-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth Hastie, Carlee Moser, Xin Sun, Jeffrey Lennox, Priscilla Y Hsue, Ronald J Bosch, Steven Deeks, Milenka V Meneses, Michael M Lederman, Peter Hunt, Timothy J Henrich, Vincent C Marconi, Sara Gianella, Effect of Immune-Modulatory Interventions on Asymptomatic Cytomegalovirus Shedding During Suppressive Antiretroviral Therapy, The Journal of Infectious Diseases, Volume 228, Issue 1, 1 July 2023, Pages 64–69, https://doi.org/10.1093/infdis/jiad060
Close - Share Icon Share
Abstract
Long-term consequences of human immunodeficiency virus (HIV) are likely the result of persistent inflammation and immune dysfunction of which cytomegalovirus (CMV) is a known contributor. We leveraged 2 AIDS Clinical Trials Group clinical trials exploring the effects of immune modulators (ruxolitinib and sirolimus) on inflammation in people with HIV on antiretroviral therapy to determine whether these interventions affected CMV shedding at various mucosal sites. Analyzing 635 mucosal samples collected, we found no significant difference in CMV levels across study arms or time points. Men had more CMV shedding than women. We did confirm an association between higher CMV DNA and immune markers associated with HIV persistence and HIV-associated mortality rates.
The long-term consequences of human immunodeficiency virus (HIV) infection for individuals on suppressive antiretroviral therapy (ART), including accelerated aging and increased end-organ damage, are likely the result of persistent inflammation and immune dysfunction [1, 2]. Two recent clinical trials explored the effect of immune modulators on inflammation in people with HIV (PWH) on suppressive ART: AIDS Clinical Trials Group (ACTG) A5336 and A5337 [3, 4].
ACTG A5336 (NCT02475655) studied ruxolitinib, a Janus kinase (JAK) 1/2 inhibitor. This was a phase IIa multicenter, randomized (2:1), open-label, parallel-arm study conducted in 14 academic sites across the United States. Ruxolitinib was chosen because many of the cytokines induced by JAK–signal transducer and activator of transcription (STAT) signaling, including tumor necrosis factor α and interleukin 6, play a role in the pathogenesis of HIV [5]. Forty healthy individuals on suppressive, stable ART were given 10 mg of ruxolitinib twice daily for 5 weeks. This study revealed that ruxolitinib decreased biomarkers of immune activation and dysregulation, inflammation, and cell survival while HIV virological measures remained unchanged. In addition, the JAK 1/2 inhibitor was well tolerated, with no significant safety events. It was concluded that further studies to elucidate the role of JAK 1/2 inhibitors in reducing systemic inflammation in PWH on suppressive ART are warranted [3].
ACTG A5337 (NCT02440789) studied sirolimus, a mechanistic target of rapamycin (mTOR) inhibitor. This was an open-label, single-arm, pilot study. Sirolimus was chosen because of a retrospective analysis showing that liver and kidney transplant recipients with HIV on immunosuppressive regimens containing sirolimus had lower HIV DNA levels [6]. A total of 32 participants were enrolled in the study, receiving oral sirolimus with an intended treatment duration of 20 weeks. The study revealed a significant decrease in biomarkers of cellular cycling and CD4+ T-cell HIV DNA after sirolimus administration [4]. Of particular interest, mTOR inhibitors, including sirolimus, have been repeatedly associated with fewer cytomegalovirus (CMV) infections in transplant recipients [7, 8]. Current data suggests that this effect is partially due to the ability of mTOR inhibitors to enhance CMV-specific T cells, not just their antiviral properties [8].
