-
PDF
- Split View
-
Views
-
Cite
Cite
Ka-Li Zhu, Hui-Xia Gao, Lin Yao, Jun Rong, Li Yang, Zhi Zhang, Ping Jiang, Li-Jun Duan, Guo-Lin Wang, Er-Hei Dai, Mai-Juan Ma, Delta Infection After Vaccination Elicits Potent Neutralizing Immunity Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron, The Journal of Infectious Diseases, Volume 226, Issue 9, 1 November 2022, Pages 1551–1555, https://doi.org/10.1093/infdis/jiac149
Close - Share Icon Share
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron (B.1.1.529) variant extensively escape neutralizing antibodies by vaccines or infection. We assessed serum neutralizing activity in sera from Delta infection after vaccination and Delta infection only against SARS-CoV-2 Wuhan-Hu-1 (WA1), Beta, Delta, and Omicron. Sera from Delta infection only could neutralize WA1 and Delta but almost completely lost capacity to neutralize Beta and Omicron. However, Delta infection after vaccination resulted in a significant increase of serum neutralizing activity against WA1, Beta, and Omicron. This study demonstrates that breakthrough infection of Delta substantially induced high potency humoral immune response against the Omicron variant and other emerged variants.
Since the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in late 2019, several SARS-CoV-2 variants of concern (VOCs), including B.1.1.7 (Alpha) in the United Kingdom (UK), B.1.351 (Beta) in South Africa, P.1 (Gamma) in Brazil, and B.1.617.2 (Delta), have successively emerged with multiple substitutions in the spike glycoprotein. Among these 4 VOCs, the Beta variant showed the greatest immune evasion from serum neutralizing antibodies [1] and compromised the efficacy of vaccines [2, 3], whereas the Delta quickly outcompeted all other VOCs since first identified in October 2020 in India and partially escaped neutralization in vitro [4]. The Omicron variant (B.1.1.529) was first detected in November 2021 in South Africa and has spread rapidly across the globe, outcompeting Delta within weeks to become the dominant circulating variant in several countries [5]. The large number of over 30 mutations in the spike protein of the Omicron resulted in extensively decreased neutralization activity of certain monoclonal and serum polyclonal antibodies elicited by infection or vaccination [6, 7].
Before the emergence of the Omicron variant, high levels of Delta infections in many countries and reduced effectiveness of vaccines in preventing infection and transmission of the Delta variant [8] might already result in tens of millions of infections in naive or vaccinated individuals. Amidst rapid expansion of Omicron infections worldwide and extensive escape from immunity elicited by vaccines and previous infection, understanding residual neutralizing activity in Delta-infected individuals against the Omicron is essential to gauge the level of protection that a specific community has against infection and mild or severe coronavirus disease 2019 (COVID-19). However, little is known about the susceptibility of Omicron to neutralizing antibodies elicited by Delta infection after vaccination and Delta infection only.
METHODS
Ethical Approval
The study was approved by the Institutional Review Board (IRB) of the Beijing Institute of Microbiology and Epidemiology (IRB number AF/SC-08/02.60 and AF/SC-08/02.124). All enrolled participants provided written informed consent.
Sera From Patients With the Delta Infection After Vaccination
Between October and November 2021, an outbreak of the Delta variant was identified, and 213 patients were reported in Shijiazhuang City, Hebei Province, China, including 39 patients under the age of 18. Among 174 patients over 18 years old, 172 patients had received 2 doses of CoronaVac or 3 doses of ZF2001 (a receptor-binding domain-based protein subunit vaccine; Anhui Zhifei Longcom), whereas 37 of 39 patients under 18 years old had not received any vaccines. Five of 213 patients had sequence-confirmed Delta infection, and the others were polymerase chain reaction (PCR)-confirmed, with symptomatic disease occurring while in isolation and in direct contact with Delta sequence-confirmed cases. At the time of discharge from the hospital, 31 patients signed an informed consent form and agreed to provide data and blood samples.
