trial in Vietnam to assess TBM survival with dexamethasone therapy (conducted prior to trial registration requirements) [29]. For consistency with the Vietnam TBM discovery cohort, only participants who received dexamethasone were included in the primary analyses. As dexamethasone may modify the association between genetic polymorphisms and TBM mortality [13], exploratory analyses were performed using data from participants enrolled in the dexamethasone trial (n = 64) who did not receive dexamethasone. No genotyping data were available for HIV-1–seropositive participants in the Vietnam cohorts. The Indonesia TBM validation cohort included 418 HIV-1–seronegative and 50 HIV-1–seropositive participants enrolled in a prospective cohort study of TBM in Indonesia, all of whom received dexamethasone therapy [32, 33]. All participants provided written, informed consent. Country and institution specific ethical approvals were obtained (Supplementary Methods) [28–33].
SNP Selection
Genotyping and SNP quality control are described in the Supplementary Methods. SNPs within and around (± 10 kb) of the MUC5B and MUC5AC gene region were identified from the TBM/PACG genome-wide case-control SNP dataset. Four independent SNPs were selected using PLINK version 1.07 [34] to calculate the linkage disequilibrium (LD) between each SNP pair and then filtering at r2 0.1 to retain independent SNPs.
CSF TNF concentrations may be associated with TBM outcomes, including mortality [11, 27]. We hypothesized that SNPs associated with significantly higher or lower CSF TNF concentrations would be more likely to be associated with TBM outcomes. As a screening step, the 4 independent SNPs identified above were evaluated for associations with log2 concentrations (pg/mL) of TNF in CSF collected at enrollment from 152 participants in the Vietnam TBM discovery cohort. Only SNPs associated with significant changes in log2 TNF CSF concentrations were carried forward.
The association between 55 MUC5AC SNPs and CSF TNF concentrations was assessed using linear regression. Cytokine concentrations and CSF leukocytes were measured as previously described and compared using standard statistical methods (Supplementary Methods) [35]. The association between SNPs of interest and CSF cytokine concentrations was confirmed using data from the Indonesian TBM validation cohort (Supplementary Methods).
Evaluation of Tuberculosis Outcomes
The association between MUC5AC SNP rs28737416 and TBM status was assessed between TBM cases and PACG controls using logistic regression based on an additive genetic model (AA vs Aa vs aa), with adjustment for the first 3 principal components using plink version 1.9 [34]. The association between MUC5AC SNP rs28737416 and pulmonary tuberculosis cases and PACG controls was assessed using the same methods.
Survival analyses were performed in the Vietnam TBM discovery cohort, Vietnam TBM validation cohort, and Indonesia TBM validation cohorts to determine if MUC5AC SNP rs28737416 genotypes were associated with TBM mortality. Time to death was evaluated using Kaplan-Meier estimates and compared by log-rank testing (survival package version 3.2-13, R version 4.0.2). Hazard ratios (HRs) were calculated using the Cox proportional hazards model. Potential confounders, including (1) weight, (2) duration of illness, (3) age, and (4) Glasgow coma score (GCS), were evaluated for associations with the outcomes of interest; those with a P value ≤ .2 were used in adjusted analyses. Information on censoring and missing data are in the Supplementary Methods. To assess if the relationship between MUC5AC SNP rs28737416 genotype and TBM mortality was mediated through changes in concentrations of CSF TNF and interferon-gamma (IFN-γ), Cox models were repeated in the Vietnam TBM discovery cohort with adjustment for concentrations of CSF TNF and/or IFN-γ, and HRs compared to those from the base models (complete case analysis, n = 152).
Evaluation of Expression Quantitative Trait Loci
The association between SNPs and mucin mRNA expression in lung tissue was evaluated using the Genotype-Tissue Expression Project (GTEx) expression quantitative trait loci (eQTL) calculator (https://www.gtexportal.org/home/testyourown, accessed 30 July 2021; Supplementary Methods) [36].
RESULTS
A MUC5AC Promoter Region SNP Is Associated With Lower Concentrations of CSF TNF and IFN-γ in TBM
A total of 126 SNPs were available in the TBM genome-wide SNP dataset within and around (± 10 kb) the MUC5B and MUC5AC gene region. Three independent SNPs were identified in MUC5B (all imputed). One independent SNP (directly genotyped) was identified in the MUC5AC promoter region (Figure 1A). None of the MUC5B or MUC5AC SNPs were in high LD with one another (Figure 1B). MUC5AC SNP rs28737416 was not in LD with TOLLIP gene SNPs (rs5743854 and rs3750920) previously linked to TBM susceptibility (Supplementary Table 1) [7, 25]. Allele frequencies for each SNP are shown for the Kinh Vietnamese population (Figure 1C) and other representative populations (Supplementary Figure 1).