With ACTG A5336 and A5337 revealing that immune modulators decreased biomarkers associated with T-cell activation, cell lifespan, immune dysregulation, and inflammation, we were interested in exploring how these interventions might affect a known driver of inflammation in PWH: CMV. Persistent asymptomatic CMV replication is frequent in PWH and is associated with increased systemic immune activation and T-cell dysfunction [9, 10]. The link between CMV and systemic inflammation is complex and has been the focus of intensive investigation. In addition to eliciting a strong CMV-specific T-cell response, CMV shedding is also associated with increased systemic inflammation and bystander cellular activation [9–13]. Higher anti-CMV immunoglobulin G (IgG) levels in plasma were also associated with steeper CD4+ T-cell decay in elite controllers and with epithelial gut damage in people with or without HIV [14, 15]. CMV replication might also be associated with increased size of HIV reservoirs [2, 9, 10].
While it seems intuitive that immunosuppressive interventions might increase CMV shedding, immune modulators, like ruxolitinib and sirolimus, could suppress viral shedding by reducing inflammation and enhancing immune response. Therefore, the objectives of the current substudy were (1) to investigate whether 2 immune modulators (ruxolitinib and sirolimus) affected CMV shedding at various mucosal sites and (2) to elucidate a possible contributing effect of CMV shedding on inflammatory outcomes in PWH.
METHODS
Study Design
ACTG A5351s was a prospective, observational, optional substudy of PWH on suppressive ART who were receiving an immune-modulatory intervention as a part of 2 approved ACTG parent protocols: A5336 (ruxolitinib vs open-label ART) and A5337 (sirolimus; single arm). Participants provided genital secretions (semen or vaginal swab samples), oral swab samples, and urine samples, which were assayed for CMV DNA. These samples were collected at study entry and longitudinally at 5 time points over 12 weeks for A5336 and 6 time points over 44 weeks for A5337. Stored plasma samples were used to assay CMV IgG at study entry in both studies. Immunological biomarkers previously generated from A5336 samples were also available for this analysis.
Participants
A subset of participants from A5336 and A5337 were coenrolled into this substudy, including PWH who were ≥18 years of age on stable, suppressive ART for ≥ 2 months before study entry. This trial was approved by the institutional review board of each institution. All participants provided written, informed consent.
Procedures
Participants were enrolled for the same duration as in their parent protocol. For A5336, participants were followed up for up to 12 weeks. For A5337, the duration of the study was 44 weeks (12 weeks before sirolimus treatment, 20 weeks on sirolimus, and an additional 12 weeks off sirolimus). A5337 participants could coenroll in this substudy at any time before the start of sirolimus treatment at week 12.
Outcomes
Primary outcome measures included frequency and levels of genital, oral, and urinary CMV DNA measured with Taqman polymerase chain reaction, as described elsewhere [16]. Secondary outcome measures included levels of immunological biomarkers previously obtained from A5336 samples and plasma CMV IgG levels at study entry [3]. High-sensitivity enzyme-linked immunosorbent assay was used to measure interleukin 6; standard enzyme-linked immunosorbent and microarray analyses were used to measure soluble CD14, tumor necrosis factor α, interleukin 1β, 7, 10, 15, and 18, and transforming growth factor β1/2/3; and flow cytometry, and microarray panels were used to measure HLA-DR/CD38, CD25, CD127, Bcl-2, Ki67, α4β7, and lymphocyte subsets.
Statistical Analysis
The analyses included all participants who coenrolled in A5351s who also met eligibility to be included in efficacy analyses for the parent trial. Analyses were conducted separately by parent study and individually by anatomic sites. CMV DNA levels from genital secretion samples were compared between A5336 arms at each time point using Wilcoxon rank sum test. In addition, the proportions with detectable CMV DNA were compared between A5336 arms at each time point, using a 2-sided mid-P Fisher exact test. Analyses of A5337 were limited to descriptive summaries owing to the small number of participants who coenrolled in A5351s. Relationships between CMV DNA and biomarkers measured in A5336 were described with Spearman correlations, with an emphasis on magnitude of effect sizes owing to the limited sample size. Individuals with undetectable CMV DNA or CMV IgG levels were assigned the lowest rank. All inferential analyses were assessed with a 5% significance level; owing to the exploratory nature of this analysis, no adjustments for multiple comparisons were made. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Thirty-seven participants from A5336 and 9 from A5337 were coenrolled in this substudy; 2 participants, 1 from each study, were excluded from our analysis population as they were not included in the efficacy analysis population in the parent study. Overall, 635 samples were collected: 509 from A5336 and 126 from A5337 participants. Of these, 190 samples were from genital secretions, 227 from oral swab samples, and 218 from urine samples. All samples were tested for CMV DNA (Supplementary Figure 1).