Sera From Patients With the Delta Infection Only
In April 2021, a cargo ship arrived at a dockyard in Zhejiang Province, China, and 3 of 20 crew members reported a fever. Subsequently, a nasopharyngeal swab was collected from each crew member to screen for SARS-CoV-2 infection, and the nasopharyngeal swab sample from 12 members, including 3 members with fever, tested positive for SARS-CoV-2 using real-time reverse-transcription PCR assay. Nasopharyngeal swabs from 2 patients with cycle threshold (Ct) values <30 were sequenced for the whole viral genome of SARS-CoV-2 and were identified as having the variant B.1.617.2 (GISAID accession ID EPI_ISL_3611059-3611060). Serum samples were obtained from 8 of 12 crew members between June and July 2021 after they were discharged from the hospital, approximately 2 months after infection.
Pseudovirus Production and Neutralization Assay
Pseudovirus particles were generated by cotransfecting HEK-293T cells (CRL-3216; American Type Culture Collection) with human immunodeficiency virus backbones expressing firefly luciferase (pNL4-3-R-E-luciferase, provided by Dr. Lin-Qi Zhang from Tsinghua University) and pcDNA3.1 vector encoding either WA1 or mutated S proteins (Beta, Delta, and Omicron) plasmid. The medium was replaced with fresh medium at 24 hours, and supernatants were harvested at 48 hours posttransfection and clarified by centrifugation at 300 ×g for 10 minutes before aliquoting and storing at −80°C until use. The SARS-CoV-2 pseudovirus neutralization assay (pVNT) was performed with target cell line HeLa cells expressing ACE2 orthologs (HeLa-ACE2, provided by Dr. Lin-Qi Zhang). Duplicate 3-fold, 8-point serial dilutions (starting at 1:30) of heat-inactivated serum were incubated with 500–1000 TCID50 of SARS-CoV-2 pseudotyped virus for 1 hour at 37°C. HeLa-ACE2 (200 000 cells/well) were subsequently added into the mixture and incubated for approximately 48 hours at 37°C with 5% CO2. Luciferase activity was then measured using GloMax 96 Microplate Luminometer (Promega). The half-maximal neutralization titers for serum were determined by luciferase activity 48 hours after exposure to the virus-serum mixture with a 4-parameter nonlinear regression inhibitor curve in GraphPad Prism 8.4.1 (GraphPad Software). Titers are reported as the serum dilution that inhibited 50% of infection (pVNT50). A samples with pVNT50 titer no more than 30 (the detectable limit) was considered negative for neutralizing antibodies and was assigned a nominal value of 10 for geometric mean titer (GMT) calculation.
Statistical Analysis
The Friedman test with false discovery rate method was used for multiple group comparisons. The Wilcoxon rank-sum test was used to analyze the difference between the 2 groups. All statistical analyses were performed using GraphPad Prism (version 8.4.2; La Jolla, CA), and all statistical tests were 2-sided with a significance level of 0.05.
RESULTS
We obtained convalescent serum samples from 28 COVID-19 patients with the Delta infection only and 31 COVID-19 patients with the Delta infection after vaccination (Table 1). In addition, considering that 26 of 28 COVID-19 patients with the Delta infection only from Shijiazhuang City were under 18 years old, convalescent serum samples from 8 COVID-19 patients with the Delta infection only and >18 years old from a cargo ship were included for the analysis (Table 1). The median age for patients with the Delta infection after vaccination was 42 years (interquartile range [IQR], 37–65; range 9–77 years), 10 years (IQR, 8–12, range 2–68 years) for patients with Delta infection only from Shijiazhuang City, and 37 years (IQR, 29–46; range 19–50 years) for patients with Delta infection only from the cargo ship (Table 1). Convalescent serum samples were collected at a median day of 47, 38, and 53 from symptoms onset to sampling for Delta infection after vaccination, Delta infection only from Shijiazhuang, and Delta infection only from the cargo ship, respectively. Among 31 patients with the Delta infection after vaccination, 22 have received 2 doses of CoronaVac, and 9 received 3 doses of the ZF2001 vaccine.