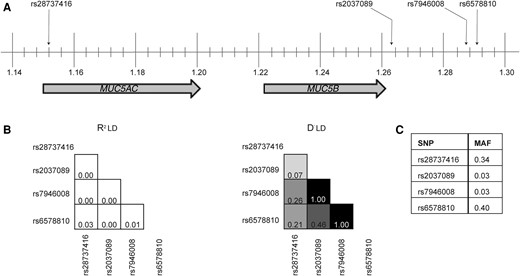
Chromosome map and linkage disequilibrium plots of independent single-nucleotide polymorphisms (SNPs) identified in MUC5B and MUC5AC gene regions. A, MUC5B and MUC5AC genes are located on band 1 of the p arm of chromosome 11; the locations of index SNPs are indicated. Vertical lines represent subband intervals of 0.004. B, Linkage disequilibrium (LD) plots showing R2 (left) and D′ (right) values for the index SNPs. The degree of shading is proportionate to the R2 and D′ values for paired SNPs. C, Minor allele frequency (MAF) for each SNP.
SNPs associated with CSF TNF concentrations may be associated with TBM mortality [11, 27]. As a screening step, we analyzed the association between selected SNPs and CSF TNF concentrations collected from participants in the Vietnam TBM discovery cohort. Using an allelic genetic model (ie, each allele was counted once), the major (C) allele in MUC5AC SNP rs28737416 was associated with a median 2.7-fold log2 higher concentration of CSF TNF compared to the minor (T) allele (P = 7.2 × 10−8). None of the other independent SNPs were associated with changes in TNF concentrations (data not shown). To attempt to localize the functional SNP in MUC5AC, the association between 55 SNPs in MUC5AC and log2 CSF TNF concentrations was assessed (Supplementary Table 2). CSF TNF was highly associated with 54/55 MUC5AC SNPs, and further analysis demonstrated that the entire MUC5AC gene is in high LD in Asian populations (Supplementary Figure 2). Thus, analyses were continued with rs28737416 as the SNP of interest.
Next, alternate genetic models (additive, AA vs Aa vs aa genotypes and dominant, AA vs Aa/aa genotypes) were compared (Figure 2A and 2B). In the dominant model, SNP rs28737416 T/T and T/C genotypes were significantly associated with lower CSF concentrations of TNF (P = 1.8 × 10−8) and IFN-γ (P = 2.3 × 10−6), and higher CSF concentrations of interleukin 2 (IL-2; P = 4.8 × 10−6) and IL-10 (P = .0047) after application of a Bonferroni corrected (0.05/10 cytokines) P value threshold of <.005 (Figure 2B). Based on the strength of the associations, the dominant model was felt to best fit the data and was used in further CSF analyses.

Evaluation of CSF cytokine expression in TBM patients with MUC5AC SNP rs28737416 using additive and dominant genetic models. The associations between MUC5AC SNP rs28737416 and log2 CSF cytokine concentrations (pg/mL) were evaluated using additive (A) and dominant genetic models (B). For the additive model, CSF cytokine concentrations were compared between participants with the T/T (shaded circles), T/C (open circles), or C/C (solid circles) genotypes using the Kruskal-Wallis test. In the dominant model, cytokine concentrations were compared between participants with a composite of the T/T and T/C genotypes (shaded circles) to participants with the C/C genotype (solid circles) using Wilcoxon rank-sum testing. For all graphs, the median is indicated by a thick, horizontal line, and the interquartile range is represented by thin, horizontal lines. To adjust for multiple comparisons, a Bonferroni correction was applied, and the significance threshold adjusted to the level of P < .005. P values for cytokines with statistically significant differences in expression after Bonferroni correction are shown. Abbreviations: CSF, cerebrospinal fluid; IFN-γ, interferon-γ; IL, interleukin; SNP, single-nucleotide polymorphism; TBM, tuberculous meningitis; TNF, tumor necrosis factor.
Principal component analysis of CSF cytokine concentrations generated 2 uncorrelated factors (R2 = 0.08), factor 1 (eigenvalue 5.46, explaining 74.1% variability) and factor 2 (eigenvalue 1.02, explaining 13.8% variability). Whereas all cytokines contributed to the variability in factor 1 (loading values ≥0.56), most of the variability in factor 2 was explained by TNF (loading value = 0.59) and IFN-γ (loading value = 0.57) (Supplementary Results and Supplementary Table 3). Higher values of factor 1 (exponentiated β [expβ] = 0.77; 95% confidence interval [CI], .63–.93; P = .007) were associated with the C/C genotype. However, the association between the C/C genotype and higher factor 2 was much stronger (expβ = 0.60; 95% CI, .49–.74; P = 2.0 × 10−6). These data suggest that CSF TNF and IFN-γ are highly correlated and associated with differences in MUC5AC SNP rs28737416 genotype.
We validated the association of rs28737416 with TNF concentrations using CSF collected from participants in the Indonesia TBM validation cohort. As in the Vietnam TBM discovery cohort, the T/T and T/C genotypes of rs28737416 were associated with lower mean log2 TNF concentrations (estimate = −0.44, P = .05) and IFN-γ (estimate = −0.67, P = .03) compared to the C/C genotype. Associations with additional CSF cytokines are presented in Supplementary Table 4. These data demonstrate the reproducibility of this genetic phenotype in an independent cohort.