The median age was 49 years (range, 25–62 years). Seventy-seven percent of participants (n = 34) were male sex at birth; 47% (n = 20) were black non-Hispanic, 36% (n = 16) white non-Hispanic, and 11% (n = 5) Hispanic. The median baseline CD4+ T-cell count was 859/μL (range, 406–1858/μL). All participants but 1 (n = 43) had plasma HIV RNA levels <40 copies/mL. The baseline CD4+/CD8+ T-cell ratio was >1 in 61% of participants. The most common ART regimen a combined efavirenz, emtricitabine, and tenofovir. All but 2 participants had a detectable plasma CMV IgG level at study entry (Table 1).
| . | Participants, No. (%)a . | |||
|---|---|---|---|---|
| . | . | A5336 . | . | |
| Characteristic . | Total (N = 44) . | Ruxolitinib (n = 26) . | No Treatment (n = 10) . | A5337 (n = 8) . |
| Age, y | ||||
| Median (IQR) | 49 (43–55) | 49.5 (46–55) | 43 (33–49) | 51 (46–545) |
| Range | 25–62 | 29–62 | 25–58 | 27–62 |
| Sex | ||||
| Male | 34 (77) | 21 (81) | 7 (70) | 6 (75) |
| Female | 10 (23) | 5 (19) | 3 (30) | 2 (25) |
| Race/ethnicity | ||||
| White non-Hispanic | 16 (37) | 9 (36) | 3 (30) | 4 (50) |
| Black non-Hispanic | 20 (47) | 12 (48) | 5 (50) | 3 (38) |
| Hispanic (any race) | 5 (12) | 3 (12) | 1 (10) | 1 (13) |
| >1 Race | 2 (5) | 1 (4) | 1 (10) | 0 (0) |
| History of intravenous drug use | ||||
| Never | 36 (82) | 20 (77) | 9 (90) | 7 (88) |
| Previously | 8 (18) | 6 (23) | 1 (10) | 1 (13) |
| BMIb | ||||
| Normal (18.5 to <25) | 10 (23) | 6 (23) | 1 (10) | 3 (38) |
| Overweight (25 to <30) | 14 (32) | 8 (31) | 4 (40) | 2 (25) |
| Obese (≥30) | 20 (45) | 12 (46) | 5 (50) | 3 (38) |
| Baseline CD4+/CD+8 T-cell ratio | ||||
| <0.5 | 1 (2) | 1 (4) | 0 (0) | 0 (0) |
| 0.5–1 | 16 (36) | 9 (35) | 3 (30) | 4 (50) |
| >1 | 27 (61) | 16 (62) | 7 (70) | 4 (50) |
| ART regimen | ||||
| EFV/FTC/TDF | 14 (32) | 7 (27) | 3 (30) | 4 (50) |
| ABC/DTG/3TC | 7 (16) | 6 (23) | 0 (0) | 1 (13) |
| FTC/RPV/TAF | 6 (14) | 3 (12) | 3 (30) | 0 (0) |
| RAL and FTC/TAF | 4 (9) | 2 (8) | 2 (20) | 0 (0) |
| TRV and RAL | 4 (9) | 3 (12) | 1 (10) | 0 (0) |
| DTG and FTC/TAF | 3 (7) | 2 (8) | 1 (10) | 0 (0) |
| Other | 6 (14) | 3 (12) | 0 (0) | 3 (38) |
| Entry CMV levels | ||||
| Genital secretion sample | ||||
| Median (IQR), copies/mL | 0 (0–38) | 0 (0–2604) | 0 (0–38) | 0 (0–0) |
| Undetectable | 27 (73) | 15 (71) | 6 (67) | 6 (86) |
| Detectable | 10 (27) | 6 (29) | 3 (33) | 1 (14) |
| Oral swab sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 41 (93) | 23 (88) | 10 (100) | 8 (100) |
| Detectable | 3 (7) | 3 (12) | 0 (0) | 0 (0) |
| Urine sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 38 (86) | 22 (85) | 8 (80) | 8 (100) |
| Detectable | 6 (14) | 4 (15) | 2 (20) | 0 (0) |
| Entry CMV IgG level | ||||