Characteristics of the Patients With SARS-CoV-2 Delta Infection After Vaccination and Delta Infection Onlya
| Characteristics . | Delta Infection After Vaccination . | Delta Infection Only . | P Value . | ||
|---|---|---|---|---|---|
| All . | Shijiazhuang . | Crew Members . | |||
| No. of subjects | 31 | 36 | 28 | 8 | |
| Age (median, IQR) | 42.0 (37.0–65.0) | 11.5 (9.0–25.0) | 10.0 (8.0–12.0) | 37.0 (29.3–45.5) | <.0001 |
| Age Group (%) | <.0001 | ||||
| >18 | 29 (93.5) | 10 (27.8) | 2 (7.1) | 8 (100) | |
| ≤18 | 2 (6.5) | 26 (72.2) | 26 (92.9) | 0 | |
| Sex (%) | .468 | ||||
| Male | 16 (51.6) | 22 (61.1) | 14 (50.0) | 8 (100) | |
| Female | 15 (48.4) | 14 (38.9) | 14 (50.0) | 0 | |
| Disease Severity (%) | .333 | ||||
| Asymptomatic | 2 (6.5) | 0 | 0 | 0 | |
| Mild | 7 (22.6) | 22 (61.1) | 22 (78.6) | 0 | |
| Moderate | 21 (67.7) | 12 (33.3) | 6 (21.4) | 6 (75.0) | |
| Severe | 1 (3.2) | 2 (5.6) | 0 | 2 (25.0) | |
| Vaccination Status (%) | |||||
| Yes | 31 (100) | 0 | 0 | 0 | |
| No | 0 | 36 (100) | 28 (100) | 8 (100) | |
| Type of Vaccine (%) | |||||
| CoronaVac | 22 (71.0) | 0 | 0 | 0 | |
| ZF2001 | 9 (29.0) | 0 | 0 | 0 | |
| Interval between 2nd or 3rd dose and symptom onset (median, IQR) | 118.0 (47.0–148.0) | NA | NA | NA | |
| Interval between symptom onset and sampling (median, IQR) | 47.0 (36.0–53.0) | 43.0 (34.0–53.0) | 38.0 (31.5–45.8) | 53.0 (53.0–87.0) | |
| Characteristics . | Delta Infection After Vaccination . | Delta Infection Only . | P Value . | ||
|---|---|---|---|---|---|
| All . | Shijiazhuang . | Crew Members . | |||
| No. of subjects | 31 | 36 | 28 | 8 | |
| Age (median, IQR) | 42.0 (37.0–65.0) | 11.5 (9.0–25.0) | 10.0 (8.0–12.0) | 37.0 (29.3–45.5) | <.0001 |
| Age Group (%) | <.0001 | ||||
| >18 | 29 (93.5) | 10 (27.8) | 2 (7.1) | 8 (100) | |
| ≤18 | 2 (6.5) | 26 (72.2) | 26 (92.9) | 0 | |
| Sex (%) | .468 | ||||
| Male | 16 (51.6) | 22 (61.1) | 14 (50.0) | 8 (100) | |
| Female | 15 (48.4) | 14 (38.9) | 14 (50.0) | 0 | |
| Disease Severity (%) | .333 | ||||
| Asymptomatic | 2 (6.5) | 0 | 0 | 0 | |
| Mild | 7 (22.6) | 22 (61.1) | 22 (78.6) | 0 | |
| Moderate | 21 (67.7) | 12 (33.3) | 6 (21.4) | 6 (75.0) | |
| Severe | 1 (3.2) | 2 (5.6) | 0 | 2 (25.0) | |
| Vaccination Status (%) | |||||
| Yes | 31 (100) | 0 | 0 | 0 | |
| No | 0 | 36 (100) | 28 (100) | 8 (100) | |
| Type of Vaccine (%) | |||||
| CoronaVac | 22 (71.0) | 0 | 0 | 0 | |
| ZF2001 | 9 (29.0) | 0 | 0 | 0 | |
| Interval between 2nd or 3rd dose and symptom onset (median, IQR) | 118.0 (47.0–148.0) | NA | NA | NA | |
| Interval between symptom onset and sampling (median, IQR) | 47.0 (36.0–53.0) | 43.0 (34.0–53.0) | 38.0 (31.5–45.8) | 53.0 (53.0–87.0) | |
Abbreviations: IQR, interquartile range; NA, not available; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
P value was calculated between Delta infection after vaccination and all of Delta infection only.