We found no significant associations between rs28737416 and total of CSF white blood cells (expβ = 1.23; 95% CI, .90–1.70; P = .19), neutrophils (expβ = 1.05; 95% CI, .83–1.34; P = .68), or lymphocytes (expβ = 1.25; 95% CI, .90–1.74; P = .18) in the Vietnam TBM discovery cohort. These data suggest that MUC5AC SNP rs28737416 T allele is associated with lower CSF concentrations of TNF and IFN-γ in individuals with TBM, independent of CSF cellularity.
MUC5AC SNP rs28737416 Is Associated With TBM Susceptibility and Mortality
Next, we analyzed the association between MUC5AC SNP rs28737416 and TBM susceptibility. In an additive genetic model, the T/T genotype was associated with higher susceptibility to TBM (OR = 1.24; 95% CI, 1.03–1.49; P = .02), but not pulmonary tuberculosis (OR = 1.11; 95% CI, .98–1.25; P = .10) (Table 1).
Association of MUC5AC SNP rs28737416 With Diagnosis of Tuberculous Meningitis and Pulmonary Tuberculosis
| Genotype . | Controlsa . | Cases . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Tuberculous meningitis | ||||
| (n = 1139) | (n = 407) | |||
| C/C | 582 (51.3) | 182 (45.4) | 1.24 (1.03–1.49) | .021 |
| T/C | 464 (40.9) | 169 (42.1) | ||
| T/T | 89 (7.8) | 50 (12.5) | ||
| Pulmonary tuberculosis | ||||
| (n = 1268) | (n = 1598) | |||
| C/C | 655 (51.6) | 662 (48.0) | 1.11 (.98–1.25) | .100 |
| T/C | 510 (40.2) | 588 (42.6) | ||
| T/T | 100 (7.9) | 129 (9.3) | ||
| Genotype . | Controlsa . | Cases . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Tuberculous meningitis | ||||
| (n = 1139) | (n = 407) | |||
| C/C | 582 (51.3) | 182 (45.4) | 1.24 (1.03–1.49) | .021 |
| T/C | 464 (40.9) | 169 (42.1) | ||
| T/T | 89 (7.8) | 50 (12.5) | ||
| Pulmonary tuberculosis | ||||
| (n = 1268) | (n = 1598) | |||
| C/C | 655 (51.6) | 662 (48.0) | 1.11 (.98–1.25) | .100 |
| T/C | 510 (40.2) | 588 (42.6) | ||
| T/T | 100 (7.9) | 129 (9.3) | ||
Data are No. (%).
The odds of being diagnosed with either tuberculous meningitis or pulmonary tuberculosis (analyzed separately) in the presence of the MUC5AC SNP rs28737416 T/T genotype was compared between cases and controls using logistic regression based on an additive genetic model. The total number of individuals with major homozygous (C/C), heterozygous (T/C), and minor homozygous (T/T) genotypes are shown.
Abbreviations: CI, confidence interval; OR, odds ratio; PACG, primary angle closure glaucoma; SNP, single nucleotide polymorphism.
Controls were generated from the same cohort of participants with PACG. The number of controls differs between groups because each dataset (ie, the tuberculous meningitis-PACG dataset and the pulmonary tuberculosis-PACG dataset) were combined prior to SNP quality control. Please see methods for additional details.
Association of MUC5AC SNP rs28737416 With Diagnosis of Tuberculous Meningitis and Pulmonary Tuberculosis
| Genotype . | Controlsa . | Cases . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Tuberculous meningitis | ||||
| (n = 1139) | (n = 407) | |||
| C/C | 582 (51.3) | 182 (45.4) | 1.24 (1.03–1.49) | .021 |
| T/C | 464 (40.9) | 169 (42.1) | ||
| T/T | 89 (7.8) | 50 (12.5) | ||
| Pulmonary tuberculosis | ||||
| (n = 1268) | (n = 1598) | |||
| C/C | 655 (51.6) | 662 (48.0) | 1.11 (.98–1.25) | .100 |
| T/C | 510 (40.2) | 588 (42.6) | ||
| T/T | 100 (7.9) | 129 (9.3) | ||
| Genotype . | Controlsa . | Cases . | OR (95% CI) . | P Value . |
|---|---|---|---|---|
| Tuberculous meningitis | ||||
| (n = 1139) | (n = 407) | |||
| C/C | 582 (51.3) | 182 (45.4) | 1.24 (1.03–1.49) | .021 |
| T/C | 464 (40.9) | 169 (42.1) | ||
| T/T | 89 (7.8) | 50 (12.5) | ||
| Pulmonary tuberculosis | ||||
| (n = 1268) | (n = 1598) | |||
| C/C | 655 (51.6) | 662 (48.0) | 1.11 (.98–1.25) | .100 |
| T/C | 510 (40.2) | 588 (42.6) | ||
| T/T | 100 (7.9) | 129 (9.3) | ||
Data are No. (%).
The odds of being diagnosed with either tuberculous meningitis or pulmonary tuberculosis (analyzed separately) in the presence of the MUC5AC SNP rs28737416 T/T genotype was compared between cases and controls using logistic regression based on an additive genetic model. The total number of individuals with major homozygous (C/C), heterozygous (T/C), and minor homozygous (T/T) genotypes are shown.