| Median (IQR), IU/mL | 31.7 (21.2–41.0) | 34.4 (25.8–41.5) | 22.1 (18.6–39.3) | 31.3 (24.9–41.1) |
| Undetectable | 5 (11) | 3 (12) | 1 (10) | 1 (13) |
| Detectable | 39 (89) | 23 (88) | 9 (90) | 7 (88) |
| . | Participants, No. (%)a . | |||
|---|---|---|---|---|
| . | . | A5336 . | . | |
| Characteristic . | Total (N = 44) . | Ruxolitinib (n = 26) . | No Treatment (n = 10) . | A5337 (n = 8) . |
| Age, y | ||||
| Median (IQR) | 49 (43–55) | 49.5 (46–55) | 43 (33–49) | 51 (46–545) |
| Range | 25–62 | 29–62 | 25–58 | 27–62 |
| Sex | ||||
| Male | 34 (77) | 21 (81) | 7 (70) | 6 (75) |
| Female | 10 (23) | 5 (19) | 3 (30) | 2 (25) |
| Race/ethnicity | ||||
| White non-Hispanic | 16 (37) | 9 (36) | 3 (30) | 4 (50) |
| Black non-Hispanic | 20 (47) | 12 (48) | 5 (50) | 3 (38) |
| Hispanic (any race) | 5 (12) | 3 (12) | 1 (10) | 1 (13) |
| >1 Race | 2 (5) | 1 (4) | 1 (10) | 0 (0) |
| History of intravenous drug use | ||||
| Never | 36 (82) | 20 (77) | 9 (90) | 7 (88) |
| Previously | 8 (18) | 6 (23) | 1 (10) | 1 (13) |
| BMIb | ||||
| Normal (18.5 to <25) | 10 (23) | 6 (23) | 1 (10) | 3 (38) |
| Overweight (25 to <30) | 14 (32) | 8 (31) | 4 (40) | 2 (25) |
| Obese (≥30) | 20 (45) | 12 (46) | 5 (50) | 3 (38) |
| Baseline CD4+/CD+8 T-cell ratio | ||||
| <0.5 | 1 (2) | 1 (4) | 0 (0) | 0 (0) |
| 0.5–1 | 16 (36) | 9 (35) | 3 (30) | 4 (50) |
| >1 | 27 (61) | 16 (62) | 7 (70) | 4 (50) |
| ART regimen | ||||
| EFV/FTC/TDF | 14 (32) | 7 (27) | 3 (30) | 4 (50) |
| ABC/DTG/3TC | 7 (16) | 6 (23) | 0 (0) | 1 (13) |
| FTC/RPV/TAF | 6 (14) | 3 (12) | 3 (30) | 0 (0) |
| RAL and FTC/TAF | 4 (9) | 2 (8) | 2 (20) | 0 (0) |
| TRV and RAL | 4 (9) | 3 (12) | 1 (10) | 0 (0) |
| DTG and FTC/TAF | 3 (7) | 2 (8) | 1 (10) | 0 (0) |
| Other | 6 (14) | 3 (12) | 0 (0) | 3 (38) |
| Entry CMV levels | ||||
| Genital secretion sample | ||||
| Median (IQR), copies/mL | 0 (0–38) | 0 (0–2604) | 0 (0–38) | 0 (0–0) |
| Undetectable | 27 (73) | 15 (71) | 6 (67) | 6 (86) |
| Detectable | 10 (27) | 6 (29) | 3 (33) | 1 (14) |
| Oral swab sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 41 (93) | 23 (88) | 10 (100) | 8 (100) |
| Detectable | 3 (7) | 3 (12) | 0 (0) | 0 (0) |
| Urine sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 38 (86) | 22 (85) | 8 (80) | 8 (100) |
| Detectable | 6 (14) | 4 (15) | 2 (20) | 0 (0) |
| Entry CMV IgG level | ||||
| Median (IQR), IU/mL | 31.7 (21.2–41.0) | 34.4 (25.8–41.5) | 22.1 (18.6–39.3) | 31.3 (24.9–41.1) |
| Undetectable | 5 (11) | 3 (12) | 1 (10) | 1 (13) |
| Detectable | 39 (89) | 23 (88) | 9 (90) | 7 (88) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; BMI, body mass index; CMV, cytomegalovirus; DTG, dolutegravir; FTC, emtricitabine; IgG, immunoglobulin G; IQR, interquartile range; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir; TRV, truvada (Emtricitabine/Tenofovir).