Characteristics of the Patients With SARS-CoV-2 Delta Infection After Vaccination and Delta Infection Onlya
| Characteristics . | Delta Infection After Vaccination . | Delta Infection Only . | P Value . | ||
|---|---|---|---|---|---|
| All . | Shijiazhuang . | Crew Members . | |||
| No. of subjects | 31 | 36 | 28 | 8 | |
| Age (median, IQR) | 42.0 (37.0–65.0) | 11.5 (9.0–25.0) | 10.0 (8.0–12.0) | 37.0 (29.3–45.5) | <.0001 |
| Age Group (%) | <.0001 | ||||
| >18 | 29 (93.5) | 10 (27.8) | 2 (7.1) | 8 (100) | |
| ≤18 | 2 (6.5) | 26 (72.2) | 26 (92.9) | 0 | |
| Sex (%) | .468 | ||||
| Male | 16 (51.6) | 22 (61.1) | 14 (50.0) | 8 (100) | |
| Female | 15 (48.4) | 14 (38.9) | 14 (50.0) | 0 | |
| Disease Severity (%) | .333 | ||||
| Asymptomatic | 2 (6.5) | 0 | 0 | 0 | |
| Mild | 7 (22.6) | 22 (61.1) | 22 (78.6) | 0 | |
| Moderate | 21 (67.7) | 12 (33.3) | 6 (21.4) | 6 (75.0) | |
| Severe | 1 (3.2) | 2 (5.6) | 0 | 2 (25.0) | |
| Vaccination Status (%) | |||||
| Yes | 31 (100) | 0 | 0 | 0 | |
| No | 0 | 36 (100) | 28 (100) | 8 (100) | |
| Type of Vaccine (%) | |||||
| CoronaVac | 22 (71.0) | 0 | 0 | 0 | |
| ZF2001 | 9 (29.0) | 0 | 0 | 0 | |
| Interval between 2nd or 3rd dose and symptom onset (median, IQR) | 118.0 (47.0–148.0) | NA | NA | NA | |
| Interval between symptom onset and sampling (median, IQR) | 47.0 (36.0–53.0) | 43.0 (34.0–53.0) | 38.0 (31.5–45.8) | 53.0 (53.0–87.0) | |
| Characteristics . | Delta Infection After Vaccination . | Delta Infection Only . | P Value . | ||
|---|---|---|---|---|---|
| All . | Shijiazhuang . | Crew Members . | |||
| No. of subjects | 31 | 36 | 28 | 8 | |
| Age (median, IQR) | 42.0 (37.0–65.0) | 11.5 (9.0–25.0) | 10.0 (8.0–12.0) | 37.0 (29.3–45.5) | <.0001 |
| Age Group (%) | <.0001 | ||||
| >18 | 29 (93.5) | 10 (27.8) | 2 (7.1) | 8 (100) | |
| ≤18 | 2 (6.5) | 26 (72.2) | 26 (92.9) | 0 | |
| Sex (%) | .468 | ||||
| Male | 16 (51.6) | 22 (61.1) | 14 (50.0) | 8 (100) | |
| Female | 15 (48.4) | 14 (38.9) | 14 (50.0) | 0 | |
| Disease Severity (%) | .333 | ||||
| Asymptomatic | 2 (6.5) | 0 | 0 | 0 | |
| Mild | 7 (22.6) | 22 (61.1) | 22 (78.6) | 0 | |
| Moderate | 21 (67.7) | 12 (33.3) | 6 (21.4) | 6 (75.0) | |
| Severe | 1 (3.2) | 2 (5.6) | 0 | 2 (25.0) | |
| Vaccination Status (%) | |||||
| Yes | 31 (100) | 0 | 0 | 0 | |
| No | 0 | 36 (100) | 28 (100) | 8 (100) | |
| Type of Vaccine (%) | |||||
| CoronaVac | 22 (71.0) | 0 | 0 | 0 | |
| ZF2001 | 9 (29.0) | 0 | 0 | 0 | |
| Interval between 2nd or 3rd dose and symptom onset (median, IQR) | 118.0 (47.0–148.0) | NA | NA | NA | |
| Interval between symptom onset and sampling (median, IQR) | 47.0 (36.0–53.0) | 43.0 (34.0–53.0) | 38.0 (31.5–45.8) | 53.0 (53.0–87.0) | |
Abbreviations: IQR, interquartile range; NA, not available; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
P value was calculated between Delta infection after vaccination and all of Delta infection only.