Abbreviations: CI, confidence interval; OR, odds ratio; PACG, primary angle closure glaucoma; SNP, single nucleotide polymorphism.
Controls were generated from the same cohort of participants with PACG. The number of controls differs between groups because each dataset (ie, the tuberculous meningitis-PACG dataset and the pulmonary tuberculosis-PACG dataset) were combined prior to SNP quality control. Please see methods for additional details.
Selected genes influence progression of M. tuberculosis disease above susceptibility [12], so we next considered whether MUC5AC SNP rs28737416 was associated with TBM mortality in 3 independent cohorts (Table 2). In the Vietnam TBM discovery cohort, a greater proportion of participants with T/T and T/C genotypes died (35/119, 30.4%) compared to those with the C/C genotype (11/89, 12.4%; P = .005) (Figure 3A), corresponding to a 61% lower risk of death among participants with the C/C genotype (HR = 0.39; 95% CI, .20–.77; P = .006). This association persisted after adjustment for age and GCS (adjusted HR [aHR] = 0.31; 95% CI, .16–.63; P = .001) and in an additive genetic model (Supplementary Figure 3). The dominant genotypic model best fit the data and was applied to subsequent analyses.
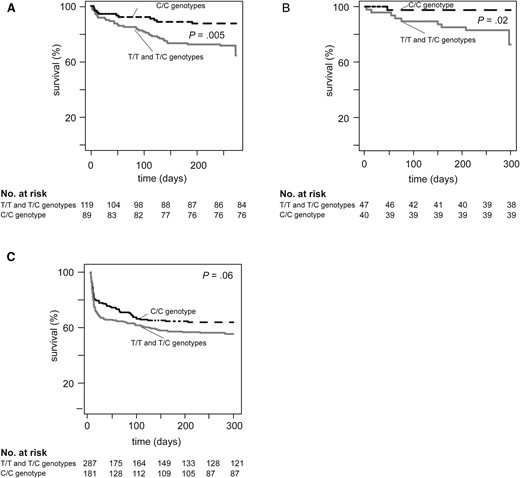
Kaplan-Meier curves comparing survival in a dominant allelic model of MUC5AC SNP rs28737416 in the tuberculous meningitis (TBM) discovery and validation cohorts among participants who received dexamethasone. Kaplan-Meier curves displaying time to death using a dominant allelic model comparing participants with the T/T and T/C genotypes to those with the C/C genotype are shown for (A) Vietnam TBM discovery cohort, (B) Vietnam TBM validation cohort, and (C) Indonesia TBM validation cohort. Displayed P values represent comparison of the survival distributions by the log-rank test.
| General characteristics . | Vietnam TBM Discovery Cohort (n = 210) . | Vietnam TBM Validation Cohort (n = 87) . | Indonesia TBM Validation Cohort (n = 468) . |
|---|---|---|---|
| Age | 43 (28–57) | 41 (25–57)a | 28 (22–37)b |
| Male sex | 131 (62.1%) | 37 (46.8%)a | 230 (55%) |
| Weight, kg | 49 (45–55) | 45 (40–49) | N/Ac |
| Disease characteristics | |||
| Duration of illness prior to presentation, d | 14 (10–30) | 17 (12–30)a | N/Ac |
| CSF WBC count, leukocytes/mm3 | 81 (28–231)d | 209 (82–375) | 155 (53–336) |
| Glasgow coma score | 15 (13–15) | 14 (13–15) | 13 (12–15)b |
| HIV seropositive | 0 (0%) | 0 (0%) | 50 (10.7%) |
| Diagnostic categorye | |||
| Definite TBM | 77 (36.5%) | 51 (58.6%) | 272 (65.1%) |
| Probable TBM | 66 (31.3%) | 27 (31.0%) | 146 (34.9% |
| Possible TBM | 68 (32.2%) | 9 (10.3%) | … |
| General characteristics . | Vietnam TBM Discovery Cohort (n = 210) . | Vietnam TBM Validation Cohort (n = 87) . | Indonesia TBM Validation Cohort (n = 468) . |
|---|---|---|---|
| Age | 43 (28–57) | 41 (25–57)a | 28 (22–37)b |
| Male sex | 131 (62.1%) | 37 (46.8%)a | 230 (55%) |
| Weight, kg | 49 (45–55) | 45 (40–49) | N/Ac |
| Disease characteristics | |||
| Duration of illness prior to presentation, d | 14 (10–30) | 17 (12–30)a | N/Ac |
| CSF WBC count, leukocytes/mm3 | 81 (28–231)d | 209 (82–375) | 155 (53–336) |
| Glasgow coma score | 15 (13–15) | 14 (13–15) | 13 (12–15)b |
| HIV seropositive | 0 (0%) | 0 (0%) | 50 (10.7%) |
| Diagnostic categorye | |||
| Definite TBM | 77 (36.5%) | 51 (58.6%) | 272 (65.1%) |
| Probable TBM | 66 (31.3%) | 27 (31.0%) | 146 (34.9% |
| Possible TBM | 68 (32.2%) | 9 (10.3%) | … |
Results are presented as median (interquartile range) for continuous variables or No. (%) for categorical variables.