Data represent no. (%) of participants unless otherwise specified.
BMI calculated as weight in kilograms divided by height in meters squared.
| . | Participants, No. (%)a . | |||
|---|---|---|---|---|
| . | . | A5336 . | . | |
| Characteristic . | Total (N = 44) . | Ruxolitinib (n = 26) . | No Treatment (n = 10) . | A5337 (n = 8) . |
| Age, y | ||||
| Median (IQR) | 49 (43–55) | 49.5 (46–55) | 43 (33–49) | 51 (46–545) |
| Range | 25–62 | 29–62 | 25–58 | 27–62 |
| Sex | ||||
| Male | 34 (77) | 21 (81) | 7 (70) | 6 (75) |
| Female | 10 (23) | 5 (19) | 3 (30) | 2 (25) |
| Race/ethnicity | ||||
| White non-Hispanic | 16 (37) | 9 (36) | 3 (30) | 4 (50) |
| Black non-Hispanic | 20 (47) | 12 (48) | 5 (50) | 3 (38) |
| Hispanic (any race) | 5 (12) | 3 (12) | 1 (10) | 1 (13) |
| >1 Race | 2 (5) | 1 (4) | 1 (10) | 0 (0) |
| History of intravenous drug use | ||||
| Never | 36 (82) | 20 (77) | 9 (90) | 7 (88) |
| Previously | 8 (18) | 6 (23) | 1 (10) | 1 (13) |
| BMIb | ||||
| Normal (18.5 to <25) | 10 (23) | 6 (23) | 1 (10) | 3 (38) |
| Overweight (25 to <30) | 14 (32) | 8 (31) | 4 (40) | 2 (25) |
| Obese (≥30) | 20 (45) | 12 (46) | 5 (50) | 3 (38) |
| Baseline CD4+/CD+8 T-cell ratio | ||||
| <0.5 | 1 (2) | 1 (4) | 0 (0) | 0 (0) |
| 0.5–1 | 16 (36) | 9 (35) | 3 (30) | 4 (50) |
| >1 | 27 (61) | 16 (62) | 7 (70) | 4 (50) |
| ART regimen | ||||
| EFV/FTC/TDF | 14 (32) | 7 (27) | 3 (30) | 4 (50) |
| ABC/DTG/3TC | 7 (16) | 6 (23) | 0 (0) | 1 (13) |
| FTC/RPV/TAF | 6 (14) | 3 (12) | 3 (30) | 0 (0) |
| RAL and FTC/TAF | 4 (9) | 2 (8) | 2 (20) | 0 (0) |
| TRV and RAL | 4 (9) | 3 (12) | 1 (10) | 0 (0) |
| DTG and FTC/TAF | 3 (7) | 2 (8) | 1 (10) | 0 (0) |
| Other | 6 (14) | 3 (12) | 0 (0) | 3 (38) |
| Entry CMV levels | ||||
| Genital secretion sample | ||||
| Median (IQR), copies/mL | 0 (0–38) | 0 (0–2604) | 0 (0–38) | 0 (0–0) |
| Undetectable | 27 (73) | 15 (71) | 6 (67) | 6 (86) |
| Detectable | 10 (27) | 6 (29) | 3 (33) | 1 (14) |
| Oral swab sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 41 (93) | 23 (88) | 10 (100) | 8 (100) |
| Detectable | 3 (7) | 3 (12) | 0 (0) | 0 (0) |
| Urine sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 38 (86) | 22 (85) | 8 (80) | 8 (100) |
| Detectable | 6 (14) | 4 (15) | 2 (20) | 0 (0) |
| Entry CMV IgG level | ||||
| Median (IQR), IU/mL | 31.7 (21.2–41.0) | 34.4 (25.8–41.5) | 22.1 (18.6–39.3) | 31.3 (24.9–41.1) |
| Undetectable | 5 (11) | 3 (12) | 1 (10) | 1 (13) |
| Detectable | 39 (89) | 23 (88) | 9 (90) | 7 (88) |
| . | Participants, No. (%)a . | |||
|---|---|---|---|---|
| . | . | A5336 . | . | |
| Characteristic . | Total (N = 44) . | Ruxolitinib (n = 26) . | No Treatment (n = 10) . | A5337 (n = 8) . |