We first tested neutralizing activity against pseudoviruses expressing the spike proteins of the Wuhan-Hu-1 (WA1) vaccine strain and the Beta, Delta, and Omicron variants in serum samples from Delta infection only. We found high levels of neutralizing activity against both the Delta and WA1 with a GMT of 1245.0 (95% confidence interval [CI], 692.2–2240) and 563.0 (95% CI, 300.3–1055.0), respectively (Figure 1a). However, serum neutralizing activity against the Beta variant was decreased to a GMT of 38.5 (95% CI, 19.7–75.4), and only 15 (41.7%) of 36 serum samples displayed detectable serum neutralizing activity. It is remarkable that only 6 (16.7%) of the 36 serum samples displayed detectable serum neutralizing activity against the Omicron, resulting in a GMT of 19.2 (95% CI, 11.4–32.5) with a 64.9-fold decrease compared with the Delta variant (Figure 1a). Further analysis showed no significant differences in antibody response between patients who were >18 years old and less than 18 years old regardless of WA1 and variants (Figure 1b). These findings suggest that almost all convalescent serum from patients with the Delta infection only loss the neutralizing activity to the Omicron, and antibody response was not affected by the age.
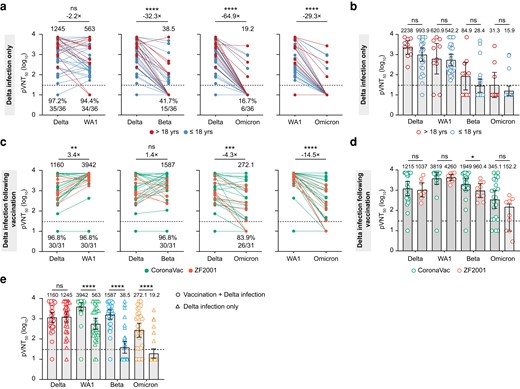
Neutralization of Delta, Wuhan-Hu-1 (WA1), Beta, and Omicron to sera from convalescent patients with Delta infection after vaccination and Delta infection only. (a) Neutralizing activity of sera from convalescent patients with Delta infection only (n = 36) sampled approximately 1 month after infection. Participants were either >18 years old (red, n = 10) or less than 18 years old (blue, n = 26). (b) Comparison of the antibody titer between patients >18 years old and patients ≤18 years old. (c) Neutralizing activity of sera from convalescent patients with Delta infection after vaccination (n = 31) sampled approximately 1 month after infection. Participants were either received CoronaVac (green, n = 22) or ZF2001 (orange, n = 9). (d) Comparison of the antibody titer between patients vaccinated CoronaVac (n = 22) and ZF2001 (n = 9). (e) Comparison of the antibody titer between patients Delta infection after vaccination and Delta infection alone by Delta (red), WA1 (green), Beta (blue), and Omicron (Orange). The neutralization titers of the sera against the indicated viral variants are expressed as 50% pseudovirus inhibitory dilution (pVNT50). Geometric mean titers (GMT) are shown above each column. The percentage of samples with neutralizing activity in pVNT50 are shown below each column. The heights of bars indicate GMT; error bars show 95% confidence intervals. The horizontal dotted line represents the limit of detection at 1:30. A 2-tailed Friedman test with a false discovery rate for multiple comparisons was performed to compare WA1, Beta, and Omicron to the Delta in a and c as well as Wilcoxon rank-sum test was performed in b, d, and e. *, P < .05; **, P < .01; ***, P < .001; and ****, P < .0001. ns, not significant.
We next determined the neutralization activity of the serum from patients with the Delta infection after vaccination against WA1, Beta, Delta, and Omicron pseudoviruses. Of note, we observed that Delta infection after vaccination enhanced antibody response against WA1 with a GMT of 3942.0 (95% CI, 2480.0–6265.0), which was significantly higher (3.4-fold) than antibody response against the Delta variant with a GMT of 1160.0 (95% CI, 641.6–2098.0) (Figure 1c). Moreover, although GMT against the Omicron variant displayed a 4.3-fold and 14.5-fold decrease compared with GMT against the Delta and WA1, respectively, the serum had a high potency to neutralize the Beta and Omicron variants. We observed that only 1 (3.2%) and 5 (16.1%) of 31 serum samples completely lost neutralizing activity against the Beta and Omicron variants, respectively (Figure 1c). We then compared antibody response between patients vaccinated CoronaVac and ZF2001 (Figure 1d). No significant differences were observed for antibody response against WA1, Beta, and Omicron between the 2 groups, whereas CoronaVac-vaccinated patients displayed a higher GMT of 1949 against Beta than ZF2001-vaccinated patients with a GMT of 960.4 (Figure 1d). We further compared antibody response between patients with the Delta infection alone and patients with the Delta infection after vaccination. We found that antibody levels against WA1, Beta, and Omicron in serum from the Delta infection after vaccination were significantly higher than the Delta infection alone (Figure 1e). In contrast, no significant difference was observed in the antibody titers against the Delta between the 2 groups (Figure 1e). These findings revealed that the Delta infection after vaccination not only boosted high potency neutralizing antibodies against WA1, but it also boosted antibody response to the Beta and Omicron variants regardless of CoronaVac or ZF2001.