Abbreviations: CSF, cerebral spinal fluid; HIV, human immunodeficiency virus; TBM, tuberculosis meningitis; WBC, white blood cell count.
n = 79 due to missingness.
n = 412 due to missingness.
Data not available.
n = 204 due to missingness.
Participant diagnostic category at study end. Diagnostic categories were defined either according to consensus criteria or study-specific criteria as described in the Supplementary Methods. Possible TBM was not defined for the Indonesia TBM validation cohort.
| General characteristics . | Vietnam TBM Discovery Cohort (n = 210) . | Vietnam TBM Validation Cohort (n = 87) . | Indonesia TBM Validation Cohort (n = 468) . |
|---|---|---|---|
| Age | 43 (28–57) | 41 (25–57)a | 28 (22–37)b |
| Male sex | 131 (62.1%) | 37 (46.8%)a | 230 (55%) |
| Weight, kg | 49 (45–55) | 45 (40–49) | N/Ac |
| Disease characteristics | |||
| Duration of illness prior to presentation, d | 14 (10–30) | 17 (12–30)a | N/Ac |
| CSF WBC count, leukocytes/mm3 | 81 (28–231)d | 209 (82–375) | 155 (53–336) |
| Glasgow coma score | 15 (13–15) | 14 (13–15) | 13 (12–15)b |
| HIV seropositive | 0 (0%) | 0 (0%) | 50 (10.7%) |
| Diagnostic categorye | |||
| Definite TBM | 77 (36.5%) | 51 (58.6%) | 272 (65.1%) |
| Probable TBM | 66 (31.3%) | 27 (31.0%) | 146 (34.9% |
| Possible TBM | 68 (32.2%) | 9 (10.3%) | … |
| General characteristics . | Vietnam TBM Discovery Cohort (n = 210) . | Vietnam TBM Validation Cohort (n = 87) . | Indonesia TBM Validation Cohort (n = 468) . |
|---|---|---|---|
| Age | 43 (28–57) | 41 (25–57)a | 28 (22–37)b |
| Male sex | 131 (62.1%) | 37 (46.8%)a | 230 (55%) |
| Weight, kg | 49 (45–55) | 45 (40–49) | N/Ac |
| Disease characteristics | |||
| Duration of illness prior to presentation, d | 14 (10–30) | 17 (12–30)a | N/Ac |
| CSF WBC count, leukocytes/mm3 | 81 (28–231)d | 209 (82–375) | 155 (53–336) |
| Glasgow coma score | 15 (13–15) | 14 (13–15) | 13 (12–15)b |
| HIV seropositive | 0 (0%) | 0 (0%) | 50 (10.7%) |
| Diagnostic categorye | |||
| Definite TBM | 77 (36.5%) | 51 (58.6%) | 272 (65.1%) |
| Probable TBM | 66 (31.3%) | 27 (31.0%) | 146 (34.9% |
| Possible TBM | 68 (32.2%) | 9 (10.3%) | … |
Results are presented as median (interquartile range) for continuous variables or No. (%) for categorical variables.
Abbreviations: CSF, cerebral spinal fluid; HIV, human immunodeficiency virus; TBM, tuberculosis meningitis; WBC, white blood cell count.
n = 79 due to missingness.
n = 412 due to missingness.
Data not available.
n = 204 due to missingness.
Participant diagnostic category at study end. Diagnostic categories were defined either according to consensus criteria or study-specific criteria as described in the Supplementary Methods. Possible TBM was not defined for the Indonesia TBM validation cohort.
In the Vietnam TBM validation cohort, more T/T and T/C participants (9/47, 19.1%) died compared to those with the C/C genotype (1/40, 2.5%; P = .02) (Figure 3B), representing an 87.1% lower risk of death from TBM (HR = 0.13; 95% CI, .02–1.02, P = .05). This association persisted after adjustment for age, weight, and GCS (aHR = 0.13; 95% CI, .02–1.13; P = .06). Analyses of participants in the placebo arm (no dexamethasone therapy) from the same clinical trial did not demonstrate a relationship between MUC5AC genotype and TBM mortality (Supplementary Results and Supplementary Figure 4A) in unadjusted analyses. However, the risk of death among those with the C/C genotype was significantly higher (HR = 4.74; 95% CI, .51–.78; P = .04) after adjustment for GCS.
In the Indonesian TBM validation cohort, a higher number of participants with the rs28737416 T/T and T/C genotypes died (127/287, 44.3%) compared to those with the C/C genotype (65/181, 35.9%, P = .06) (Figure 3C), corresponding to a 20.1% lower risk of death among participants with the C/C genotype (HR = 0.80; 95% CI, .66–.99; P = .04). This association was attenuated after adjustment for age and GCS (aHR = 0.87; 95% CI, .69–1.09; P = .22). In sensitivity analyses using only HIV-seronegative participants, more participants with the T/T and T/C genotypes (106/252, 42.1%) died compared to those with the C/C genotype (56/166, 33.7%, P = .08). The resultant risk of death was 24.8% lower among participants with the C/C genotype, and of borderline statistical significance (HR = 0.75; 95% CI, .54–1.04; P = .08) (Supplementary Figure 4B). Data on the proportional hazards assumptions for all cohorts is included in the Supplementary Results.