| Age, y | ||||
| Median (IQR) | 49 (43–55) | 49.5 (46–55) | 43 (33–49) | 51 (46–545) |
| Range | 25–62 | 29–62 | 25–58 | 27–62 |
| Sex | ||||
| Male | 34 (77) | 21 (81) | 7 (70) | 6 (75) |
| Female | 10 (23) | 5 (19) | 3 (30) | 2 (25) |
| Race/ethnicity | ||||
| White non-Hispanic | 16 (37) | 9 (36) | 3 (30) | 4 (50) |
| Black non-Hispanic | 20 (47) | 12 (48) | 5 (50) | 3 (38) |
| Hispanic (any race) | 5 (12) | 3 (12) | 1 (10) | 1 (13) |
| >1 Race | 2 (5) | 1 (4) | 1 (10) | 0 (0) |
| History of intravenous drug use | ||||
| Never | 36 (82) | 20 (77) | 9 (90) | 7 (88) |
| Previously | 8 (18) | 6 (23) | 1 (10) | 1 (13) |
| BMIb | ||||
| Normal (18.5 to <25) | 10 (23) | 6 (23) | 1 (10) | 3 (38) |
| Overweight (25 to <30) | 14 (32) | 8 (31) | 4 (40) | 2 (25) |
| Obese (≥30) | 20 (45) | 12 (46) | 5 (50) | 3 (38) |
| Baseline CD4+/CD+8 T-cell ratio | ||||
| <0.5 | 1 (2) | 1 (4) | 0 (0) | 0 (0) |
| 0.5–1 | 16 (36) | 9 (35) | 3 (30) | 4 (50) |
| >1 | 27 (61) | 16 (62) | 7 (70) | 4 (50) |
| ART regimen | ||||
| EFV/FTC/TDF | 14 (32) | 7 (27) | 3 (30) | 4 (50) |
| ABC/DTG/3TC | 7 (16) | 6 (23) | 0 (0) | 1 (13) |
| FTC/RPV/TAF | 6 (14) | 3 (12) | 3 (30) | 0 (0) |
| RAL and FTC/TAF | 4 (9) | 2 (8) | 2 (20) | 0 (0) |
| TRV and RAL | 4 (9) | 3 (12) | 1 (10) | 0 (0) |
| DTG and FTC/TAF | 3 (7) | 2 (8) | 1 (10) | 0 (0) |
| Other | 6 (14) | 3 (12) | 0 (0) | 3 (38) |
| Entry CMV levels | ||||
| Genital secretion sample | ||||
| Median (IQR), copies/mL | 0 (0–38) | 0 (0–2604) | 0 (0–38) | 0 (0–0) |
| Undetectable | 27 (73) | 15 (71) | 6 (67) | 6 (86) |
| Detectable | 10 (27) | 6 (29) | 3 (33) | 1 (14) |
| Oral swab sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 41 (93) | 23 (88) | 10 (100) | 8 (100) |
| Detectable | 3 (7) | 3 (12) | 0 (0) | 0 (0) |
| Urine sample | ||||
| Median (IQR), copies/mL | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Undetectable | 38 (86) | 22 (85) | 8 (80) | 8 (100) |
| Detectable | 6 (14) | 4 (15) | 2 (20) | 0 (0) |
| Entry CMV IgG level | ||||
| Median (IQR), IU/mL | 31.7 (21.2–41.0) | 34.4 (25.8–41.5) | 22.1 (18.6–39.3) | 31.3 (24.9–41.1) |
| Undetectable | 5 (11) | 3 (12) | 1 (10) | 1 (13) |
| Detectable | 39 (89) | 23 (88) | 9 (90) | 7 (88) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; BMI, body mass index; CMV, cytomegalovirus; DTG, dolutegravir; FTC, emtricitabine; IgG, immunoglobulin G; IQR, interquartile range; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir; TRV, truvada (Emtricitabine/Tenofovir).
Data represent no. (%) of participants unless otherwise specified.
BMI calculated as weight in kilograms divided by height in meters squared.