DISCUSSION
The rapid and widespread growth of the Omicron virus poses an urgent challenge to public health. Lower neutralizing antibody titers have been associated with an increased risk of symptomatic COVID-19 [9], indicating that completely lost or limited neutralizing activity against the Omicron may increase risk of infection and higher burden of disease [10]. Our study demonstrates significant resistance of the Omicron variant to serum neutralizing activity induced by the Delta infection only. However, despite the significant escape of the Omicron to antibody response, recent studies have shown that the majority of T-cell responses induced by infection or vaccination remain capable of recognizing the Omicron variant and previously emerged variants [11]. Whether cellular immunity will be effective as a second-level defense in preventing severe disease after Omicron infection in the absence of a potent neutralizing antibody response remains to be determined [12].
More importantly, after vaccination, patients with the Delta infection effectively induced a substantial increase in serum neutralization against vaccine-matched WA1 virus and the Beta and Omicron variants. Given the similarity of the booster antigens for the infection with live virus and booster vaccination with messenger ribonucleic acid (mRNA), our findings are consistent with recent studies that a single-dose mRNA in vaccinated individuals enhanced the ability to neutralize the Omicron [6, 7, 13]. The enhanced neutralizing activity to variants may be due to the broad and potent neutralizing activity of antibodies produced the evolved memory B cells were recruited into the plasma cell compartment [14, 15]. However, higher levels of neutralizing serum activity might not necessarily prevent SARS-CoV-2 infection, as indicated by reports of Omicron infections in individuals who had received a booster vaccine. Our data, taken together, suggest that Delta infection after vaccination could generate a strong antibody response in previously vaccinated individuals, not only when considering the antibodies levels but also when examining results of the neutralizing activity to variants.
Limitations of our study include that the antibodies were measured approximately 1 month after the Delta infection in previously vaccinated individuals. Longitudinal follow-up will be required to determine the durability of the neutralizing antibody response to the Omicron. Our analysis was limited to small sample size individuals with Delta infection after receiving the ZF2001 vaccine and more children in patients with Delta infection only. Measures of T- and B-cell responses can shed further light on whether Delta infection after vaccination might be sufficient for augmenting T- and B-cell memory against the Omicron.
CONCLUSIONS
In summary, Omicron causes widespread escape from neutralization by serum obtained the Delta infection only, meaning that previously Delta-infected individuals will have little protection from infection with Omicron. In contrast, the Delta infection after vaccination can induce robust neutralization against the immune evasive Omicron variant.
Notes
Acknowledgments. We thank all study subjects for their participation in our studies.
Author contributions. M.-J. M. conceived the study. H.-X. G., J. R., L. Y., Z. Y., P. J., and E.-H. D. collected serum samples. L. Y. and K.-L. Z. performed serology assays. L. Y., K.-L. Z., and M.-J. M. analyzed the data. M.-J. M. drafted the manuscript. All authors reviewed and approved the final manuscript.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the official position of Anhui Medical University, Beijing Institute of Microbiology and Epidemiology, and The Fifth Hospital of Shijiazhuang. All the authors have declared no relationships or activities that could appear to have influenced this work.
Financial support. This work was funded by grants from the Beijing Natural Science Foundation (L202038) and the Natural Science Foundation of China (81773494 and 81621005).
References
Author notes
K.-L. Z., H.-X. G., and L. Y. contributed equally to this study.
E.-H. D. and M.-J. M. are senior authors and contributed equally to this study.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.