A model building approach was used to test if the association between MUC5AC rs28737416 genotype and TBM mortality was mediated by CSF concentrations of TNF and IFN-γ in the Vietnam discovery cohort. In the base model, the C/C genotype was associated with a 71% lower risk of death from TBM (HR = 0.29; 95% CI, .13–.66; P = .003; Table 3). Adjustment for CSF TNF (HR = 0.38; 95% CI, .16–.89; P = .03), IFN-γ (HR = 0.36; 95% CI, .15–.84; P = .02), or both (HR = 0.38, 95% CI, .16–.90; P = .03) attenuated this relationship. As a confirmatory step, the association between TBM mortality and CSF TNF and IFN-γ concentrations was assessed. The risk of death from TBM was 32% lower for each log2 increase in CSF TNF (HR = 0.68; 95% CI, .53–.86; P = .002) and 12% lower for each log2 increase in IFN-γ (HR = 0.88; 95% CI, .80–.96; P = .007). Together, these data suggest that MUC5AC SNP rs28737416 is associated with TBM susceptibility and mortality, and that this relationship is only modestly mediated by changes in CSF TNF and IFN-γ concentrations.
Mediation of the Association Between MUC5AC rs28737416 and TBM Mortality by Concentrations of CSF TNF and IFN-γ in the Vietnam Discovery Cohort
| Model . | HR (95% CI) . | P Value . |
|---|---|---|
| Base model | 0.29 (.13–.66) | .003 |
| Adjusted for log2 TNF | 0.38 (.16–.89) | .03 |
| Adjusted for log2 IFN-γ | 0.36 (.15–.84) | .02 |
| Adjusted for log2 TNF and log2 IFN-γ | 0.38 (.16–.90) | .03 |
| Model . | HR (95% CI) . | P Value . |
|---|---|---|
| Base model | 0.29 (.13–.66) | .003 |
| Adjusted for log2 TNF | 0.38 (.16–.89) | .03 |
| Adjusted for log2 IFN-γ | 0.36 (.15–.84) | .02 |
| Adjusted for log2 TNF and log2 IFN-γ | 0.38 (.16–.90) | .03 |
The Cox proportional hazards model was used to calculate the risk of death (hazard ratio) associated with having the C/C genotype compared to a composite of the C/T and T/T genotypes for participants in the Vietnam discovery cohort for whom CSF cytokine data and genotyping data were available (complete case analysis, n = 152). All models were adjusted for age, Glasgow coma score, and duration of illness prior to presentation. Adjustment for additional variables to assess for mediation was performed as indicated in the row headings. Significant P values are shown in bold.
Abbreviations: CI, confidence interval; HR, hazard ratio; IFN-γ, interferon-γ; TBM, tuberculosis meningitis; TNF, tumor necrosis factor.
Mediation of the Association Between MUC5AC rs28737416 and TBM Mortality by Concentrations of CSF TNF and IFN-γ in the Vietnam Discovery Cohort
| Model . | HR (95% CI) . | P Value . |
|---|---|---|
| Base model | 0.29 (.13–.66) | .003 |
| Adjusted for log2 TNF | 0.38 (.16–.89) | .03 |
| Adjusted for log2 IFN-γ | 0.36 (.15–.84) | .02 |
| Adjusted for log2 TNF and log2 IFN-γ | 0.38 (.16–.90) | .03 |
| Model . | HR (95% CI) . | P Value . |
|---|---|---|
| Base model | 0.29 (.13–.66) | .003 |
| Adjusted for log2 TNF | 0.38 (.16–.89) | .03 |
| Adjusted for log2 IFN-γ | 0.36 (.15–.84) | .02 |
| Adjusted for log2 TNF and log2 IFN-γ | 0.38 (.16–.90) | .03 |
The Cox proportional hazards model was used to calculate the risk of death (hazard ratio) associated with having the C/C genotype compared to a composite of the C/T and T/T genotypes for participants in the Vietnam discovery cohort for whom CSF cytokine data and genotyping data were available (complete case analysis, n = 152). All models were adjusted for age, Glasgow coma score, and duration of illness prior to presentation. Adjustment for additional variables to assess for mediation was performed as indicated in the row headings. Significant P values are shown in bold.
Abbreviations: CI, confidence interval; HR, hazard ratio; IFN-γ, interferon-γ; TBM, tuberculosis meningitis; TNF, tumor necrosis factor.
SNP rs28737416 Is Associated With mRNA Expression of MUC5AC But Not MUC5B
To explore potential mechanisms for the association between mucins and TBM, the association between rs28737416 and MUC5B and MUC5AC mRNA expression in healthy lung tissues was assessed using public datasets [36]. Participants with the T/T genotype had significantly higher median normalized MUC5AC mRNA expression compared to participants with the T/C or C/C genotypes (0.11, 0.06, and −0.05, respectively; normalized effect size [NES] −0.14, P = .009). There was no association between rs28737416 and MUC5B mRNA expression in lung tissues (NES, −0.021, P = .67).