Effect of Immune Modulators on CMV Levels
Overall and at any time point, most participants had undetectable CMV levels at all 3 sample sites (Figure 1A). CMV DNA was detected in 14 participants (31%) in genital secretion samples, 11 (25%) in oral swab samples, and 10 (23%) in urine samples. As described elsewhere, CMV DNA was detected more commonly in samples obtained from men than in those from women [17]. Among the 10 enrolled women, only 1 was positive across the 3 sample types; however, among men, 13 (38%), 10 (29%), and 9 (26%) had detectable CMV DNA in genital secretion, oral swab, and urine samples, respectively.
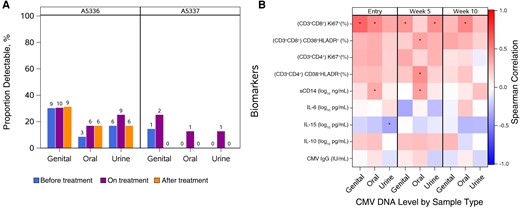
A, Proportion of individuals with detectable cytomegalovirus (CMV) by parent study, study visit, and sample type. In A5336, 36 people provided oral swab and urine samples across study visits; 30, 33, and 29 provided genital secretion samples (semen or vaginal swab samples) before, during, and after treatment, respectively. In A5337, 8 people provided oral swab samples across the study visits; 8 provided urine samples before and during treatment but only 5 after treatment; and 7, 8, and 5, respectively, provided genital secretion samples before, during, and after treatment. B, Correlations between CMV DNA level obtained from 3 sites and serum inflammatory biomarkers in A5336 participants at 3 time points: entry, week 5, and week 10. *P < .05. Abbreviations: IgG, immunoglobulin G; IL-6, interleukin 6; IL-10, interleukin 10; IL-15, interleukin 15; sCD14, soluble CD14.
There was no significant difference in CMV DNA levels between study arms in A5336 (P > .36 for both Wilcoxon and Fisher test) (Supplementary Figure 2). In A5337, differences were not observed in CMV DNA levels across time points (Supplementary Figure 3); comparisons are limited to descriptive summaries.
Association Between CMV and Markers of Immune Activation and Inflammation
Subsequently, we examined correlations between CMV DNA levels and plasma CMV IgG at study entry and immunological markers collected as part of A5336, pooling across arms to increase sample size and power. We observed a consistent positive correlation between CMV DNA and the percentages of Ki67+ cells among CD4+ and CD8+ T cells (r values ranging from 0.11 to 0.62; P values, from .58 to <.001) and of CD38+HLA-DR+ cells among CD8+ T cells (r values ranging from 0.14 to 0.37; P values, from .50 to .03) across time points and sample sites. Plasma CMV IgG levels at study entry were not correlated with CMV DNA from any sample type or time point (Figure 1B and Supplementary Table 1).