DISCUSSION
This is the first study to link polymorphisms in a mucin gene with M. tuberculosis disease. Specifically, SNP rs28737416 T allele was associated with lower CSF concentrations of TNF and IFN-γ in participants with TBM, higher MUC5AC mRNA in lungs, and higher susceptibility and risk of death from TBM among participants receiving dexamethasone. Mortality from TBM was higher among participants with the C/C genotype who did not receive dexamethasone, suggesting that dexamethasone may modify the association between MUC5AC and TBM mortality survival [13]. The relationship of lower CSF TNF and IFN-γ and higher TBM mortality is consistent with other studies of TBM in persons receiving dexamethasone, which is the current standard of care [11, 27]. However, concentrations of CSF TNF and IFN-γ only modestly mediate the effect of MUC5AC on TBM mortality. Together, these findings suggest that MUC5AC may play an important role in TBM pathogenesis (Figure 4).
![Hypothesis to explain the underlying mechanism linking MUC5AC polymorphisms to TBM mortality. Based on mRNA expression findings from the Genotype-Tissue Expression Project (GTEx) portal, participants with the protective (C/C) rs28737416 MUC5AC genotype may have lower expression of MUC5AC in the lungs compared to those with the susceptible (T/T and T/C) genotypes. Altered MUC5AC expression may enhance mucosal barrier breakdown [37], potentially facilitating dissemination of M. tuberculosis and altering systemic adaptive immune signaling. In the presence of dexamethasone therapy, this results in lower concentrations of CSF TNF and IFN-γ and higher risk of death from TBM, a finding that is consistent with observations among participants with the susceptible LTA4H genotype [11, 27]. The figure was created with BioRender.com. Abbreviations: CSF, cerebrospinal fluid; IFN-γ, interferon-γ; TBM, tuberculous meningitis; TNF, tumor necrosis factor.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/228/3/10.1093_infdis_jiad050/1/m_jiad050f4.jpeg?Expires=1749843525&Signature=ckKpq0KX6~mHJcmND9AMY7SlQ8HixOtPzHX4BeTdDOZyVWQfN5bmTuLUqdi7dQMMp915gMlLklOcU1K2Qb3gXx2W7lNCorur8hZEMrmIP8oVsAkP-2lyjUVEdwNR2ky5OYwJ1gqoEAODFTQ-2UfWNSlLof~a~72gBZ5cWUVRMYF8xYGjZZPYE9PSNMVUd~bwzA4laXXBE-rlPk60pOLo3oEVc37~vGaSubwJAFQflrmjBTEk3gOZ57wIKeI6lq-7erhMzPm4zaGKv5~7oQNS-B3G8gIlg6Dms3wvy4xI9yPTaFMX-a1svT7edgsTpeM98XdzFXiNM8YdCn0pgw10CQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Hypothesis to explain the underlying mechanism linking MUC5AC polymorphisms to TBM mortality. Based on mRNA expression findings from the Genotype-Tissue Expression Project (GTEx) portal, participants with the protective (C/C) rs28737416 MUC5AC genotype may have lower expression of MUC5AC in the lungs compared to those with the susceptible (T/T and T/C) genotypes. Altered MUC5AC expression may enhance mucosal barrier breakdown [37], potentially facilitating dissemination of M. tuberculosis and altering systemic adaptive immune signaling. In the presence of dexamethasone therapy, this results in lower concentrations of CSF TNF and IFN-γ and higher risk of death from TBM, a finding that is consistent with observations among participants with the susceptible LTA4H genotype [11, 27]. The figure was created with BioRender.com. Abbreviations: CSF, cerebrospinal fluid; IFN-γ, interferon-γ; TBM, tuberculous meningitis; TNF, tumor necrosis factor.
Several studies have demonstrated a role for MUC5AC in pulmonary health and disease. For example, MUC5AC is protective against viral infection in mice [21, 22]. However, MUC5AC overexpression is linked to ventilator-associated lung injury in humans [37]. However, this study did not demonstrate an association between the SNP of interest and susceptibility to pulmonary tuberculosis, suggesting that the mechanisms limiting M. tuberculosis progression in the lung may differ from those that prevent extrapulmonary dissemination. Indeed, polymorphisms in TLR2 have also been associated with susceptibility to TBM, but not pulmonary tuberculosis [10]. Alternatively, the TBM susceptibility phenotype may be more penetrant (and thus more easily detected in small cohorts) than the pulmonary tuberculosis phenotype.
How MUC5AC influences TBM pathogenesis is uncertain. One possible explanation is that MUC5AC influences dissemination of M. tuberculosis. Alteration of MUC5AC expression may lead to mucosal barrier disruption [21, 37], which is required for passage of M. tuberculosis-infected alveolar macrophages through the respiratory epithelium during initial infection [38]. Thus, variants that alter MUC5AC expression could facilitate bacterial spread to the CSF. Additionally, enhanced disruption of the mucosal barrier by induced MUC5AC may negatively impact immune control of M. tuberculosis. In murine models of mechanical lung injury, MUC5AC promoted neutrophilic infiltration [37]. In human M. tuberculosis infection, neutrophilic infiltrates are associated with active disease, caseous necrosis, exacerbated pathogenesis, and may favor severe disease phenotypes [39].