DISCUSSION
Despite achieving viral suppression with ART, PWH have significantly earlier onset of comorbid conditions than persons without HIV [18]. With the population of PWH aging, it is pertinent to explore whether limiting CMV replication in PWH affects levels of inflammation and long-term morbidity [13, 17, 18]
Leveraging 2 ongoing clinical trials of immune modulators, we were interested in determining if the observed effects on immunological outcomes might be partially attributable to an impact on CMV shedding. This hypothesis was based on research demonstrating CMV shedding to be a driver of systemic inflammation and immune dysfunction in PWH [1, 2, 9, 10].
Overall, our study confirmed that CMV DNA was most commonly found in genital secretion samples (29% of genital vs 11% of oral swab and 18% of urinary samples) and more frequently in men than in women. Based on these observations, future trials designed to evaluate the presence of CMV DNA should collect genital secretion samples, when possible, to optimize the ability to find this virus in male participants. Other mucosal sites may be more appropriate for female participants.
Furthermore, we did not find a significant effect on CMV DNA shedding with ruxolitinib or sirolimus in PWH on suppressive ART. The absence of an increase in CMV shedding is a noteworthy observation for the safety profile of these immune modulators in individuals with CMV infections.
Our study did confirm an association between higher CMV DNA and increased expression of Ki67 in CD4+ and CD8+ T cells, as well as higher levels of CD38+HLA-DR+ CD8+ T cells, previously described by our group and others [19]. These associations could have implications for the role of CMV in HIV persistence through expansion of activated cycling CD4+ T cells and CD8+ T cells, both drivers of inflammation, and through a lower CD4+/CD8+ T-cell ratio, which is predictive of poor clinical outcome. One additional noteworthy observation was the consistent, negative association between CMV DNA shedding and interleukin 15. This proinflammatory cytokine is generated in response to viral infection but is also involved in enhancing viral control [20, 21]. Research is underway to investigate its therapeutic potential [22, 23].
Unfortunately, the modest sample size of 44 participants and the high rate of undetectable CMV DNA at mucosal sites limited our power to observe any significant differences. Eligibility criteria restricted trial participation to a unique population of generally healthy PWH on suppressive ART who are less likely to shed CMV, potentially making CMV-associated inflammation and CMV effects less observable. Finally, we cannot establish causality in our correlative analysis, which was pooled across study arms.
Further research is warranted to explore whether limiting CMV replication in PWH, through immunization or CMV-targeted antivirals, affects levels of inflammation [24, 25] and long-term morbidity. Several studies are already investigating the effect of anti-CMV interventions on HIV immune outcomes, including ACTG A5355 (ClinicalTrials.gov NCT05099965), a randomized, placebo-controlled trial investigating the safety and immunogenicity of a CMV vaccine in CMV seropositive PWH, and ACTG A5383 (ClinicalTrials.gov NCT04840199), a randomized, controlled trial evaluating the effects of letermovir in CMV-seropositive PWH on ART.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the James B. Pendleton Charitable Trust, the Center for AIDS Research Translational Virology (TV) Core, and all study participants and enrolling sites, as well as the parent study teams.
Author contributions. E. H., C. M., J. L., P. Y. H., S. D., M. M. L., P. H., T. J. H., V. C. M., and S. G. assisted with study design. J. L., P. Y. H., S. D., T. J. H., and V. C. M. chaired the parent studies and assisted with enrollment and recruitment of study participants. M. V. M. assisted with data generation. E. H., C. M., X. S., R. J. B., and S. G. assisted with data analysis. E. H., C. M., and S. G. wrote the primary manuscript, which was reviewed and edited by all authors.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants UM1 AI068634, UM1 AI068636, and UM1 AI106701).
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