In this study, participants with the rs28737416 T/T or T/C genotype demonstrated lower TNF and IFN-γ concentrations in the CSF. How MUC5AC variants alter CSF cytokine expression is unclear, as MUC5AC is not expressed in the central nervous system or within M. tuberculosis-infected myeloid cells [40], and suggests that MUC5AC may modulate systemic immune responses. Indeed, mucin binding to Pseudomonas aeruginosa influences host defense, and O-linked glycans on mucin chains may influence regulatory protein binding and signaling [41]. Furthermore, there is evidence that mucins influence immune homeostasis in the gastrointestinal tract and lungs by binding to Siglec-family receptors or lectin-receptors on macrophages or dendritic cells, respectively [42]. However, the role of MUC5AC in immune homeostasis and inflammatory signaling has not been directly studied. Future directions to understand the role of MUC5AC on systemic inflammation include measuring plasma cytokine levels in persons with and without MUC5AC polymorphisms during infection and assessing for MUC5AC binding to potential receptors using immunoprecipitation and mass spectrometry [43].
It is important to acknowledge that we did not identify the causal SNP influencing MUC5AC expression or function. However, rs28737416 was associated with MUC5AC mRNA expression in public datasets from healthy individuals, and this SNP is in strong LD with MUC5AC eQTLs measured from lungs during asthma exacerbations and with asthma susceptibility alleles [20, 44]. Additionally, the risk of death for the susceptible MUC5AC genotype in the Vietnam TBM discovery cohort (HR = 2.6[1/0.39], as this study reported the HR for the protective genotype) was similar to that of the susceptible genotype for other genetic variants (LTA4H, HR 2.5 and CD43, HR = 2.8) associated with TBM mortality [11, 12]. Future directions to explore these findings include (1) fine mapping to evaluate MUC5AC expression in lung epithelial cells at homeostasis and during exposure to M. tuberculosis-infected macrophages; (2); using site-directed mutagenesis to generate promoter variants (including rs28737416, which is in the promoter region) and assess for functionality in vitro using reporter gene expression; and (3) developing polygenic risk scores based on known susceptibility alleles for TBM, and investigating the combined effects of these alleles on TBM mortality [45].
This study should be interpreted in the context of several limitations. First, there is a potential for confounding due to population structure in the highly homogenous Vietnamese population. However, adjustment for principal components representative of genetic substructure did not significantly alter the results (data not shown), and findings were confirmed in an independent Indonesian cohort. Furthermore, the T/T genotype was present in several diverse populations, including populations of African descent. Given the high rates of tuberculosis disease in Africa, it would be intriguing to assess the association between MUC5AC and TBM in an African cohort in the future. Second, the association between MUC5AC genotypic variants and mortality in the Indonesia TBM validation cohort was not as strong as in the Vietnamese cohorts, possibly due to differences in illness severity at presentation, sample sizes, LD patterns across ethnicity, or environmental influences. However, the similar trend provides additional evidence for an association between MUC5AC and TBM. Third, some participants may have been misclassified at enrollment with TBM. However, TBM diagnosis was based on prespecified criteria, which generally results in random misclassification, which would bias away from finding an association. Fourth, MUC5AC is also expressed in the gastrointestinal tract, and TBM pathogenesis may be modified by changes in gut microbiota that are influenced by MUC5AC [46].
In summary, polymorphisms in MUC5AC are associated with higher lung MUC5AC mRNA expression, lower TNF and IFN-γ in the CSF, and higher TBM susceptibility and mortality in the presence of dexamethasone. These data provide evidence that respiratory mucins play a role in TBM pathogenesis, possibly by facilitating dissemination of M. tuberculosis or influencing the underlying host immune response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the participants in the parent studies used in these analyses. We also acknowledge the clinical, laboratory, and administrative staff at each study site for their dedication and assistance with this project. Finally, we would like to acknowledge the Genotype-Tissue Expression Project (GTEx), which supports the platform on which eQTL analyses were performed. At the time of publication, the GTEx Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by the National Cancer Institute, the National Human Genome Research Institute, the National Heart, Lung, and Blood Institute, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke. The data used for the analyses described in this manuscript were obtained from the GTEx Portal eQTL calculator on 30 July 2021.
Financial support. This work was supported by the National Institute of Child Health and Human Development (grant number K23HD100221 to M. C. S.); the National Institute of Allergy and Infectious Diseases (grant numbers R01 AI136921 to J. A. S., R01 AI145781 to A. v. L., V. K., and R. v. C., and 1P30AI168034-01 to the Seattle TB Research Advancement Center, which supports S. S.); the University of Washington Center for AIDS Research (grant number AI027757 to M. C. S.); the Wellcome Trust (grant numbers 206724/Z/17/Z Intermediate Fellowship in Public Health and Tropical Medicine to N. T. T. T., and 106680/B/14/Z Major Overseas Program Funding to G. T.); and the National Health and Medical Research Council, Australia (grant number APP1056689 to S. J. D.).
References
Author notes
Presented in part: Society for Mucosal Immunology Meeting, July 2022, Seattle, WA.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




